Keywords
Polymeric nanoparticles; Salting out method; Control/ Living radical polymerization; Ring opening polymerization; Macro initiator; Radio sensitizer; Spin penetration; Desolvation of macromolecules; Micro tube pumping; Enhanced permeation and retention effect
Introduction
Since the genesis of Nanotechnology and nanoparticles, the field of polymeric nanoparticles (PNP) is broadly expanding and playing a crucial role in a wide spectrum of areas ranging from medicine to biotechnology, pollution control to environmental technology [1]. Nanoparticles (10 to 1000 nm) are formulated or engineered to carry an array of moieties in a con-trolled targeted manner and to achieve a greater pharmacological response [2,3]. Targeted and tumor-directed therapies are required to improve the outcome and reducing the toxicity [4]. With this, conjugation of drug-loaded nanoparticles along with targeting moieties can be used for receptor-mediated and targeted delivery [5-7].
The use of nanoparticles has a number of advantages viz. targeting, enhancements of therapeutic effect, enhancing permeability, dose reduction and toxic effects as well as availability in parenteral, pre-orals and topical [8,9]. Among the new drug delivery systems, polymeric nanoparticles have been considered as favorable and rising carriers for anticancer agents [10]. Moreover, these polymeric colloidal systems, after i.v. administration, may extravasate solid tumours and infected sites, where the capillary endothelium is defective, thus the tutor site is passively targeting via drug loaded nanoparticles [11].
Polymeric nanoparticles offer significant edge over other nanocarrier, primarily since a tremendous versatility in polymer matrices allows for tailoring the nanoparticle properties. In addition, ease of production, ease of surface modification, encapsulation efficiency, payload protection, slow or fast polymer degradation and stimuliresponsive polymer erosion for temporal control over the drug release, and can easily be scaled-up and manufacturing under Current Good Manufacturing Practices (cGMP) guidelines [12].
The polymer matrix of the nanoparticles must meet several requirements such as biocompatibility, easily biodegradable, better mechanical strength, and easy processing. For controlled release, the best known class of biodegradable materials is the poly (lactide- co-glycolide) s (PLGAs). Following the trend of green polymers, widely accepted and used are biopolymers viz. bovine serum albumin (BSA), human serum albumin (HAS), collagen, gelatin, and hemoglobin have been explored regarding their application to drug delivery systems [13,14].
Advantage of Nano Formulation
In comparison to conventional cancer treatments, the nano scale of these particulate systems also lowers the irritant reactions at the injection site [2].
1. Nanotechnology-based delivery systems can also protect drugs from degradation.
2. Due to decrease in the size the available enhanced product may vary in their physical properties.
3. Reduction in dose frequency.
4. Economic and Patient compliance.
5. Insoluble drugs can also be delivered using Nano-based delivery systems.
6. Nano-based systems can also incorporate previously rejected drugs or drugs with administration issues.
7. Due to specific pathophysiological feature of the diseased tissues, drug targeting is refined and improved.
8. An ideal targeting system should have longer circulating time and optimum concentration at target site.
9. Its pharmacological activity is not affected by longer circulating time.
10. Enhanced permeability and retention effect are characteristic features of tumor, aiding drug delivery.
11. Macrophages (liver and spleen) are passively targeted by drugs.
12. Blood Brain Barrier is the most efficient and challenging natural barrier for CNS targeting drugs which are Lipophilic. For such drugs nanotechnology is the simplest solution as such drugs reach the target site via ultra-filtration process due to its nano-size.
13. Enhance the oral bioavailability of the agents that are not effectively used orally.
Conventional Method of Preparation
Conventionally, two approaches are employed in synthesis of NPs: Pre-formed polymers dispersion method; and Monomer polymerization (Figure 1) [15].
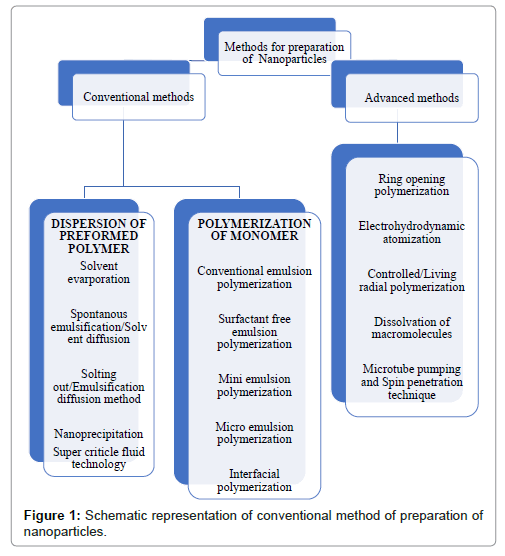
Figure 1: Schematic representation of conventional method of preparation of nanoparticles.
Pre-formed polymer dispersion method
Many approaches have been suggested to prepare biodegradable NPs from polymers (PLA, PLG, PLGA and poly) E-caprolactone)) by dispersing the preformed polymers [16,17].
Solvent evaporation method: This method involves, formation of an emulsion, using aqueous and organic phase. The polymer is completely dissolved in the organic solvent (e.g., Dichloromethane, chloroform, ethyl acetate). The drug is dispersed into the above preformed polymer solution. The aqueous solution is added to make an emulsion making an O/W (oil/water) emulsion. To prevent phase separation surfactant/emulsifying agents (gelatin, poly(vinly alcohol], polysorbate-80,) are added. The end step is to evaporate the organic phase either by temperature and pressure or by continuous stirring [18].
Spontaneous emulsification/solvent diffusion technique: This is an upgraded approach of the conventional solvent techniques (evaporation) [19], in which the oil phase consists of a water-soluble organic solvent (acetone, methanol) and water insoluble solvent (dichloromethane, chloroform) as oil phase. Aqua is used as the aqueous phase. Upon mixing the water soluble organic phase spontaneously diffuse into the water and thus precipitating out the water insoluble organic phase contain the drug as polymeric nano-particles. The remaining aqueous phase is then subjected to evaporation resulting in polymeric NP. The formation of NP’s depends upon the Oil-to-polymer ratio and offers advantages over the conventional methods.
Salting out/emulsification–diffusion method: Bindschaedler et al. [20] first disclosed a modified version of emulsion process that results in a salting-out process which avoids surfactants and chlorinated solvents. Polymer and drug are firstly dissolved in a water-miscible solvent such as acetone, it is then emulsified more like Ouzo effect [21], into aqueous gel containing a salting agent (electrolyte: MgCl2, CaCl2; non-electrolyte: Sucrose). Dilute it with sufficient water to facilitate the acetone diffusion into aqueous phase resulting in salting out as nanosphere/particles. Both the solvent and salting agent are removed via cross-flow filtration. The selection of appropriate salting agent is vital as it plays an important role in the encapsulation process of making the nanoparticles [22,23].
Nanoprecipitation: This approach is also known as solvent displacement method. The preformed polymer dis-solved into the organic layer is precipitated out by diffusion of organic solvent into the aqueous medium by either using surfactant [24]. The polymer (PLA), is dissolved in a water-miscible solvent of intermediate polarity, it’s diffusion into aqueous phase leading to the precipitation of nanoparticles [25].
Production of NPs using supercritical fluid technology: Conventional methods (solvent evaporation, coacervation and in situ polymerization) involve the use of toxic solvents and/or surfactants. The current green approach is the use of Super-critical fluids. This method utilizes CO2 in its supercritical state where it provided the ad-vantage of both liquid and gas, and above all is environment safe. Easy regulation of temperature, pressure, higher purity of NP’s and minimum-to-no solvent residue are the attractive features of this approach [11].
