Introduction
Regional nerve blocks and application of opioids are important tissues in acute and chronic pain management[1,2,3]. Repeated doses or continuous administration of these drugs are required as local anaesthetics and opioids have a limited duration of actionp[4,5]. These techniques are usually more invasive, more expensive or time consuming. Controlled drug release methods such as the utilization of liposomal or biodegradable polymer microencapsulation are technique used for increasing the duration of action and decreasing the toxicity of drug[6,7].
Tramadol is a centrally acting analgesic with both opioid and non opioid effects, which is associated with little respiratory depression8. Tramadol has a relatively short duration of action requiring repeated doses(9,10). Tramadol is a water soluble drug. Such therapy leads to poor patient compliance. Therefore, slow release preparation seemed to be a logical approach in tramadol therapies. One of the methods of sustained drug delivery system is by micro encapsulation which is micro particulate drug delivery system.(4,5)
Ethylcellulose and tramadol were selected as model encapsulation material and a model drug, respectively. Ethylcellulose is a water insoluble polymer and widely used in pharmaceutical as a well material for sustained release microspheres. This is due to high safety, good stability; easy fabrication and cheapness. Various hydrophilic drugs have been prepared in form ethyl cellulose microspheres. Tramadol is water soluble drug so ethylcellulose was selected polymer. Microspheres were prepared by w/o/w multiple emulsion solvent evaporation method11. Drug: polymer ratio and volume of External phase were two variables selected for this method
Materials & Method
Materials: Tramadol HCl ( Morvel Laboratories, Mehsana, Gujarat), Ethylcellulose, Polyvinyl alcohol ( S D fine chemicals , Mumbai) Dichloromethane, concentrated Hydrochloric acid, (Allied chemical corporation ,Baroda),
Method:
Preparation of microspheres:
Ethyl cellulose microspheres were prepared using a w/o/w multiple emulsion solvent evaporation technique. Ethyl cellulose dissolved in 20 ml Dichloromethane. 500 mg Tramadol Hydrochloride dissolved in 1.2 ml water. Drug solution was added into the polymeric solution and stirring with high speed using magnetic stirrer. So formation of primary emulsion. Primary emulsion was drop wise added into the 1% PVA solution (1 gm of PVA dissolved in 100 ml distilled water) and stirring the resulting solution at1600 RPM using the propeller till the dichloromethane evaporate. Formation of microspheres which were collected by vacuum filtration, washed the microspheres with 2 times 100 ml distilled water and remove the PVA residue. The microspheres collected in filter paper dried at room temperature at 24 hrs.
Study Design for Optimization of Process Parameters (32 Factorial Design)
Batches were prepared to optimize process parameters for preparation of microspheres, according to a 3 2 factorial design as follows: 2 independent variables ( drug : polymer ratio and volume of External Phase ( 1 % PVA) and 3 levels of study, as indicated.
Evaluation of microspheres
Polymer –Drug compatibility: it was done by Differential scanning colorimetry (Perkin Elmer Instrument Pyris-1 DSC )
Percentage Yield

Percentage encapsulation efficiency
30 mg of microspheres was dissolved in dichloromethane to prepare a 10-ml solution. The Tramadol HCl was extracted 2 times from dichloromethane using 25 ml of distilled water each time. Extraction was carried out using a separating funnel. Each time, the separating funnel was handshaken for 15 minutes and then allowed to equilibrate for 10 minutes. The absorbance of each aqueous extract was measured at 272.5 nm using a spectrophotometer against a blank of distilled water.
2Particle size analysis:
It was done by particle size analyzer.
Model: Laser Diffraction Particle size analyzer
Make: Sympatec, Germany
Particle range: 0.1 to 875 micrometer
Surface morphology:
it was done by scanning electron microscope
Model: ESEM TMP+ EDAX
Make: Philips, Netharland
Detector: secondary and back scattered electron detector
In vitro drug release:
The drug release rate was determined using USP dissolution apparatus ?.The 400 mg micropshere placed in basket. Dissolution media was use as 500 ml 0.1 M HCl, 50rpm, 37 ±0.50C.At the end of predetermine time intervals (e.g 1.2.3.4.5.6.7.and 8h), aliquots(5ml)were removed from each dissolution vessel and filter through 0.4µm what man filter papers. An equal volume of drug free medium (5ml) were added into the in to each dissolution vessel, to maintain a constant volume of medium during the dissolution test. The percentage drug released at each time point was quantified by ultraviolet spectroscopy at a ëmax of 272.5nm.
