Introduction
Urinary tract infection (UTI) is a common and important clinical problem in childhood. Urinary tract infection may lead to renal scarring, hypertension, and end-stage renal disease. Although children with UTI tend to present with fever, it is often difficult on clinical grounds to distinguish UTI from other febrile illness in developing countries [1,2]. This makes (UTI) as one of the most often missed diagnosis in the paediatric wards in developing countries. UTI whether symptomatic or asymptomatic have greater significance in childhood than in adults as most renal scars occur after such infections within the first five years of life [3].
Several studies have reported varying prevalence rates of UTI in children ranging from 3.3 in USA to 37.5% in Pakistan [4,5]. Clinical features of urinary tract infection vary depending on the age at occurrence. Newborns and infants present with non specific symptoms and signs which include fever, hypothermia, jaundice, vomiting, poor feeding, irritability and weight loss [5,6]. Abdominal or flank pain, vomiting, fever, urinary frequency, dysuria, urgency and enuresis are common in pre-school children [7]. Fever has been reported as the commonest symptom of UTI in children with frequency of up to 91% [5].
The diagnosis of UTI in children posses a big challenge to the clinicians and several reasons have been responsible for the difficulties in establishing the diagnosis of UTI in children include; non specific clinical presentation and the difficult in getting urine sample for laboratory investigations [8,9]. A clean midstream catch is widely used method in older children, in infants uncontaminated urine sample can best be obtained by suprapubic aspiration from the bladder [9].
The predominant bacteria causing UTI in children are gram negative bacteria, which form part of fecal flora and colonize the perineum. Escherichia coli strains are the commonest isolated uropathogens in children followed by other enteric gram negative bacteria especially Klebsiella pneumoniae, Proteus spp and Pseudomonas spp [9,10]. The sensitivity pattern of the uropathogens is changing and is posing a growing problem in the empiric treatment of UTI in children [11]. Amoxicillin and cotrimoxazole which are recommended by WHO as first line treatment for UTI are no longer effective [9,10,12]. There is an increase resistance of the community Escherichia coli isolates to gentamicin, cephalosporins and quinolones [13].
This study was done to establish the magnitude of UTI among febrile children and to determine the common uropathogens and their susceptibility pattern. Also the study evaluated the usefulness of the dipstick urinalysis in the diagnosis of UTI in children. The antibiotic sensitivity pattern is expected to serve as a guide in improving the management of UTI in children and thereby avoiding long term complications associated with UTI.
Methods
Study design
The cross-sectional study was conducted at Bugando Medical Centre (BMC), a tertiary hospital in Northwestern part of Tanzania from October 2010 to February 2011. All children with fever aged from 2 months to 60 months with no indwelling catheters were serially recruited into the study. The sample size was calculated using Kish and Lisle formula. Since no study has been done in our setting the prevalence of 50% was fitted in the formula.
Laboratory procedure
Mid-stream clean catch urine (MSU) was obtained in all children above 2 and those below 2 years who were able to provide MSU [3,14]. Suprapubic aspiration was done for children below 2 years after cleaning the skin with iodine alcohol antiseptic(Sigma Aldrich, France). Urine specimens were collected into a sterile container (HiMedia Laboratories. Pvt. Ltd, India) on the same day of recruitment. Within an hour of specimen collection, 1μl and 10μl standard quantitative loops were used to inoculate all urine samples on Cysteine lactose electrolyte deficient Agar (CLED), Mackonkey and Blood agar plates (Thermofisher UK, England). Plates were incubated for 24hr at 37oC. Few drops of centrifuged urine were examined by Laboratory technicians using 40x objective of light microscope (Olympus), a minimum of 10 high power fields were scanned for white blood cells (WBCs). Results were reported in terms of number of cells / high power field (HPF). The criterion of ≥ 5WBC/HPF was used to indicate positive microscopy [14]. Dipstick urinalysis was done as recommended manufacturer (Yercon Diagnostic Co., Ltd, Jilin China).