The NP’s are prepared via two methods, RESS (Rapid Expansion Supercritical Solution), RESOLV, SAS (solvent anti solvent).
RESS method is a simple approach which utilizes Supercriticald CO2 as a solvent. The material of interest is completely dissolved into it, and drawn out of a nozzle by varying its temperature/pressure, thus precipitating out the NP’s and CO2 released as a gas. This is a clean technique as no residue of solvent remains. It is highly favourable for bio-erodible drug loaded polymers. The major drawback of this technique is the limited molecular mass (10,000) and solubility in SCCO2 [26,27].
RAS, a simple, but significant modification to RESS involves spreading of the supercritical solvent into a liquid solvent and also termed as RESOLV [28]. The liquid solvent apparently suppresses the particle growth in the expansion jet, thus making it possible to obtain primarily nano-sized particles.
In SAS method, is widely accepted for the drug-polymer which cannot be dissolved into SC-CO2. The drug-polymer is dissolved in a solvent which favours the SC-CO2. When the SC-CO2 is introduced into the solvent-drug-polymer mixture, at high pressures, enough antisolvent will enter into the liquid phase so that the solvent power will be lowered and the solute precipitates. After precipitation, when the final operating pressure is reached, the anti-solvent flows through the vessel so as to strip the residual solvent. This method, also called as gas antisolvent (GAS) technique [29].
Polymerization of monomers
Conventional emulsion polymerization: In this conventional system, the ingredients are comprised of surfactant, water, a monomer of low water solubility, and a water-soluble initiator. Colloidal stabilizers may be electrosteric, electrostatic, steric or exhibiting both stabilizing mechanisms. Initiation appears when a monomer molecule dissolved in the continuous aqueous phase of collides with an initiator molecule that might be a free radical or ions. Alternatively, through high-energy radiation the monomer molecule can be changed into an initiatingradical. Formation of solid particles and phase separation can occur before or after the termination of the polymerization reaction [30].
Surfactant-free emulsion polymerization: The varying quantities of surfactants are being utilized in the process of conventional emulsion polymerization systems, but there is need to eliminate those surfactants from the final product. Removal of surfactants increases the cost of production and is a slow process. Moreover, increasing energy and environmental concerns cannot be effectively addressed be-cause of these drawbacks. As a substitute, emulsion polymerization has been performed in the absence of added emulsifier, often referred to as emulsifier-free, surfactant-free, or soap-less emulsion polymerization [31,32]. This technique has received considerable attention, to be used as a simple, green process for PNP production without the addition of stabilizing surfactant as well as for its subsequent removal.
Mini-emulsion polymerization: A distinctive formulation used in mini-emulsion polymerization consists of monomer mixture water, initiator co-stabilizer, and surfactant. A low molecular mass compound acts as the co-stabilizer and also the use of a high-shear device (ultrasound, etc.) is the main difference between mini-emulsion polymerization and emulsion polymerization.
The critically stabilized Mini-emulsions require a high-shear to reach a steady state and have an interfacial tension much greater than zero. With various co-stabilizers and initiator combi-nations versatile PNPs are well developed in the present era [33].
Micro-emulsion polymerization: The new and effective approach that has attracted significant attention for preparing nano sized polymer particles is a micro-emulsion polymerization. Although microemulsion polymerization and emulsion appear similar the reason being both methods can produce colloidal polymer particles with high molar mass but they are entirely different when compared kinetically. Micro-emulsion polymerization exhibits two reaction rate intervals, whereas in emulsion polymerization three are detected. Both the average number of chains per particle and particle size are considerably smaller in micro-emulsion polymerization [34]. In the pro-cess of micro-emulsion polymerization a water soluble initiator is added to the aqueous phase of swollen micelles that are thermodynamically stable micro emulsions. The polymerization starts with the spontaneous formation of thermodynamically stable state and relies on high quantities of surfactant systems, which possess an interfacial tension, close to zero at the oil/water interface.
Interfacial polymerization: The Interfacial polymerization is one of the well-established methods used for the preparation of polymeric nano-particles [35]. It involves step i.e., polymerization of two reactive monomers or agents, which are dissolved respectively in two phases (i.e., dispersed-phase and a continuous phase), and the reaction takes place at the interface of the two liquids [36].
Preparation of nanoparticles can also be done by polymerization of monomers (Figure 2). Poly (alkylcyanoacrylates), PACA, being biodegradable is used to formulate nanoparticles by polymerization method. Here, the cyanoacrylic monomer under vigorous and continuous stir-ring is added to an aqueous solution of surface-active agents (polymerization medium) at ambient temperature to polymerize the alkyl cyanoacrylate. Drug is then dissolved in the polymerization medium either before the addition of the monomer or at the finish of the polymerization reaction. The NP suspension is then purified by ultracentrifugation or by re-suspending the particles in an isotonic surfactant free medium [37].
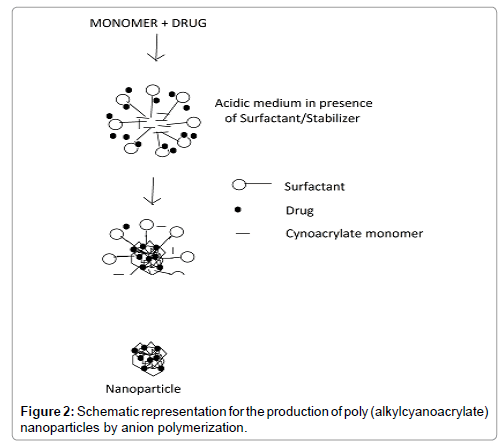
Figure 2: Schematic representation for the production of poly (alkylcyanoacrylate) nanoparticles by anion polymerization.
Different Methods for Preparation of Nanoparticles
Drug loading into the NPs is achieved by two methods: firstly, by adsorbing the drug after the formation of NPs by incubating them in the drug solution or secondly, by incorporating the drug at the time of NP production. It is thus evident that a large amount of drug can be entrapped by the process of incorporation when compared to the adsorption [38,39].
The kind of surface-active materials and stabilizers has an effect on drug loading [40]. Beside adsorption and incorporation, a novel method of drug loading for the water-soluble drugs was proposed by Yoo et al. [41-43]. In this method, drug was chemically conjugated into NPs, spontaneous emulsion solvent diffusion method was used to prepare conjugates of doxorubicin-PLGA and doxorubicin-loaded PLGA nanoparticles and found to have good encapsulation efficiency of 96.6% as well as 3.5% loading of nano-particles (Figure 3a).
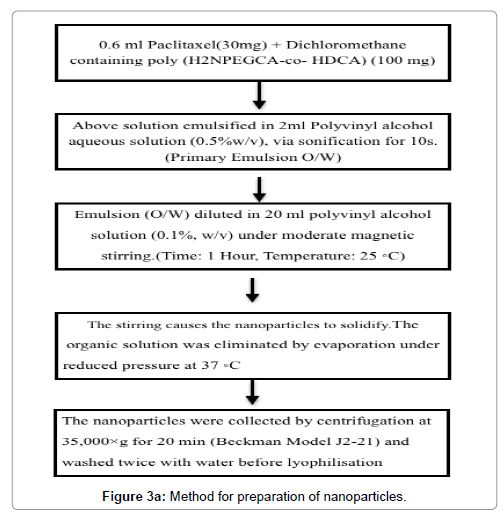
Figure 3a: Schematic representation for the production of poly (alkylcyanoacrylate) nanoparticles by anion polymerization.
