Keywords
|
| Alliance partnerships; Consultants; Discovery collaborations; Economics; Entrepreneurship; Pharmacological intervention; Preclinical research; Therapeutics discovery; Universities |
Abbreviations
|
| IP: Intellectual property; R&D: Research and development |
University-Industry Partnerships: Lifelines for Drugdiscovery Research?
|
| Technological, commercial, and regulatory pressures along with spiraling risks and costs are major challenges that threaten the very sustainability of today’s pharmaceutical industry. In response to a longterm decline in its research and development (R&D) productivity, the industry has been compelled to seek new drug-discovery paradigms in an attempt to recapture a level of robustness that offers adequate return on capitalized investment and stanches leaky pipelines [1,2]. Some so-called strategic management initiatives thus instituted (e.g., downsizings; mergers and acquisitions [3]) have brought such negative fallout that they bring to mind this sentiment from novelist Ernest Hemingway (1899-1961): “It was a brilliant cure, but we lost the patient.” Corrosive winds are also battering basic research in institutions of higher learning. Ballooning of public research funding during the boom decades of the 1960’s-1980’s fostered an explosive growth of university science departments and produced unprecedented numbers of new doctoral-level scientists capable of promulgating this growth. Over the last fifteen years, however, steep reductions in both public research support and demand for tenured science faculty have relegated many doctoral-level university researchers to a “perpetual postdoc syndrome” [4] or “postdoc pileup” [5] with “atrisk” employment prospects [6]. A legacy professorate has offered a mirage of intent-- but little decisive action-- toward remediating this untenable supply-demand imbalance while continuing to rely upon (pre)doctoral scientists to conduct the bulk of academic research [6,7]. |
| These disruptive circumstances have increasingly compelled both the pharmaceutical industry and academia to reach beyond its respective canonical realm of product or knowledge production and join forces in preclinical, research-based drug-discovery collaborations. The lure of public-private discovery alliances rests mainly with the synergy between university research as a proven source of technological innovation and insights into biological/pathological processes and industry’s traditionally deep pockets and later-stage development and commercialization expertise (e.g., human trials, marketing) [4,8-10]. Demands from policymakers, government funding agencies, advocacy groups, and patients that biomedical research yield public-health benefits have further intensified interest in fostering translational science through public-private discovery alliances [2,11-15]. |
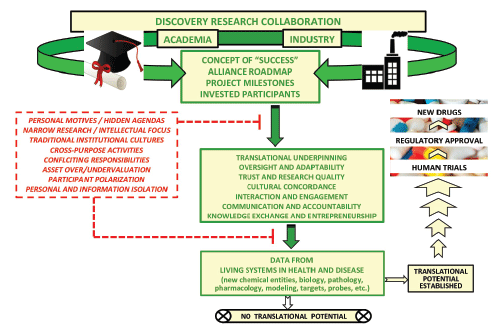
| This article will first discuss the proposition that cultural differences between academia and industry can create a disparate view of “success” regarding discovery collaborations between these stakeholders. Three elements will then be considered for their ability to foster value creation from university-industry preclinical discovery partnerships: an operational roadmap articulating collaboration parameters; well- defined project milestones that both signal progress and invite oversight and improvement; and participants incentivized for and committed to advancing the collaboration (Figure 1). The intent is not to proffer a purported “how-to” or “best-practices” guide, which seems all but impossible, given the variety of public-private drug-discovery enterprises and their goals [4,8,10]. Rather, this posture reflects the author’s extensive involvement as industry and academic scientist, educator, R&D leader, and independent consultant in building and sustaining effective public-private discovery partnerships propelled by team science that bridges institutional and disciplinary boundaries. |
Cultural Countercurrents and the Concept of “Success” in Research-based University-Industry Discovery Collaborations
|
| Deeply embedded conventions and historical memories distinguish university vs. pharma-industry research and create cultural dichotomies that can undermine public-private drug-discovery collaborations. As elaborated elsewhere [4,9,16], the academic setting classically aims at knowledge creation and dissemination by researchers focused on a defined field of study. The pharmaceutical industry takes a consumer/patient-driven posture wherein research is but one element among many others [e.g., unmet medical need, room for intellectual property (IP) protection, commercial opportunity] in a discovery campaign aimed at introducing a proprietary, market-attractive, and therapeutically impactful new treatment into the clinic whose realized sales revenue would support future drug invention. The academic reward structure is highly rank-conscious and individualized, wellestablished “products” of career accomplishment consisting of quality publications, grant/research-council funding, and next-generation scientists. In marked contrast, pharma-industry researchers are salaried as contributors to