FT-IR study: it was done by Fourier Transfer infrared spectrophotometer
(Perkin Elmer, Spectrum GX FTIR System )
Result and Discussion:
Polymer drug Compatibility:
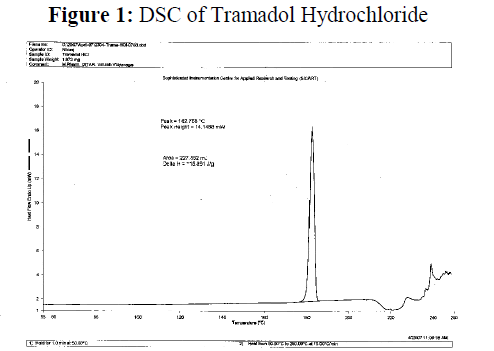
Figure 1: DSC of Tramadol Hydrochloride
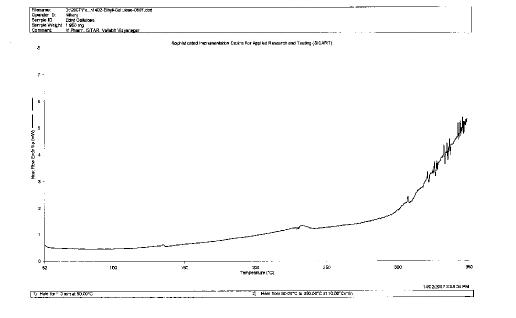
Figure 2: DSC of Ethylcellulose
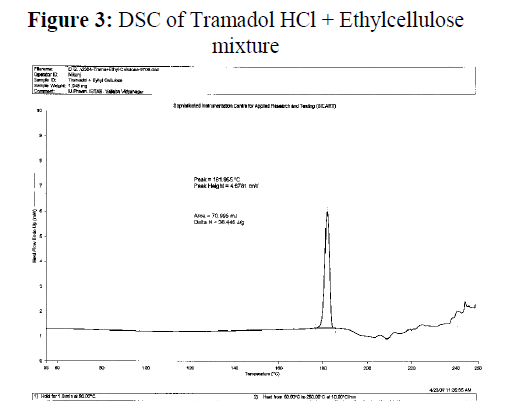
Figure 3: DSC of Tramadol HCl + Ethylcellulose mixture.
Polymer-Drug compatibility: polymer –Drug compatibility study was carried out by using differential Scanning Colorimetry. Ethylcellulose did not show any peak in DSC spectra. Tramadol Hydrochloride show one peak at 182.768°C. Physical mixture of Tramadol HCl: Ethylcellulose was found to contain one peak at 181.965°C. This peak does not much deviate from peak of standard Tramadol HCl . Therefore polymer and drug was found compatible with each other
The effect of formulation factors on characteristics of Tramadol HCl -loaded Ethyl cellulose microspheres. The effect of independent variables on yield, particle size, % encapsulation efficiency and % drug release was evaluated.
| Sl. No |
Batch no |
% yield |
%encapsulation efficiency |
Particle size (µm) |
| 1 |
EC1 |
68.0 % |
24.4 %±0.004583 |
74 |
| 2 |
EC2 |
74.1 % |
28.3%±0.009539 |
84 |
| 3 |
EC3 |
77.4 % |
31.1 %±0.003606 |
132 |
| 4 |
EC4 |
63.7 % |
19.0 %±0.004 |
64 |
| 5 |
EC5 |
67.2 % |
24.1 %±0.009849 |
78 |
| 6 |
EC6 |
73 .0% |
28.0 %±0.006928 |
118 |
| 7 |
EC7 |
59.5 % |
12.0 %±0.003606 |
46.2 |
| 8 |
EC8 |
63.0 % |
16.4 %±0.004359 |
64.3 |
| 9 |
EC9 |
70.0 % |
21.0 %±0.008544 |
102 |
Percentage Yield
Here P value for X1 and X2 were less than 0.05. So it indicates that drug: polymer ratio and volume of external phase (1% PVA) were significant effect on % yield. Here % yield was ranges from 59.51% to 77.4%. Here higher %yield obtained was in batch EC3 and lower % yield obtained was in Batch EC7.Here it was clear that when increase the drug: polymer ratio, increase the % yields. Volume of External phase decrease, increase the % yield. It was due to when increase the drug: polymer ratio, increase the viscosity of primary emulsion so, decrease the drug loss, so % yield is increase. Volume of external phase was increase so increase drug loss, so % yield was decreases.