Using the ring opening polymerisation technique, studies show the preparation of H2N-PEG-PLA, which uses H2N-PEG as micro initiator [44] (Figure 3b).
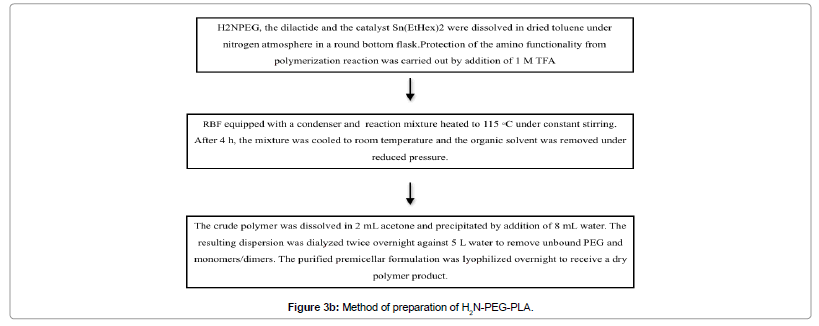
Figure 3b: Schematic representation for the production of poly (alkylcyanoacrylate) nanoparticles by anion polymerization.
Electro hydrodynamic atomization
This new method was first utilised by Xie et al. [45] who developed nanoparticles by electro hydrodynamic atomization (EHDA) of a PLGA solution using acetonitrile. This basic phenomenon has been laid by sir Raileigh who described the possibility to diffuse the fluid in small electrically charged droplets under the influence of an electrostatic field. Due to the electrostatic charge on droplets, maximum charge is displayed on the surface of the particles which further produces repulsion and thus, nanoparticle forms.
Controlled/Living Radical Polymerization (C/LRP)
The recent emergence of controlled or ‘living’ radical polymerization (C/LRP) processes has opened a new area using an old polymerization technique [46-48]. Implementation of C/LRP in the industry is important for developing aqueous dispersed systems that result in the formation of polymeric nanoparticles with reduced particle size and narrow size distribution.
Among the available controlled/living radical polymerization methods, mainly three approaches are presently successful and extensively studied viz. nitroxide-mediated polymerization (NMP) [49-52], atom transfer radical polymerization (ATRP) [53,54] and reversible addition and fragmentation transfer chain polymerization (RAFT) [55,56].
Dialysis
Dialysis offers an easy and most effective method for the preparation of small, narrow-distributed PNP [57-59]. Polymer is solubilised in an organic solvent and placed inside a dialysis tube with proper molecular weight cut-off. Dialysis was carried out against a non-solvent miscible with the former miscible and the displacement of the solvent inside the membrane is followed by the developmental stage of polymer aggregation due to less solubility and the formation of homogeneous suspension (Figure 4). It is thought that it may be based on a mechanism similar to that of nano-precipitation [60]. A number of polymer and copolymer nanoparticles [61,62] were obtained by this method.
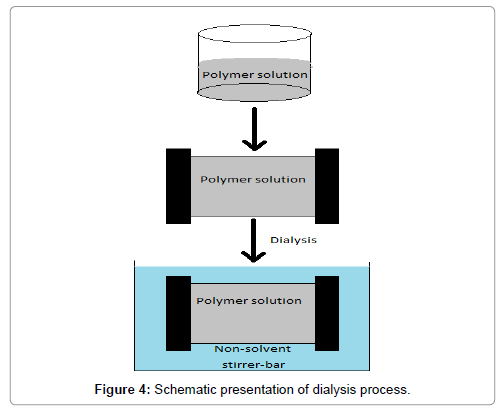
Figure 4: Schematic presentation of dialysis process.
Nanoparticles Produced by Desolvation of Macromolecules
Another technology that is useful for a wide range of polymers is based on addition of a desolvating agent or by desolvation by charge and pH changes, (ethanol or concentrated in-organic salt solutions) (Figure 5).

Figure 5: PNP preparation by desolvation technique.
The main advantage of this process is that it does not require high temperature and, therefore, may be useful when heat sensitive drugs are used [63]. This process offers the advantage of producing nanoparticles directly in aqueous suspension, but, use of potentially toxic com-pounds such as glutaraldehyde and desolvating agents requires subsequent purification [64]. Nano-particle produced by the desolvation process, unfortunately have comparatively low yield [65]. In case of gelatine, different methods such as the two-step desolvation method [66,67] have been applied to produce nanoparticles.
High Pressure Homogenization
Now a day, production of nano-particles is through widely spreaded proven technology known as High-pressure homogenization (HPH). A formulation of paclitaxel is prepared by solvent-free method using serum albumin (3%-4%) known as Nab-paclitaxel which is con-verted to nanonization by utilising HPH technology, resulting in a colloidal solution [55]. This solvent-free 130 nm particle produced for paclitaxel, has been designed to overcome the problems associated with Kolliphor EL [68-70].
Micro Tube Pumping and Spin Penetration Techniques
This technique used to achieve controlled drug release, as per literature paclitaxel (Ptx)-loaded poly (lactic-co-glycolic acid) (Ptx- PLGA-NPs), (PLGA) nanoparticles were prepared by the emulsionsolvent evaporation method and then transferred to the luminal surface and inside ePTFE vascular grafts through micro tube pumping and spin penetration techniques [71].
Loading of Ptx-PLGA-NPs to an ePTFE Graft
Before loading, silicon tube was connected in the ePTFE vascular graft which has a same in-ternal diameter (6 mm) as that of the external diameter of that silicon tube. The aqueous solution containing Ptx- PLGA-NPs of various concentrations was circulated through the tube and the graft with a micro tube pump at a flow rate of 1.91 mL/min for 30 min. Then, the graft with the Nanoparticles on its luminal surface was fixed in a polypropylene tube vertically connected to a directly driven stirrer. The NPs of the luminal surface of the graft were infiltrated into the inner part of the graft due to centrifugal force in action arise from spinning of the direct driven stirrer (1000 rpm) for 10 min. The above process was repeated three times, and then the NP-loaded grafts were freeze-dried overnight [71].
Targeting to Cancer Cell
The delivery of nanoparticles to specific sites can be through size dependant passive targeting or by active targeting in which, passive targeting depends on both tumor structure and the structure of surrounding inflamed tissues (reference). The nanoparticulate delivery systems may exploit a characteristic of solid tumors by the enhanced permeability and retention (EPR) effect in which tumor tissues demonstrate several distinctive characteristics such as hyper vasculature, defective vascular architecture and a deficient lymphatic tissue/system.
Drainage leads to accumulation of macromolecules and retention in tumor cells for a longer period of time (Figure 6). Active targeting has been performed to achieve a high degree of selectivity to specialized tissues and to enhance the uptake of NP’s into target areas such as cancer cells and angiogenic micro capillaries growing around malignant cells (Figure 7). Nano-particles are modified to target basic characteristics of cancer cells such as accelerated proliferation and particular antigen presentation [72]. Nano-particulate delivery systems utilize specific targeting agents for cancer cells and minimize the uptake of the anticancer agents with the help of normal cells, enhance the entry and release of the agent in tumor cells. These delivery systems include the anticancer agent, a targeting moiety, and a carrier and penetration enhancer. The types of molecules which are capable of specifically recognizing and binding to other biological molecules are antibodies, enzymes, receptor ligands, and receptors. In all cancer therapies, targeting over surface modification provides numerous approaches for increasing treatment specificity and accuracy while reducing toxicity to healthy cells [2].