a team effort aimed at helping invent marketable drugs. Thus, the core unit in academic science research, a laboratory group run in top-down fashion by the principal investigator, does not routinely operate with levels of collaborative interaction and crossdisciplinary teamwork essential to drug discovery as necessitated by the challenges of applying basic research to address therapeutic/medical challenges. Nor does the typical academic research operation afford much opportunity to train scientists in matters extremely important to discovery such as decision theory, collaborator relations, and risk and project management [17,18]. Such organizational and functional distinctions constitute a challenging backdrop to discovery research collaborations across university-industry boundaries that are often underappreciated, despite the dwindling ability of both universities and pharmaceutical companies to conduct research as self-sustaining “closed shops.” |
| Differences in operating principles and paradigms between universities vs. pharma-industry research may shape the meaning of “success” in discovery collaboration between these constituencies. The compounding effect of the considerable lead-time (~ 10-15 years) required in bringing a new chemical entity to market as a drug and the extreme rarity of those new chemical entities that do gain regulatory approval renders it remote that success in an early-stage university-pharma research partnership would be realized as a retained breakthrough therapeutic-- although this is the ultimate aim of the pharmaceutical industry [19,20]. Rather, success would more likely take the form of research output that holds promise for enhancing the later-stage pipeline. Such accomplishments could include: creating enabling technologies, new chemical matter, molecular probes, or disease models; deepening knowledge about disease pathology/ treatment; validating therapeutic modalities and targets; enabling candidate entry into/progression through human trials [21,22]. Misconceptions surrounding the scope and translational potential of preclinical university-industry discovery alliances can invite discord among collaborators about the fundamental laboratory findings themselves: i.e., their over-valuation by academics whose expertise lies with procuring knowledge rather than setting it onto a translational arc and their undervaluation by industry professionals whose expertise and vision may be further down the critical path toward market. |
The Collaboration Roadmap as Enabling Operational Profile
|
| Commitment to an explicit operational profile or roadmap by all participating institutions and individuals is a critical element for setting public-private discovery collaboration on a trajectory for success. The roadmap would articulate such key parameters as: the alliance’s purpose and goals, projected milestones and deliverables, risk-reward apportionment and sharing, overall project timeline, oversight for assessing progress and addressing problems, IP ownership, prospective commercialization terms, and the means to avoid/deal with exigencies such as knowledge, expertise, and resource gaps. Perhaps most critically, the roadmap should establish unambiguously the means of frequent, open, high- quality communication among collaborators and the routes for making and executing decisions. The collaboration roadmap should be sufficiently detailed to align and empower both individuals and institutions as co-collaborators for a shared purpose within the mutual context of the academia-industry discovery alliance. |
| The roadmap should attempt to minimize proactively any issues sufficiently contentious to steer the collaboration away from progress. Such tensions may arise out of the fundamentally opposing mindsets of academia and industry (vide supra). For example, the highly competitive and lucrative marketplace for proprietary therapeutics invites absolute levels of circumspection and information control in pharma/biotech that are anathema to academia’s necessity for prompt knowledge dissemination in support of career advancement through data presentation/publication. As illustrated elsewhere in detailed case- history presentations [16,23], the resulting dissonances can be assuaged by negotiating and setting parameters regarding publication embargo periods and IP boundaries/transfer mutually agreeable to both academic and industry stakeholders. |
| The very process of roadmap planning and negotiation can signal early forewarnings of attitudes that can jeopardize a discovery collaboration, especially those surrounding the inherently unpredictable nature of scientific progress (e.g., participant inflexibility). This benefit has been appreciated by a former United States President, General Dwight Eisenhower (1890-1969): “In preparing for battle I have always found that plans are useless, but planning is indispensable.” Involvement of both university and industry participants in crafting the roadmap and shaping it into a responsive collaboration can help break down institutional barriers and inculcate focus, cooperation, inclusivity, and trust among participants for project advancement and coping productively with change. |