A diagnosis of UTI was made when there were at least 10 5 colony forming unit (CFU)/ml of MSU and any colony count for suprapubic urine [14]. High colony counts with more than one species of bacteria were considered as contamination. For contaminated specimens, culture was repeated. Identification of the bacterial isolates was done using in-house biochemical testing [14,15]. Disc diffusion method was used to determine susceptibility of the isolates as previously described [16]. Using normal saline individual colonies were suspended to 0.5 Mc- Farland and using sterile cotton swabs the suspensions were inoculated on Muller Hinton Agar (Thermofisher, UK) and incubated at 37oC for 18-24hr. For the quality control, Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used. Antibiotics tested included ampicillin (30 μg), cephalexin (30μg), cefaclor (30 μg), co-trimoxazole (SXT) (1.25/23.75μg), nitrofurantoin (300mcg), ceftriaxone (30 μg), ceftazidime (30 μg), gentamicin (10 μg), ciprofloxacin (5 μg) and cefepime (30 μg
Data analysis
Data were entered into Microsoft excel software and analyzed using STATA version 11 (College Station, Texas, USA). Continuous data were recoded as mean (standard deviation {SD}) and median (range) where appropriate. Chi-square or Fisher exact tests were used to establish statistical difference in proportions for categorical data. Univariate and multivariate logistic regression analysis were done to determine factors predicting urine culture positive as UTI. Factors with p value of ≤ 0.05 were considered statistically significant.