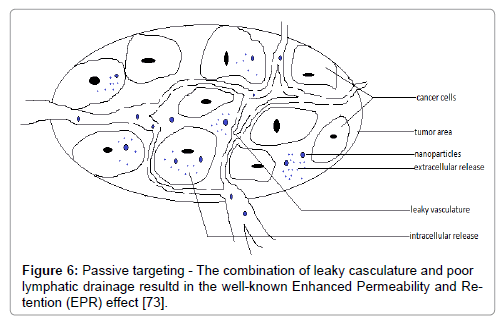
Figure 6: Passive targeting - The combination of leaky casculature and poor lymphatic drainage resultd in the well-known Enhanced Permeability and Retention (EPR) effect [73].
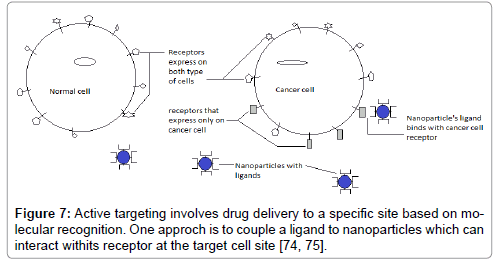
Figure 7: Active targeting involves drug delivery to a specific site based on molecular recognition. One approch is to couple a ligand to nanoparticles which can interact withits receptor at the target cell site [74, 75].
Passive targeting- The combination of leaky vasculature and poor lymphatic drainage results in the well-known Enhanced Permeability and Retention (EPR) effect (Figure 6) [73].
Active targeting involves drug delivery to a specific site based on molecular recognition. One approach is to couple a ligand to nanoparticles which can interact with its receptor at the target cell site (Figure 7) [74-94].
Ligands that Binds to the Cancer Cell Receptors
Cancer cells have many types of receptors and they are used for targeting purpose. These receptors and their ligands are given below (Table 1). Some agents are used as theragnostic agents, for therapeutic and diagnostic purpose, which are targeted to the specific cell by bonded ligand [95].
| Cancer Cell Receptors |
Ligands |
References |
| Toll-like receptors |
MPLA |
[76] |
| C-type lectins |
Mannan |
[77] |
| Siglec |
Anti-Siglec-7 polyclonal antibody |
[78] |
| Claudins |
CPE30 |
[79] |
| αvβ1 integrins |
RGD/RGDp
LDV/LDVp |
[80] |
| STAT3 |
JSI-124 |
[81] |
| ICAM-1 |
Clabl |
[82] |
| Folate receptor |
Folate |
[83] |
| Specific receptor of lymphatic metastatic tumors |
LyP-1 |
[84] |
| Prostate specific receptor |
PSMA |
[85] |
| αvβ3 integrins |
RGD |
[86] |
| Nucleolin |
AS1411 |
[87] |
| HER-2 |
rhuMAbHER2 |
[88] |
| Transferrin receptor |
Lactoferrin |
[89] |
| Opioid receptor |
Simil-opioid peptide (g7) |
[90] |
| Opioid receptor |
Simil-opioid peptide (g7) |
[91] |
| Specific brain receptor |
Pep TGN |
[92] |
| Antibody binding site on cell |
Monoclonal antibody |
[93] |
| Proteins, Phospholipids, Sugar on the cell |
Aptamer |
[94] |
Table 1: Ligands that binds to the cancer cell receptors.
Functionalization of Polymer with Ligand for Target Specific Delivery
The modifications in surface of nanoparticulate include coating/ linking with linking with folate, antibodies, adjuvants, proteins, ligands, antigens, enzymes, pH sensitive agents, and a plethora of other substances [1]. PLGA-based nanoparticles grafted with the RGD-peptidomimetic (RGDp), or RGD peptide would target the tumor endothelium and would further enhances the anti-tumor efficacy of PTX. According to in-vitro studies, it was found that RGD-grafted nanoparticles were more associated to Human Umbilical Vein Endothelial cells (HUVEC) on binding to αvβ3 integrin than non-targeted nanoparticles. In-vivo studies demonstrated that the targeting of RGD and RGDp-grafted nanoparticles to tumor vessels as well as the effective retardation of TLT tumor growth and extended survival chances of mice treated by PTX-loaded RGD-nanoparticles when compared to non-targeted NP’s. Hence, the targeting of anti-cancer drug to tumor endothelium by RGD-labelled NP is a promising approach [96].
It was concluded that vitamin E TPGS (d-α-Tocopheryl polyethylene glycol 1000 succinate) has great advantages for the manufacture of controlled release polymeric nanoparticles of paclitaxel and other anti-cancer drugs. Nanoparticles of with narrow distribution can be obtained with drug encapsulation efficiency as high as 100% and the release kinetics can also be controlled [97].
The targeting characteristics of Polystyrene-latex nanospheres (PSL-NS, mean diameter, 85 nm) coated with (LPS, high affinity to hepatocytes) was evaluated at hepatocytes and PSL-NS surfaces [98]. Hepatocytes were adhered specifically with the lactosylpolystyrene coated dishes made of the same materials as PSL-NS. Surface modification of biodegradable and long-circulating polymeric nanoparticles has been achieved mainly by two methods: (i) surface coating with hydrophilic polymers/surfactants; and (ii) use of hydrophilic segments to develop biodegradable copolymers.
PEG and PEO-coated NPs
A model for repulsion of proteins from the solid substrate was proposed by Joen et al. [99] which delivers a basis for the prevention of opsonin deposition. The PEG with high surface density and long chain lengths are necessary for low protein adsorption and above all coated PEG increases the systemic circulation of nanoparticles. The nanoparticles shows lower up-take in the liver but shows higher uptake in spleen because of removal of PEG coating so it is useful in spleen targeting [100].
An attempt was made to study the effect of surface density of PEO on the compliment consumption by using diblock polymer of PLA and polyethylene oxide (PLA–PEO) Vittaz et al. [101]. It was observed that as the PEO density on the surface of the Nanoparticles increases with the decrease in compliment consumption due to steric repulsion of the surface to proteins.
Poloxamine and Poloxamer Coated NPs
In a study conducted by Illum and Davis [102], long-circulating NP’s of polystyrene and poly (methyl methacrylate) were developed with a coating of poloxamer and poloxamine. It was observed that hepatic uptake decreased about 20% with poloxamer-188 and 40% with poloxamer-338, whereas a decrease about 60% was observed for poloxamine-908 coated na-noparticles. As a result poloxamine was considered more effective coating material to avoid the liver capture of rabbits as compare to poloxamer [103].
Cyclodextrin/Carbohydrate Coated NPs
Mononuclear phagocyte system (MPS) is the natural defence mechanism by which the body prevents any unwanted entry of pathogens. Many times nanoparticles are subjected to MPS leading to its phagocytosis or elimination. Incorporation of carbohydrate coating over the nanoparticles of interest was found to by-pass the MPS uptake, as reported by Cho et al. [104]. For DNA, targeted delivery NPs of PLA and poly (L-lysine)-grafted-polysaccharide were developed [105]. They were found to be resistant to self-aggregation or clumping and adsorption (non-specific) of serum proteins.
The nanoparticles whose surface was coated with Cyclodextrin was found to increase loading efficiency of water soluble drugs and also the bioavailability of drugs that are poorly water soluble and are considered for targeted delivery. Another approach to achieve higher loading capacity of drug and to increase the stability of parent Cyclodextrin the Poly (isobutylcy-anoacrylate), PIBCA nanoparticles, coated with hydroxypropyl or natural Cyclodextrin are used.