| A roadmap engenders valuable collateral benefits to a discovery collaboration that can strengthen bonds among stakeholders. These include establishing common ground between academic and industry domains, leveraging required expertise across disciplines, and codifying the product-oriented, translational nature of the alliance. Recognition of shared purpose and context, in turn, curbs the potential for cultural collapse to threaten collaboration viability, fosters a collective spirit guarding against individuals or institutions promoting conflicting or self-interests that may undercut the collaboration, and helps ensure that administrative and research standards are recognized and met (Figure 1). A conscious expectation for integrity and data stringency to industry standards is made particularly important by the alarming reports of irreproducibility of published results from purported translational models [24] and the vast differences in the degrees of rigor, data quality, and cross-validation necessary for securing a publication, grant award, or patent vs. propelling translational drugdiscovery research [4,21]. |
Milestones as Signposts for Advancement and Improvement
|
| No matter how cogent its operational plan, any collaborative project runs its course over a finite lifecycle-- a concept often difficult for participating individuals and institutions to embrace. Indeed, needless prolongation of discovery research projects may critically undermine pharma R&D [2,25]. These considerations mandate that discrete milestones be identified and integrated into the collaboration roadmap as operational guidance regarding advancement toward articulated project goals and the need for project review, refinement, or termination. Periodic milestone assessment is critical to judging progress and maintaining collaborator focus. Milestones also facilitate ongoing vigilance, accountability, and competitive due diligence by making explicit the relationship between immediate research activities and overarching project goals (Figure 1). Discrete milestones articulating exit and renewal strategies are useful, especially since financial support from industry to academia in early-stage discovery collaborations is typically rendered within a circumscribed contractual period for accomplishing specific tasks/goals, in contrast to multi-year, renewable government grants. |
| In these stringent economic times, it is tempting for faculty and academic institutions to engage in discovery research collaborations with industry predicated upon garnering funds to support laboratory/ university activities and/or help validate the purported translational relevance of ongoing university research for attracting grant awards, launching start-ups, and/or enhancing investigator/institutional prestige. Industry pressures to increase productivity and competitive profile may tempt companies to partner with universities to embargo some academic research without a strong, immediate discovery commitment. Clearly defined and strategically positioned project landmarks serve as proactive warnings against such orthogonal, if not opposing, hidden-agenda motives from suffocating a universityindustry discovery research collaboration and keep the parties involved on notice that project advancement is linked to concrete, discoveryrelevant expectations. |
The Human Element as Collaboration Firepower
|
| Regardless of how liberal it’s financial and material resources and well-articulated the operational roadmap, a university-industry discovery collaboration depends critically upon the individuals involved. In the author’s experience, empowered stakeholders committed to addressing the nuanced multidimensional problems presented by drug innovation and not merely expounding a skill or profession (experimentation, management) are essential for setting and boosting collaboration achievement level (Figure 1). Yet factors involving the collaborators themselves that may impede alliance progress are not always given due concern in light of the all too common-- but gravely flawed-- assumption that establishing a collaboration roadmap and milestones inevitably translates into tangible discovery drive from participants. In this regard, a decisive motivational force can take the form of a risk-reward profile that requires concrete investment in the collaboration (the proverbial “skin in the game”) by all participating individuals and constituencies with associated consequences and impactful, progress-related rewards. |
| Collaboration progress may be undercut from the mere fact that the mission of a research-based academia-industry discovery partnership is rarely the primary, let alone sole, duty of the parties involved. By virtue of having been awarded research funding, an academic laboratory owes its existence to meeting the time-sensitive obligations of conducting the financed studies and satisfying the funding agency’s oversight requirements (e.g., progress reports). A wide range of scholarly activities (teaching, mentoring, writing grant proposals and manuscripts, etc.) is part of an educator’s role. Likewise, in addition to activities directly related to generating and profiling potential therapies, industry personnel involved in discovery R&D often shoulder myriad intramural supervisory, administrative, and business responsibilities independent of any collaborative research. A mandate for active, joint project participation and open communication among individuals from both academia and pharma/biotech can help reduce confounding influences from stakeholder commitments outside of the collaboration and establish a common ground for due diligence as to the need for project adjustment or termination in response to, for instance, emerging data or new technologies. |