Ethical considerations
This study was approved by BMC/Well Bugando University College of Health Sciences Ethics Review Board. An informed consent was obtained from parents and guardians before collection of urine specimens.
Limitation
Due to the study design and limited funds complication of UTI using imaging were not investigated.
Results
Three hundred and seventy febrile children were enrolled in this study; of these 194 (52%) were men and 176 (48%) were female (table 1). The median age was 18 (range 2-60) months. Two hundred and thirty (62%) of children were aged ≤ 24 months. The mean temperature of children was 38.4oC (SD 0.6oC) and the mean duration of fever before admission was 5 days (SD 3.2 days). The MSU and suprapubic aspiration urine specimens were obtained from 196 (53%) and 174 (47%) respectively (table 1). The overall prevalence of UTI by culture was 39.7%. Female children had higher prevalence of UTI than male children (89/176 versus 58/194, p<0.001). Of 174 suprapubic specimens 83 (47.7%) had positive culture compared to 64(32.7%) of MSU p=0.003. The age ≤24 months was associated with higher prevalence of UTI than age above 24 months (104/230 versus 43/140, p=0.006) table 1.
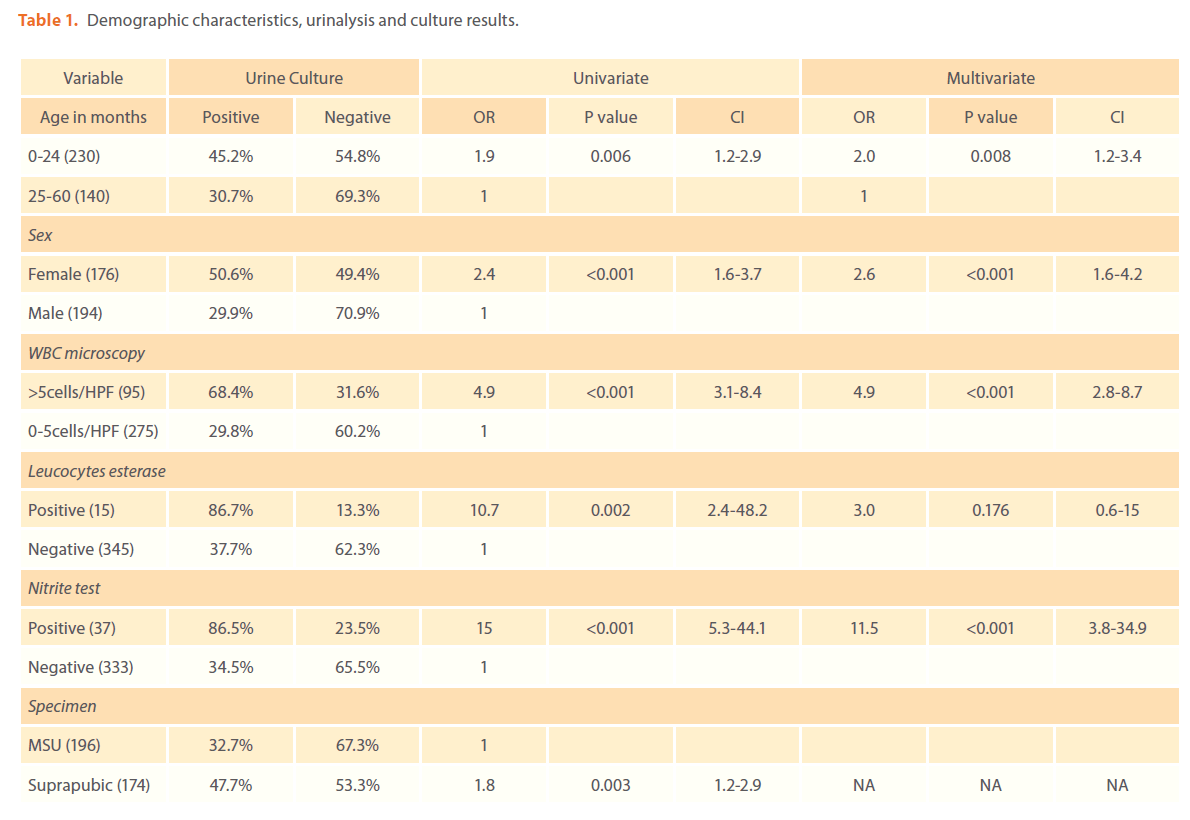
Table 1. Demographic characteristics, urinalysis and culture results.
Clinical characteristics