Polysorbate-Coated NPs to Penetrate the Blood-Brain Barrier
Blood Brain Barrier is the most efficient barrier system in the entire human body, making it difficult for the transportation of drugs (hydrophilic and similar) across it. Thus it becomes mandatory to enhance the drug transport. There have been suggested mechanisms to enhance the drug transport across BBB (Figure 8) via coated NPs such as:
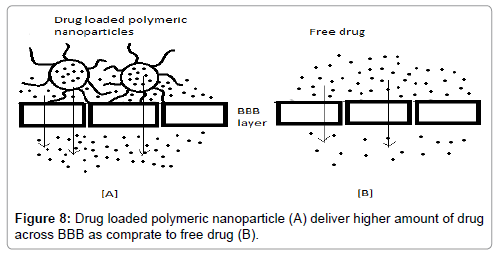
Figure 8: Drug loaded polymeric nanoparticle (A) deliver higher amount of drug across BBB as comprate to free drug (B).
(i) Binding to inner endothelial lining of brain capillary, thus, particles deliver drugs by providing a difference in concentration gradient, thus improving the passive diffusion
(ii) By phagocytosis [106].
Studies show a higher permeation across the biological matrices and membranes, when nano-particles are coated with surfactants, e.g., polysorbate-80 coated NP’s [107]. It was show-cased (Troster et al.) [108], a 9-fold increase in accumulation of radioactivity in the cerebral area post I.V. administration using polysorbate-80 coated radioactive (14C)-PMMA nanoparticles. A 60-fold increase in brain concentration was reported (Gulyaev et al. [109], when doxorubicin was bound to polysorbate-80 coated PBCA nanoparticles which were systemically administered.
Formulation of Actively Tragetting Nanoparticles (ATN) prepared (solvent evaporation) by conjugating transferrin to PEG-coated biodegradable polycyanoacrylate NP, for the enhanced delivery of Paclitaxel. Resulting, Avg. Encapsulation Efficiency=93.4 ± 3.6%; Particles Size=101.4 ± 7.2 nm; Zeta potential=(-13.6 ± 1.1 mV) [110-128].
Patent Invention and Future Prospect
Nanotechnology holds an advantage in formulating nature derived products (Table 2). Nanoparticles-mediated natural products delivery diminishes the toxicity to healthy cells [129]. Studies claim a considerable improvement in treatment using nanotechnology in comparison to the conventional methods for cancer such as chemopreventive/ therapeutic approaches. Nano-technology also portrays a promising future in the areas of diagnosis, imaging, and therapeutics. A large of research in nanotechnology is dedicated to enhance the science of nanoparticles as a drug delivery system. Considerable amount of Construct, based on principles of nanotechnology have presently on going clinical development and also pre-clinical development, with a few having achieved FDA approval.
| Drug |
Summaryof Invention |
References
|
| Docetaxel |
Docetaxel nanoparticle coated with PLGA bock copolymer conjugated with low mol. Wt. PSMA ligand. |
[111] |
| Curcumin |
Polymeric nanoparticle coated N-isopropylacryl amide (NEPAAM), acrylic acid (AA), and at least one vinyl monomer selected from the group consisting of vinyl acetate, 4-vinyl benzoic acid etc.. |
[112] |
| Dexomethasone |
Dexomethasone coated woth chitosan chloride and/or chitosan glutamate by ultrasound sonication. |
[113] |
| Beclomethasone |
Beclomethasone coated with a diblock poly (lactic) acid-poly (ethylene) glycol copolymer; 1,2 distearoyl-sn-glycero-3-phosphoethanolamine poly(ethylene)glycol copolymer by ring opening polymerization techniques (ROMP). |
[114] |
| Curcin |
Curcin planetary ball milled (PBM) nanoparticles coated alginate, cellulose, collagen, starch and PEG conjugated with folate. |
[115] |
| Mitoxantrone |
Mitoxatrone coated with polyethylenes, polycarbonates, polyanhydrides etc. Conjugated with PSMA. |
[116] |
| Carbazitaxel |
Carbazitaxel coated with diblock poly (lactic) acid-poly(ethylene)glycol conjugated with a low-molecular weight PSMA ligand. |
[117] |
| Calcium channel blocker |
Drug Np comprise hydroxy-terminated or epoxide-terminated and/or activated multiblock copolymer modifying the surface by lyophilization technique to produce a physically adsorbed coating and epoxy-derivatization to functionalize the surface. |
[118] |
| Paclitaxel |
Paclitaxel coated with PLGA conjugated with galactosamine targeting ASGP receptor by emulsion/solvent evarporation method |
[119] |
| Gefitinib |
Gefitinib nanoparticle drug delivery systems including a modified PLGA-b-PEG block copolymer. |
[120] |
| Paclitaxel |
Paclitaxel coated with Poly(2-(dimethylamino)ethyl methacrylate-co-methacrylic acid(PDM) targeting to cancer cell. |
[121] |
| 5-flurouracil |
5-flurouracil coated by bovine serum albumin conjugated with folate by coacervation method. |
[122] |
| Paclitaxel |
Paclitael coated with Human serum albumin by emulsion solvent evarporation method |
[123] |
| Paclitaxel |
Gelatin PLGA nanoparticles containing paclitaxel coated with bioadhesive molecules |
[124] |
| Ganciclovir |
Hollow protein nanoparticles containing ganciclovir encapsulating thymidine kinase (HSV1tk) modified to display a hepatitis B virus surface-antigen for hepatocyte recognition |
[125] |
| Ganciclovir |
Nanoparticle of Ganciclovir containing thymidine kinase modified with epidermal growth factor receptor |
[126] |
| Cetuximab |
Composition and method for targeting cancer cell by administering Cetuximab in combination with tetrac or triac |
[127] |
| Resveratrol |
Resveratrol coated with polymer functionalized with RGD peptide, which is recognized by 8vß3 receptor |
[128] |
Table 2: Positions of the collinear equilibrium points.
Achieving a forefront in the application of nanotechnology, with a focus on herbal/naturally derived products will aid us in building drug systems with viable and tangible results against numerous diseases [130,131]. There has been voiced a concern regarding the universal safety as well as environmental effects and common health effects on those included into manufacturing sciences, these points in questions will be addressed in due course of time [132].
Conclusion
The Polymeric nanoparticles are manufactured and modified by surface modification reaction for target specific drug delivery and it can also be used for delivering hydrophobic drug. The surface modification of polymeric nanoparticle is used to improve the systemic circulation, drug loading and enhancement in controlled drug delivery. Certainly, modification in surface is useful in achieving these goals. From the chemistry viewpoint, it is important to synthesize polymers and copolymers to meet the hydrophilic and hydrophobic properties. Production of Nanoparticles using the eco- friendly processes like supercritical fluids is a promising area of research to develop products which are free from the unwanted toxic residual solvents. Various patents are on targeting of polymeric nanoparticles. Different types of methods are used for preparation of nanoparticles and improve drug loading in dosage form (PNP). Nanotechnology is promising in Novel Drug Delivery System and it is emerging field in Pharmaceutical field.
References
- Zhang Q, Chuang KT (2001) Adsorption of organic pollutants from effluents of a kraft pulp mill on activated carbon and polymer resin. Adv Environ Res 5:251-258.
- Natalie P, Praetorius, Tarun K (2007) MandalEngineered Nanoparticles in Cancer Therapy. Recent Patents on Drug Delivery and Formulation 1: 37-51.
- Vilar G, Tulla-Puche J, Albericio F (2012) Polymers and Drug Delivery Systems. Current Drug Delivery9: 367-394.
- Kedar U,Phutane P, Shidhaye S, Kadam V (2010) Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine: Nanotechnology, Biology, and Medicine 6:714-729.