| Senior personnel are usually charged with negotiating, managing, and fronting an academia-industry discovery research collaboration, perhaps in conjunction with ancillary support from within (e.g., licensing, IP and legal professionals) and outside of (e.g., independent consultants) the collaborating institutions [4,8]. In academia, a hierarchical, autocratic management style predominates in which the principal investigator, a ranking academic expert in a particular field of study, defines his/her laboratory’s research focus. In this topdown archetype, university bench scientists performing research in support of an industrial collaboration likely include graduate students and postdoctoral fellows who may be inappropriately considered incapable of participating in project activities beyond what the author considers “turn-the-crank” science [6,7]. Furthermore, those graduate students and postdoctoral fellows run the risk of finishing their training period without having received any reward for their contributions to the discovery collaboration’s progress, whereas industry employees at all levels would (minimally) receive regular salaries for their contributions. These circumstances can unfairly marginalize university bench scientists, although they operate at the front-line regarding such matters as experimental design and conduct, data analysis, and emerging research problems. As a countermeasure to these hazards, some academic laboratories/institutions have developed a reward structure for collaboration participants at all career levels that includes active participation in potential spin-out companies and commercialization activities [26], whereas others exclude (post) doctoral trainees from collaborative drug-discovery research [27]. |
| In conjunction with a shared risk-reward profile, opportunities for all participants in collaborative discovery research to observe strategic discussions and present results under established confidentiality parameters and appropriate mentorship to project audiences can serve as potent motivational incentives. Such active project participation tangibly integrates academic researchers and their contributions within the broader collaborative arena and enhances appreciation of overall project parameters and issues related to therapeutics invention. Interactions with current and former pharma/biotech doctoral scientists offers another valuable resource for educating academic scientists collaborating with industry about real-world, productoriented research requirements, standards, and operating principles. In light of the apparent skills gap in pharmaceutical R&D [28,29], the career-preparation and -empowering potential associated with these activities cannot be ignored. As statesman Georges Clemenceau (1841- 1928) opined: “War is too important to be left to the generals.” |
Conclusion: A View to the Future
|
| Few drugs have been identified, researched, and developed exclusively within the public or private sector [19,20,30]. Preclinical discovery collaborations between universities and the pharmaceutical industry are increasingly prominent components of the ongoing global re-think of drug hunting [4,8-10]. These considerations, along with intense pressures from various quarters to improve new-drug quality and yield [2,11-15], suggest that the paradigm of academiaindustry discovery collaborations will exert even greater influence on pharmaceutical R&D discovery campaigns, particularly in terms of increasing their therapeutic reach, as informed by advances in such fields as predictive and diagnostic biomarker identification [31] and precision medicine [32]. |
| Multi-stakeholder discovery consortia have emerged that integrate multiple academic and industrial research partners [8,9,33]. The organizational and operational complexities of academia-industry discovery alliances are anticipated to increase even further, but not without difficulty. For example, the burgeoning of university spinoff companies ostensibly for moving laboratory findings closer to the clinic and the growing number of in-house agencies designed to foster university innovation and promote technology commercialization beyond campus boundaries [4,9,10] can serve to impede discovery collaborations simply by virtue of the multiplicity of individuals involved, many of whom lack commercial drug-discovery experience and are thus deficient in the research, management, and strategic proficiencies required by team-oriented, interdisciplinary R&D. Well-recognized as key capabilities of agile drug hunters, these skills are not routinely inculcated by or practiced in research-intensive academic environments [17,18]. Given the promulgation of researchfocused university “drug discovery” entities (especially in North America and Europe) [34,35], this proficiency shortfall suggests an intensifying need for the academic sector involved in drug discovery to employ researchers and administrators with pharma/biotech industry experience who also have the ability to mentor researchers-in-training. |