As temperature increases from 38oC, the risk of UTI increases but this was significant only on univariate logistic regression analysis (table 2). Diarrhea (OR 2.3, p=0.001) and prolonged duration of fever more than 5 days (OR 1.6, p= 0.04) were found to predict positive urine culture on both univariate and multivariate logistic regression analysis (table 2). Other factors such failure to thrive; dysuria, vomiting, abdominal pain and irritability were not significantly associated with UTI (table 2).
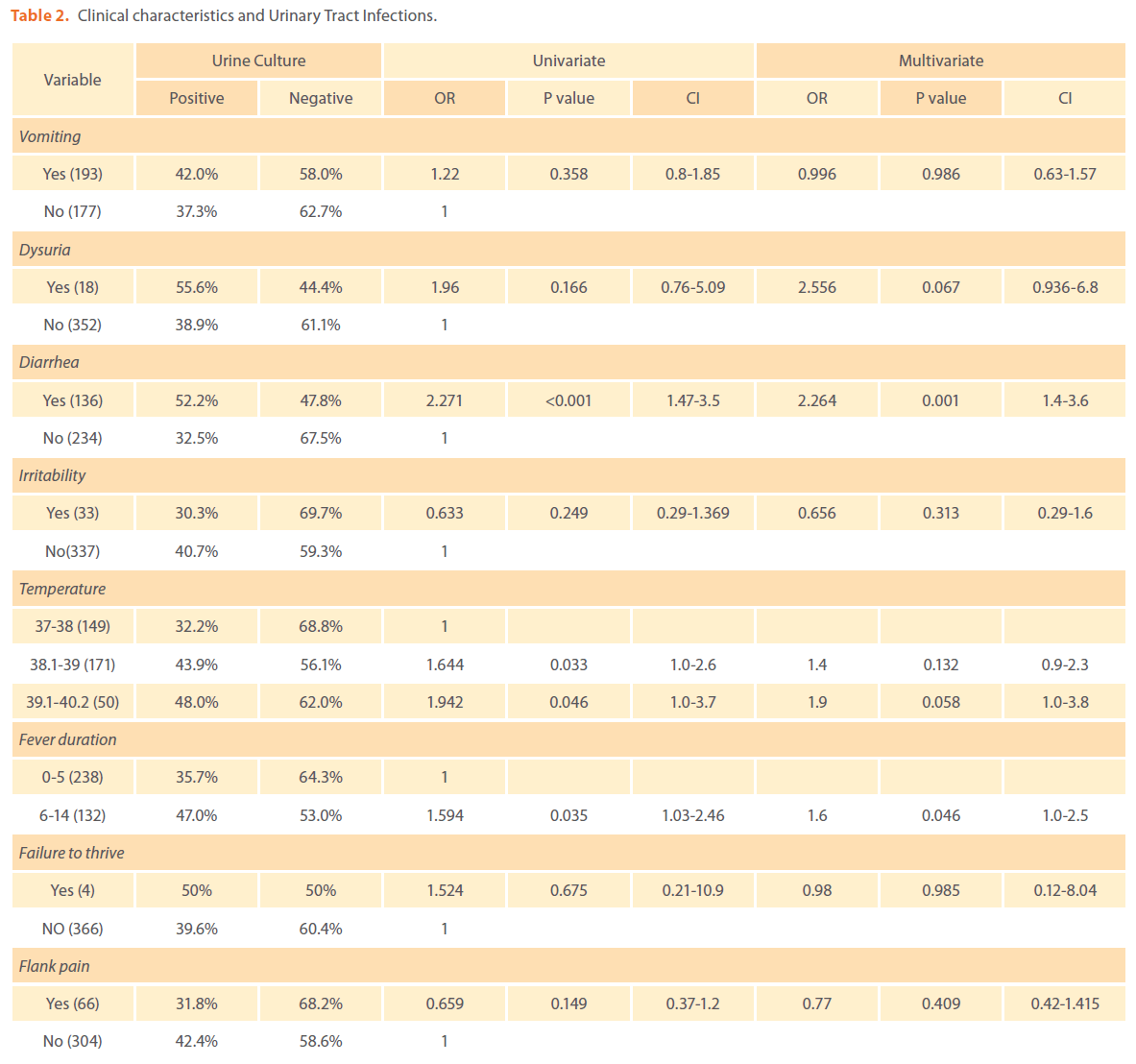
Table 2. Clinical characteristics and Urinary Tract Infections.
Bacterial isolates and susceptibility pattern
Of 147 positive culture, 134 (91%) were gram negative and 12 (9%) gram positive. Escherichia coli 64 (43.5%) was the commonest bacteria isolated followed by Klebsiella pneumoniae 52 (35.4%). Gram positive bacteria isolated were Enterococcus spp (8) and Staphylococcus aureus (4). Resistance rates of Escherichia coli were ampicillin (98.4%), co-trimoxazole (95.3%), augmentin (87.5%), cephalexin (61%), cefaclor (43.8%), gentamicin (21.9%), ceftriaxone (14%), nitrofurantoin (12.5%), ciprofloxacin (11.6%), ceftazidime (11%) and cefepime (3.1%). Furthermore, resistance rates of Klebsiella pneumoniae were ampicillin (100%), co-trimoxazole (100%), augmentin (86.5%), cephalexin (63.5%), cefaclor (58.8%), gentamicin (38.5%), ceftriaxone (46%), nitrofurantoin (21%), ciprofloxacin (19.2%), ceftazidime (33%) and cefepime (21%). Staphylococcus aureus were 100% resistant to cephalexin, cefaclor, ampicillin and 75% resistance to augmentin and cloxacillin.