- Sharma S, Singh A (2011) Nanotechnology Based Targeted Drug Delivery: Current Status and Future Prospects for Drug Development. Intech Publisher,pp: 427-462.
- Mu L, Feng SS (2003) A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. Journal of Controlled Release86:33-48.
- Liontos M, Eleutherakis-Papaiakovou E, Koutsoukos K, Zagouri F, Meletios AD, et al. (2013) The Role of Paclitaxel Albumin-Stabilized Nanoparticle Formulation (Nab-Paclitaxel) in the Treatment of Breast Cancer: A Short Review. Global Journal of Breast Cancer Research1:36-42.
- Cismaru L, Popa M (2010) Polymeric nanoparticles with biomedical applications. Rev RoumChim55:433-442.
- Abhilash M (2010) Potential applications of Nanoparticles. Int J Pharm Bio Sci 1:1-12.
- Fonseca C, Simoes S, Gaspar R (2002) Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. Journal of Controlled Release83: 273-286.
- KayserOA, Lemke L, Hernández-Trejo N (2005) The Impact of nanobiotechnology on the development of new drug delivery systems. Current Pharmaceutical Biotechnology6:35.
- USFDA (2006) Center for Drug Evaluation and Research. Guidance for Industry, Scale-up and Post Approval Changes: Chemistry, Manufacturing and Control.
- Jalil R, Nixon JR (1990) Biodegradable poly [lactic acid] and poly [lactide-co-glycolide] microcapsules: problems associated with preparative techniques and release properties. J Microencapsul7:297-325.
- Jain N, Jain R, Thakur N, Gupta BP, Jain DK, et al. (2010) Nanotechnology: A Safe and Effective Drug Delivery System. Asian Journal of Pharmaceutical and Clinical Research3:159-165.
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2011) Review Biode-gradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release70:1-20.
- Vauthier C, Beanabbou S, Spenlehauer G, Veillard M, Couvreur P (1991) Methodology of ultradispersed polymer system.STP Pharm Sci 1:109-116.
- Sun Sang K, Yoon Sung N, Jong Suk L, Bong Seok K, Sang Hoon H, et al. (2002) Preparation and characterization of coenzyme Q10-loaded PMMA nanoparticles by a new emulsification process based on microfluidization Colloids and Surfaces A: Physicochemical and Engineering Aspects 210:95-104.
- Scholes PD, Coombes AGA, Illum L, Davis SS(1993) The preparation of sub-500 nm poly[lactidecirculationco-glycolide] microspheres for site-specific drug delivery. J. Control. Rel25:145-153.
- Murakami H, Yoshino H, Mizobe M, Kobayashi M, Takeuchi H, et al. (1996) Preparation of poly[D, L-lactide-codelivery glycolide] latex for surface modifying material by a double coacervation method. Proced. Intern. Symp. Control. Rel. Bioact. Mater23:361-362.
- Bindschaedler C, Gurny R, Doelker E (1990) Process for preparing a powder of water-insoluble polymer which can be redispersed in a liquid phase, the resulting powder and utilization thereof. US Patent 4,968,350.
- Ganachaud F, Katz JL (2005) Nanoparticles and nanocapsules created using the ouzo effect: spontaneous emulsification as an alternative to ultrasonic and high-shear devices. ChemPhysChem6:209-216.
- Allemann E, Gurny R, Doelker E (1992) Preparation of aqueous polymeric nanodispersions by a reversible salting-out process: influence of process parameters on particle size. Int J Pharm87:247-253.
- Galindo-Rodriguez SA, Allemann E, Fessi H, Doelker E (2004) Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res 21:1428-1439.
- Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S (1989) Nanocapsule formation by interfacial deposition following solvent displacement. Int J Pharm55:R1- R4.
- Quintanar-Guerrero D, Allemann E, Fessi H, Doelker E (1998) Preparation techniques and mechanism of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm24:1113-1128.
- Mishima K, Matsuyama K, Tanabe D, Yamauchi S (2000) Microencapsulation of proteins by rapid expansion of super-polycritical solution with a nonsolvent. AIChE J46:857-865.
- Tom JW, Debenedetti PG, Jerome R (1994) Preparation of poly[L-lactic acid] and compo-site poly[L-lactic acid]-pyrene by rapid expansion of supercritical solution. J. Super-crit. Fluids7:9-29.
- Sun YP, Rolling HW, Bandara J, Meziani JM, Bunker CE (2002) Preparation and pro-cessing of nanoscale materials by supercritical fluid technology. In: Supercritical fluid technology in materials science and engineering: synthesis, proper-ties, and applications,Marcel Dekker, New York pp:491-576.
- Randolph TW, Randolph AD, Mebes M, Yeung S (1993) Submicron-sized biodegradable particles of poly[L-lactic acid] via the gas antisolvent spray precipitation process. Bio-technol. Prog9:429-435.
- Kreuter J (1982) The mechanism of termination in heterogeneous polymerization. J Polym Sci Polym Lett Ed20:543-545.
- Hearn J, Wilkinson MC, Goodall AR, Chainey M (1985) Kinetics of the surfactant-free emulsion polymerization of styrene: the post nucleation stage. J Polym Sci PolymChem Ed23:1869-1883.
- Liu G, Liu P (2010) Synthesis of monodispersed crosslinked nanoparticles decorated with surface carboxyl groups via soapless emulsion polymerization. Colloid Surf A 354:377-381.
- Wang S, Wang X, Zhang Z (2007) Preparation of polystyrene particles with narrow particle size distribution by gamma-ray initiated miniemulsion polymerization stabilized by polymeric surfactant. EurPolym J43:178-184.
- Puig JE (1996) Microemulsion polymerization [oil-in water]. In: Polymeric materials encyclopedia, CRC Press, USA. 6: 4333-4341.
- Landfester K, Musyanovych A, Mailander V (2010) From polymeric particles to multifunc-tionalnanocapsules for biomedical applications using the miniemulsion process. J Polym Sci Part aPolymChem48:493-515.
- Karode SK, Kulkarni SS, Suresh AK, Mashelkar RA (1998) New insights into kinetics and thermodynamics of interfacial polymerization. ChemEng Sci53:2649-2663.
- Behan N, Birkinshaw C, Clarke N (1999) A study of the factors affecting the formation of poly[n-butylcyanoacrylate] nanoparticles. Proced. Intern. Symp. Control. Rel. Bioact. Mater26:1134-1135.
- Alenso MJ, Losa C, Calvo P, Vila-Jato JL (1991) Approaches to improve the association of amikacin sulphate to poly[cyanoacrylate] nanoparticles. Int. J. Pharm68:69-76.
- Ueda M, Iwara A, Kreuter J (1998) Influence of the preparation methods on the drying re-lease behavior of loperamide-loaded nanoparticles. J. Microencapsulation15:361-372.
- Egea MA, Gamisani F, Valero J, Garcia ME (1994) Entrapment of cisplatin in-to biodegradable polyalkylcyanoacrylate nanoparticles. Farmaco49: 211-217.
- Yoo HS, Oh JE, Lee KH, Park TG (1999) Biodegradable nanoparticles containing doxo-rubicin-PLGA conjugate for sustained release, Pharm. Res16:1114-1118.
- Xua Z, Gu W, Huang J, Sui H, Zhou Z, et al. (2005) In vitro and in vivo evaluation of actively targetable nanoparticles for paclitaxel delivery. International Journal of Pharmaceutics288:361-368.
- Miller T, Breyer S, Van Colen G, MierW, Haberkorn U, et al. (2013) Premature drug release of polymeric micelles and its effects on tumor targeting. International Journal of Pharmaceutics445:117-124.