| Among university scientists involved in research areas allied to drug discovery, the ever-present scramble for external funding and the primacy of grant awards and publications as gold-standard career assets perpetuates the status quo of an inward-looking academic domain. So-called academic drug- discovery units notwithstanding [34,35], university postures based on ossified operational and faculty- reward paradigms can short-circuit translational discovery collaborations. This context, along with the Bayh-Dole Act (i.e., United States Patent and Trademark Law Amendment Act of 1980) and related legislation allowing discoveries made with federal funding to be patented by universities, small businesses, or non-profit institutions in preference to the government, may foster an attitude within academia that regards university inventions and spin-off companies primarily as potential sources of financial gain and faculty recognition and less so as enablers of therapeutics discovery. Yet very few academic inventions per se have resulted in profitable drugs [20,36,37]. To foster and strengthen academic interactions with industry for discovery purposes, the author envisions that universities will need to jettison many traditional ivorytower paradigms, adapt more flexible and inclusive collaborative research models, and embrace internal funding and risk-sharing mechanisms and investment strategies. A more holistic view on the part of academicians involved in drug discovery is required that continually reaches far beyond purported experimental disease models and incorporates clinical thinking about human disease and its treatment. As a corollary, integration of collaborative discovery activities into faculty/staff reward and advancement criteria and trainee (student, postdoctoral) development needs to be improved substantially. Some initiatives along these lines have already been adopted by select discovery-oriented academic institutions and incorporated into their pharma/biotech collaborations, as evidenced by real- world examples and case studies published elsewhere [8,16,27,33]. |
| Although universities actively seek research collaborations with the private sector [38], at present (large) pharmaceutical companies seem more intensively occupied with identifying good-fit academic partners for discovery team science. Several international pharmaceutical concerns have relocated their global R&D centers to urban hubs harboring a concentration of research-intensive universities and medical schools, established R&D sites staffed by both academic and industry scientists, and/or instituted information-mining operations specifically for leveraging portfolio-relevant knowledge from academia (e.g., Ref. [39-41]). These types of activities are predicted to grow in number, scale, and geographic reach, engendering increasingly global academia-industry discovery collaborations with parameters well beyond classic paradigms such as fee-for-hire of academic services and licensing/acquisition of university IP. Future public-private discovery collaborations are anticipated to integrate multiple therapeutic areas across university laboratories, departments, and academic institutions with long-term (i.e., multi-year) support commitment from all academic and industry partners (e.g., Ref. [42]). |
| The increasing prominence of and challenges presented by university-industry discovery alliances suggest an expanding role for third-party scientists academically credentialed and industry-practiced in the art of cross-disciplinary drug hunting for bridging productand knowledge-oriented researchers as independent consultants. For example, as a scientist with ongoing experience in both domains, the author is frequently asked by universities to provide guidance about contemporary industry practices and therapeutic trends. Similarly, the author’s activities as evaluator of university inventions for their therapeutic significance and/or corporate portfolio alignment have increased appreciation by pharma professionals as to how fundamental insights into pharmacological and pathological phenomena can be worthy of collaborative commitment and support. |
| Given the 10-to-15-year lead-time between “maybe” and “market” in drug discovery [1,2], continued scrutiny of extant and future academia-industry research collaborations for their role in helping generate breakthrough therapies is likely to proffer new suggestions for enriching such enterprises-- an outcome that itself could be considered a measure of collaboration success. In this spirit, poet Walter (“Walt”) Whitman (1819-1892) may deserve the last word: “What is accomplished is very important. But the spirit in which it is accomplished is equally important.” |
Figures at a glance
|
 |
| Figure 1 |
|