Physical examination: Urinalysis (Leukocyte esterase, nitrate test and WBC microscopy)
The prevalence of UTI by WBC urine microcopy, nitrite and leucocytes esterase tests was 25.6%, 4%, 10% respectively. Out of 147 specimens with positive culture 65 (44.2%), 13(8.8%) and 32 (21.7%) had positive WBC urine microscopy; leukocyte esterase test and nitrate test respectively (table 3) and their specificities were 86.5%, 99.1% and 97.8% respectively (table 3). The positive predictive value of WBC urine microscopy, leukocyte esterase and nitrate test were 68.4%, 86.6% and 86.4% respectively. The combined sensitivity of urine WBC microscopy and leukocytes esterase test; WBC microscopy and nitrite test and leukocytes esterase and nitrite test were 49.2%, 56.4% and 28.6% respectively.
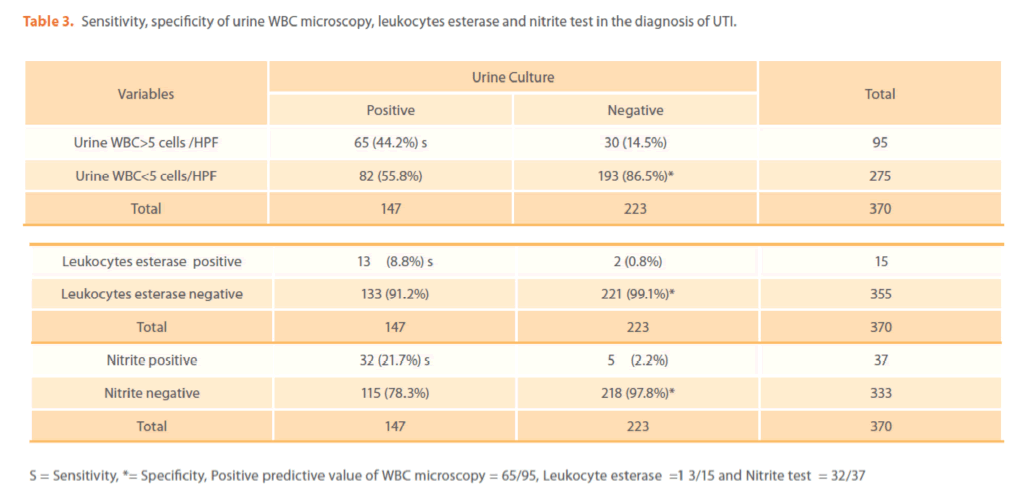
Table 3. Sensitivity, specificity of urine WBC microscopy, leukocytes esterase and nitrite test in the diagnosis of UTI.
Discussion
This study involves 370 febrile children attending Bugando Medical Centre; the median age of these children was 18 months as in the previous studies majority of children were below 24 months [17]. This age group is vulnerable to infectious diseases and forms a large group of admitted children in the developing countries [17]. High prevalence of UTI of 39.7% was observed in this study in contrast to other studies where the prevalence of UTI in children ranged from 3.3%- 7.5% [2,4,6,18,19]. The prevalence in this study is almost similar to the study in Pakistan in which 37.5% of febrile children were found to have UTI [5]. A significant proportion of female in this study had UTI compared to male (p<0.001) and this has also been observed previously [6,18,19]
In the present study other factors that predicted UTI were age below or equal 24 months, prolonged fever of more than 5 days, positive urine WBC microscopy, positive nitrite test and diarrhea. These factors can be used in developing countries to predict UTI in children in place where urine culture is not routinely done. Diagnosis of UTI in children poses a significant challenge due to the fact that most of the clinical characteristics in children suspected to have UTI are not reliable, in the present study as in other studies [20] dysuria, flank pain, vomiting, failure to thrive, irritability were not statistically significantly associated with UTI. There is no a specific sign or symptom that can predict the presence of UTI in infants and children. Combinations of findings, including a prior history of UTI, should be taken into account when making a decision to evaluate for UTI [20,21]. The limitation in this study is that other causes of fever where not investigated.