- Tessmar JK, Mikos AG, Gopferich A (2002) Amine-reactive biodegradable diblockcopol-ymers. Biomacromolecules3:194-200.
- Jingwei X, Liang K, Yiyong P, Jinsong H (2006) Fabrication of Micro- and Nano-chitosan Particles Using Electrohydrodynamic Atomization. Journal of Colloid and Interf Sci302: 103-112.
- Matyjaszewski K, Xia J (2001) Atom transfer radical polymerization. Chem Rev101:2921-2990.
- ZetterlundPB, Kagawa Y, Okubo M (2008) Controlled/living radical polymerization in dispersed systems. Chem Rev108:3747-3794.
- Nicolas J, Charleux B, Guerret O, Magnet S (2005) Nitroxide-mediated controlled free-radical emulsion polymerization using a difunctional water-soluble alkoxyamine initiator. Toward the control of particle size, particle size distribution, and the synthesis of triblock copolymers. Macromolecules38:9963-9973.
- Braunecker WA, Matyjaszewski K (2007) Controlled/living radical polymerization: features, developments, and perspectives. Prog Polym Sci32:93-146.
- Cunningham MF (2008) Controlled/living radical polymerization in aqueous dispersed systems. Prog Polym Sci33:365-398.
- Nicolas J, Ruzette AV, Farcet C, Gerard P, Magnet S, et al. (2007) Nanostructured latex particles synthesized by nitroxide-mediated controlled/living free-radical polymerization in emulsion. Polymer48:7029-7040.
- Farcet C, Lansalot M, Charleux B, Pirri R, Vairon JP (2000) Mechanistic aspects of nitrox-ide-mediated controlled radical polymerization of styrene in miniemulsion, using a water-soluble radical initiator. Macromolecules33:8559-8570.
- Li W, Matyjaszewski K, Albrecht K, Moller M (2009) Reactive surfactants for polymeric nanocapsules via interfacially confined miniemulsion ATRP. Macromolecules42:8228-8233.
- Min K, Matyjaszewski K (2005) Atom transfer radical polymerization in microemulsion. Macromolecules38:8131-8134.
- Rieger J, Zhang W, Soffelbach F, Charleux B (2010) Surfactant-free RAFT emulsion polymerization using poly [N, N-dimethylacrylamide] trithiocarbonate macromolecular chain transfer agents. Macromolecules43:6302-6310.
- Zhou X, Ni P, Yu Z (2007) Comparison of RAFT polymerization of methyl methacrylate in conventional emulsion and miniemulsion systems. Polymer48:6262-6271.
- Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S (1989) Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm55:1-4.
- Jeong YI, Cho CS, Kim SH, Ko KS, Kim SI, et al. (2001) Preparation of poly[dl-lactide-co-glycolide] nanoparticles without surfactant. J ApplPolym Sci80:2228-2236.
- Jeon HJ, Jeong YI, Jang MK, Park YH, Nah JW (2000) Effect of solvent on the preparation of surfactant-free poly[dl-lactide-co-glycolide] nanoparticles and norfloxacin release characteristics. Int J Pharm207:99-108.
- Prasad Rao J, Geckeler KE (2011) Polymer nanoparticles: Preparation techniques and size-control Parameters. Progress in Polymer Science36:887-913.
- Jung SW, Jeong YI, Kim YH, Kim SH (2004) Self-assembled polymeric nanoparticles of poly[ethylene glycol] grafted pullulan acetate as a novel drug carrier. Arch Pharm Res27:562-569.
- Errico C, Bartoli C, Chiellini F, Chiellini E (2009) Poly (hydroxyalkanoates)-based polymeric nanoparticles for drug delivery. BioMed Research International 2009.
- Ibrahim H, Bindschaedler C, Doelker E, Buri P, Gurny R (1992) Aqueous nanodispersions prepared by a salting-out process. Int J Pharm87:239-246.
- Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F (2006) Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine 2: 8-21.
- El-Samaligy MS, Rohdewald P (1983) Reconstituted collagen nanoparticles, a novel drug carrier delivery system. J Pharm Pharmacol 35:537-539.
- Coester C, Langer K, Briesen HV, Kreuter J (2000) Gelatin nanoparticles by two step desolvation-a new preparation method, surface modifications and cell uptake. J Mi-croencapsul17:187-193.
- Zillies J, Coester C (2004) Evaluating gelatin based nanoparticles as a carrier system for double stranded oligonucleotides. J Pharm Pharm Sci 7:17-21.
- Zwiorek K, Kloeckner J, Qagner E, Coester C (2004) Gelatin nanoparticles as a new and simple gene delivery system. J Pharm Pharm Sci7: 22-28.
- Lu Z, Yeh TK, Tsai M, Au JLS, Wientjes MG (2004) Paclitaxel-loaded gelatinnanoparti-cles for intravesical bladder cancer therapy. Clin Cancer Res10:7677-7684.
- Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, et al. (2002) Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clinical Cancer Research 8: 1038-1044.
- Hyun Jung L, Hye Yeong N, Byung Ha L, DaeJoong K, Jai Young K, et al. A Novel Technique for Loading of Paclitaxel-PLGA Nanoparti-cles onto ePTFE Vascular Grafts, Biotechnol. Prog23: 693-697.
- Moghimi SM, Hunter AC, Morray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53: 283-318.
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65:271-284.
- Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced drug delivery reviews 60:1615-1626.
- Allen TM (2002) Ligand-targeted therapeutics in anticancer therapy. Nature Reviews Cancer 2: 750-763.
- Liu G, Zhang L, Zhao Y (2010) Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clinical & Experimental Immunology 160: 168-175.
- Hamdy S, Haddadi A, Shayeganpour A, Samuel J, Lavasanifar A (2011) Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles. Pharmaceutical research 28: 2288.
- Scott CJ, Marouf WM, Quinn DJ, Buick RJ, Orr SJ, et al. (2008) Immunocolloidal targeting of the endocytotic siglec-7 receptor using peripheral attachment of siglec-7 antibodies to poly (lactide-co-glycolide) nanoparticles. Pharmaceutical research 25: 135-146.
- Rajapaksa TE, Stover-Hamer M, Fernandez X, Eckelhoefer HA, Lo DD (2010) Claudin 4-targeted protein incorporated into PLGA nanoparticles can mediate M cell targeted delivery. Journal of Controlled Release 142:196-205.
- Fievez V, Plapied L, des Rieux A, Pourcelle V, Freichels H, et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. European Journal of Pharmaceutics and Biopharmaceutics 73: 16-24.
- Molavi O, Ma Z, Hamdy S, Lavasanifar A, Samuel J (2009) Immunomodulatory and anticancer effects of intra-tumoral co-delivery of synthetic lipid an adjuvant and STAT3 inhibitor, JSI-124. Immunopharmacology and immunotoxicology 31: 214-221.
- Chittasupho C, Xie SX, Baoum A, Yakovleva T, Siahaan TJ, et al. (2009) ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. European journal of pharmaceutical sciences37: 141-150.
- Liang C, Yang Y, Ling Y, Huang Y, Li T, et al. (2011) Improved therapeutic effect of folate-decorated PLGA–PEG nanoparticles for endometrial carcinoma. Bioorganic & medicinal chemistry19:4057-4066.
- Luo G, Yu X, Jin C, Yang F, Fu D, et al. (2010) LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. International journal of pharmaceutics 385: 150-156.
- Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ (2008) Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt (IV) prodrug-PLGA–PEG nanoparticles. Proceedings of the National Academy of Sciences. 105: 17356-17361.
- Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, et al. (2009) Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. Journal of Controlled Release140:166-173.
- Guo J, Gao X, Su L, Xia H, Gu G, et al. (2011) Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32: 8010-8020.
- Chen H, Gao J, Lu Y, Kou G, Zhang H, et al. (2008) Preparation and characterization of PE38KDEL-loaded anti-HER2 nanoparticles for targeted cancer therapy. J. Control. Release 128: 209-216.
- Hu K, Shi Y, Jiang W, Han J, Huang S, et al. (2011) Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: preparation, characterization and efficacy in Parkinson's disease. Int. J. Pharm 415: 273-283.
- Tosi G, Costantino L, Rivasi F, Ruozi B(2007) Targeting the central nervous system: in vivo experi-ments with peptide-derivatized nanoparticles loaded with Loperamide and Rhoda-mine-123. J. Control. Release122: 1-9.
- Costantino L, Gandolfi F, Tosi G, Rivasi F, Vandelli MA, et al. (2005) Peptide-derivatized biodegradable nanoparticles able to cross the blood–brain barrier. J Control Release108: 84-96.
- Tosi G, Vergoni AV, Ruozi B, Bondioli L, Badiali L, et al. (2010) Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: in vivo pharmacological evidence and biodistri-bution. J. Control. Release145:49-57.
- Nellis DF, Ekstrom DL, Kirpotin DB, Zhu J, Andersson R, et al. (2005) Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnology progress 21: 205-220.
- Eligngton AD, Szostak JW (1990) In vitro selection of RNA molecule that binds to specific ligands. Nature 346:818-822.
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, et al. (2012) PLGA-based nanoparticles: an overview of biomedical applications. Journal of controlled release 161: 505-522.
- Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, et al. (2009) Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. Journal of Controlled Release 140:166-173.
- Mu L, Feng SS (2003) A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. Journal of controlled release 86: 33-48.
- Shinoda T, Maeda A, Kojima S, Kagatani S, Konno Y, et al. Nanosphere coated with lactosylpolystyrene polymer as a targeting carrier to hepato-cytes, Drug Deliv6: 147-151
- Joen SI, Lee JH, Andrade JD, de Gennes PG (1991) Protein-surface interactions in the presence of polyethylene oxide. I. Simplified theory. J Colloid Interf Sci142: 149-158.
- Peracchia MT, Fattal E, Desmaele D, Bensard M, Noel JP, et al. (1999) PEGylatedpolycyanoacrylate nanoparticles for in-travenous administration and spleenic targeting.J Control Rel60:21-128.
- Vittaz M, Razile D, Spenlehauer G, Verrecchia T, Veillard M, et al. (1996) Effect of PEO surface density on long-circulating PLA–PEO nanoparticles whichare very low complement activators, Biomaterials17:1575-1581.
- Illum L, Davis SS (1983) Effect of the nonionic surfactant poloxamer 338 on the fate and deposition of polystyrene microspheres following intravenous administration. J Pharm Sci72: 1086-1089.
- Illum L, Davis SS, Muller RH, Mak E, West P (1993) The organ distribution and circu-lation time of intravenously injected colloidal carriers sterically stabilized with a block copolymer poloxamine 908. Int. J. Pharm89:25-31.
- Cho CS, Jeong YI, Ishihara T, Takei R, Park JU, et al. (1997) Simple preparation of nanoparticles coated with carbohydrate-carrying polymers. Biomaterials18: 323-326.
- MaruyamaAT, Ishihara SW, Kim T (1997) Nanoparticle DNA carrier with poly [L-lysine] grafted polysaccharide copolymer and poly [D, L-lactic acid], Bioconjug. Chem8: 735-742.
- Alyautdin R, Gothier D, Petrov V, Kharkevich D, Kreuter J (1995) Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly[butyl cyanoacrylate] nanoparticles. Eur. J. Pharm. Biopharm41: 44-48.
- Cavallaro G, Fresta M, Giammona G, Puglisi G, Villari A (1994) Entrapment of b-lactams antibiotics in polyethylcyanoacrylate nanoparticles: studies on the possible in vivo application of this colloidal delivery system.Int. J. Pharm111: 31-41.
- Troster SD, Muller U, Kreuter J (1990) Modification of the body distribution of poly[methyl methacrylate] nanopartinocles in rats by coating with surfactants. Int. J. Pharm61: 85-100.
- Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, et al. (1999) Significant transport of doxorubicin into the brain with polysorbate 80-coated nanopar-ticles. Pharm. Res16: 1564-569.
- Sui, Y, Ye Q(2004) CN1528452A.
- Stphene EZ, Mir Mukkaram A (2013) EP 2644192 A1.
- Maitra A, Pramanik D (2011) inventors; The Johns Hopkins University, assignee. Smart polymeric nanoparticles which overcome multidrug resistance to cancer therapeutics and treatment-related systemic toxicity. United States patent application.
- David B, Owen C, Sinead R,Lidia T (2012) A polymeric nanoparticle WO 2012052565 A1.
- Sabnis A, Troiano G (2012) Therapeutic Polymeric Nanoparticles Comprising Corticosteroids and Methods of Making and Using Same. United States patent application US 13/523,034.
- Lillard Jr JW, Singh S, Singh R (2013) Morehouse School of Medicine, assignee. Delivery system for specifically targeting cancer cells and method of use thereof. United States patent application US 13/569,941.
- Zale SE, Ali MM (2012) Bind Biosciences, Inc., assignee. Cancer cell targeting using nanoparticles. United States patent US 8,246,968.
- Stphene EZ (2012) Drug loaded polymeric nanoparticles and methods of making and using same WO 2012166923 A2.
- Vinod L, Robert J, Cunxian S (1996) Surface-modified nanoparticles and method of making and using same WO 1996020698 A2.
- Sung HW, Hsu HK(2006) US2006115537A1.
- Basu S, Harfouche R, Soni S, Sengupta S (2010) The Brigham, Women's Hospital, Inc., assignee. polymeric nanoparticles with enhanced drug-loading and methods of use thereof. United States patent application US 13/147,755.
- Geckeler K, Yeonju L (2012) Paclitaxel-loaded polymeric nanoparticle and prepa-ration thereof WO 2012138013 A1.
- Russell J, McEwan GJ (2002) EP1206251A1.
- Desai Yang A, Louie L, Yao Z, Soon-ShiongP, MagdassiS, et al. (2004) US20046749868B1.
- Au JL, WientjesMG (2006) US2006034925A1.
- Kuroda S, Tanizawa K, Kondo A, Ueda M, Senoo S (2003) JP2003286199A.
- Kuroda S, Tanizawa K, Kondo A, Ueda M, Senoo S, et al. (2004) JP2004002313A.
- Hung-Yun L, Faith BD, Paul JD (2010) Combination treatment of cancer with cetuximab and tetrac. WO 2010120506 A1.
- Faith BD, Paul JD, Shaker AM, Hung-Yun L (2010) Small molecule ligands of the integrin rgd recognition site and method of use. WO 2010075332 A1.
- Zhang L, Gu FX, Chan JM(2008) Nano-particles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther 83: 761-769.
- Kawasaki ES, Player A (2005) Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine 1: 101-109.
- Blagosklonny MV (2005) How cancer could be cured by 2015. Cell Cycle 4: 269-278.
- Dhruba J, Bharali B, Imtiaz A, Siddiqui S, VaqarM, et al. (2011) A Mousa, Nanoparticle Delivery of Natural Products in the Prevention and Treatment of Cancers: Current Status and Future Prospects. Cancers 3:4024-4404.
18815