The other challenge in the diagnosis of UTI in children is specimen collection; in the present study 174 (47%) of children underwent suprapubic puncture to obtain urine specimen of these 47.7% had positive urine culture compared to 32.7% of MSU specimens (p=0.003), similar findings has been observed before [22]. Suprapubic aspiration is highly recommended as specimen of choice in children below 2 years, this specimen has high yield when compared to other urine specimens [22]. In this study about 95% (data not shown) of urine samples with significant bacteriuria had single specie of bacteria, this is due to clear instructions given to parents/guardian before urine collection and in most cases nurses assisted the mother to obtain the urine sample. Among the isolated bacteria, gramnegative bacteria were the most predominant uropathogens. Escherichia coli was predominant specie isolated, followed by Klebsiella pneumoniae [18,23]; in UTI infecting organisms are commonly derived from the patient’s own faecal flora, for the children up to approximately age 5 years are predisposed to UTIs, partly because of periurethral colonization by E coli, Enterococcus spp, and Proteus species [23].
Escherichia coli and Klebsiella pneumoniae in the present study showed multidrug resistance to ampicillin, augmentin, cotrimoxazole, cephalexin, cefaclor and gentamicin. These are commonly antibiotics used in Tanzania for treatment of UTI in children; the high resistance rate to these antibiotics observed in this study poses great challenges in the treatment options. Third generation cephalosporins could be used with the guidance of laboratory results, the commonly used third generation cephalosporins in our setting is ceftriaxone; 14% and 46% of Escherichia coli and Klebsiella pneumoniae were found to be resistance to ceftriaxone [24]. The other option is the use of ciprofloxacin and nitrofurantoin, the resistance rate to these drugs in the present study were 11.6% and 12.5% to Escherichia coli and 19.2% and 21% to Klebsiella pneumoniae respectively. These findings support the need of continuous surveillance of drug resistance patterns and the use of routine culture and susceptibility in the diagnosis and management of UTI in developing countries. Prolonged duration of fever for more than 5 days was significantly associated with the isolation of multidrug resistance isolates (data not shown); this shows the impact of self medication and lack of guidelines for antibiotics use in primary health facilities [24].
This study also investigated the sensitivity and specificity of rapid test in the diagnosis of UTI in children, these information are useful in developing countries where culture is not routinely done. Low sensitivity but high specificity of leukocytes esterase test, WBC urine microscopy and nitrite test were observed in this study [25, 26]. This implies that the use of these tests in the diagnosis of UTI in children would result in large number of false negative with few false positive. The highest combined sensitivity of 56.4% was observed when nitrite test and urine WBC microscopy are used in series. These tests should be used in combination with urine culture in the diagnosis of UTI in children; in the place with limited culture facilities. In this study as demonstrated previously [24] positive nitrite and leukocytes esterase test will indicate UTI with specificity of 97.8% and 99.1% respectively. In pediatric patients, urine cultures should be sent to the laboratory because approximately 10-20% of pediatric patients with UTI have normal urinalysis results [24-26] this is confirmed in this study.
Conclusion
High prevalence of UTI is observed among febrile children in our setting and is predicted by prolonged fever, diarrhea, female sex, age below or equal to 24 months, positive nitrite test and WBC urine microscopy. Multidrug resistance Escherichia coli and Klebsiella pneumoniae were the most commonly isolated bacteria. Due to low sensitivity of rapid tests urine culture should be routinely performed to diagnose UTI in febrile children.
189
References
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER (2003) Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 348:195.
- Jeena PM, Coovadia HM, Adhikari M (1996) Probable association between urinary tract infections (UTI) and common diseases of infancy and childhood: a hospital-based study of UTI in Durban, South Africa. J Trop Pediatr 42(2):112-4.
- Downs SM (1999) Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement.Pediatr 103(4):e54
- Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS (1998) Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatr 102(2):e16.
- Anisur R, Jahanzeb M, Siddiqui TS, Idris M (2008) Frequency and clinical presentation of UTI among children of Hazara Division, Pakistan. J Pak Med Assoc 20(1):63-5.
- Shaikh N, Morone NE, Bost JE, Farrell MH (2008) Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 27(4):302-8.
- Kaushal RK, Bansal S, Sharma VK, Sood A, Goyal A (2003) Urinary tract infection among children presenting with Fever. Indian J Pediatr 40(3):269-70.
- Musa-Aisien AS, Ibadin OM, Ukoh G, Akpede GO (2003) Prevalence and antimicrobial sensitivity pattern in urinary tract infection in febrile under-5s at a children’s emergency unit in Nigeria. Ann Trop Pediatr 23(1):39-45.
- Brown BJ, Asinobi AO, Fatunde OJ, Osinusi K, Fasina NA (2003) Antimicrobial sensitivity pattern of organisms causing urinary tract infection in children with sickle cell anaemia in Ibadan, Nigeria. West Afr J Med 22(2):110-3.
- Adeyemo AA, Gbadegesin RA, Onyemenem TN, Ekweozor CC (1994) Urinary tract pathogens and antimicrobial sensitivity patterns in children in Ibadan, Nigeria. Ann Trop Pediatr 14(4):271-4.
- Hari P, Mantan M, Bagga A (2003) Management of urinary tract infections. Indian J Pediatr 70(3):235-9.
- Yen CW, Chen DH (1999) Urinary tract infection in children. J MicrobiolImmunol Infect 32(3):199-205.
- Grüneberg RN (1994) Changes in urinary pathogens and their antibiotic sensitivities, 1971-1992. J AntimicrobChemother 33: 1–8
- Murray, Baron, Pfaller, Tenover (1995) Manual of Clinical Microbiology. 6th edition.American Society of Microbiology Press, Washington DC.
- Mshana SE, Kamugisha E Mirambo M, Chakraborty T, Lyamuya EF (2009) Prevalence of multiresistant gram-negative organisms in a tertiary hospital in Mwanza, Tanzania. BMC Res Notes 2:49
- Clinical and Laboratory Standards Institute (2006) Performance standards for antimicrobial disk susceptibility tests. Approved standard.In Ninth edition Document M2-A9 Clinical and Laboratory Standards Institute, Wayne, PA.
- Hori H, Watanabe M, Sakurai M (1993) Infectious diseases in African children. ActaPediatrJpn 35(6):553-8.
- Okwara FN, Obimbo EM, Wafula EM and Murila FV (2004) Bacteraemia, Urinary Tract Infection and Malaria in Hospitalized Febrile Children in Nairobi: Is there an Association? East Afr Med J 81: 47-51
- 19.Hoberman A, Chao HP, Keller DM, Hickey R, Davis HW, Ellis D (1993) Prevalence of urinary tract infection in febrile infants. J Pediatr 1993 123(1):17-23.
- Zorc JJ, Levine DA, Platt SL, Dayan PS, Macias CG, Krief W (2005) Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatr 116(3):644-8.
- Shaikh N, Morone NE, Lopez J, Chianese J, Sangvai S, D’Amico F (2007) Does this child have a urinary tract infection? JAMA 298(24):2895-904
- Quigley R (2009) Diagnosis of urinary tract infections in children. CurrOpinPediatr 21(2):194-8.
- Ronald A (2002) The etiology of urinary tract infection: Traditional and emerging pathogens. Am J Med 113 (suppl 1A):14S-19S.
- Weisz D, Seabrook JA, Lim RK (2010) The Presence of Urinary Nitrites Is a Significant Predictor of Pediatric Urinary Tract Infection Susceptibility to First- and Third-Generation Cephalosporins. J Emerg Med 39(1):6-12.
- Bachur R, Harper MB (2001) Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch PediatrAdolesc Med 155(1):60-5.
- Goldsmith BM, Campos JM (1990) Comparison of urine dipstick, microscopy, and culture for the detection of bacteriuria in children. ClinPediatr (Phila) 29(4):214-8.