Percentage Encapsulation efficiency
Here % Encapsulation Efficiency ranges from 12.0% to 31.1 %. Here higher % encapsulation efficiency obtained was in batch EC3 and lower % encapsulation efficiency obtained was in Batch EC7. % encapsulation efficiency was increase with increasing the drug: polymer ratio and decreasing the volume of external phase. Here P value for X1 and X2 were less than 0.05. so it indicate that drug: polymer ratio and volume of external phase (1%w/v PVA) were significant effect on % Encapsulations efficiency. It was due to when increase the drug : polymer ratio , increase the viscosity of primary emulsion so, decrease the drug loss by diffusion, so % Encapsulations efficiency is increase. Volume of external phase was increase so increase drug loss by diffusion , so % yield was decreases.
Particle size
P value for X1 and X2 were less than 0.05. so it indicate that drug: polymer ratio and volume of external phase (1%w/v PVA) were significant effect on particle size.
Here particle size ranges from 46.2 to 132 micrometer. Here higher particle size obtained was in batch EC3 and lower particle size obtained was in Batch EC7. Particle size was increase with increasing the drug: polymer ratio and decreasing the volume of external phase. The droplet size of primary emulsion was increase with increase the drug: polymer ratio, so finally particle sizes of microspheres were increase. The droplet size of the secondary emulsion may decrease because of a decrease in the frequency of collision of droplets with an increase in the volume of the external phase of the secondary emulsion. The decrease in the particle size of microspheres associated with an increase in the volume of the external phase of the secondary emulsion.
SEM study

Figure 4 (a): SEM images of Tramadol Hydrochloride microspheres

Figure 4 (b): SEM images of Tramadol Hydrochloride microspheres.
All the microspheres were spherical in nature its surface was smooth observed in SEM report. As noted the volume of external phase was increased, aggregation of microspheres was decreased.
| Time (hr) |
EC1 |
EC2 |
EC3 |
%
EC4 |
Drug Rele
EC5 |
ase
EC6 |
EC7 |
EC8 |
EC9 |
| 0 |
0.00% |
0.00% |
0.00% |
0.00% |
0.00% |
0.00% |
0.00% |
0.00% |
0.00% |
| 1 |
38.78% |
35.16% |
32.59% |
41.22% |
38.10% |
36.65% |
42.03% |
41.97% |
38.01% |
| 2 |
50.05% |
45.14% |
42.73% |
57.89% |
57.3% |
45.15% |
59.45% |
57.68% |
49.04% |
| 3 |
60.35% |
54.88% |
49.38% |
66.81% |
64.59% |
57.26% |
69.45% |
64.99% |
58.55% |
| 4 |
67.84% |
59.38% |
55.13% |
75.97% |
69.64% |
62.65% |
79.16% |
71.09% |
64.22% |
| 5 |
73.61% |
65.58% |
58.78% |
82.06% |
75.44% |
65.60% |
86.59% |
77.69% |
70.46% |
| 6 |
78.34% |
72.91% |
61.86% |
86.20% |
79.52% |
67.41% |
90.71% |
81.88% |
73.26% |
| 7 |
83.19% |
76.46% |
63.66% |
90.06% |
81.66% |
69.41% |
95.61% |
84.34% |
76.17% |
| 8 |
86.21% |
80.16% |
66.35% |
93.47% |
83.81% |
72.41% |
99.20% |
88.20% |
78.24% |
| T50(min) |
119 |
150 |
190 |
98 |
99 |
155 |
90 |
98 |
125 |
3.4: Percentage In vitro drug release.
Table No: Percentage dug release.
P value for X1 and X2 were less than 0.05. so it indicate that drug: polymer ratio and volume of external phase (1%w/v PVA) were significant effect on % in vitro drug release.% in vitro drug release was increase with decreasing the drug: polymer ratio and increasing the volume of external phase. Here in-vitro drug release was initially bursting effect followed by sustained. Here when increase the drug: polymer ratio, particle size of microspheres was increase. As particle size of microspheres was increase, decrease the surface area of microspheres, so finally drug release was decrease. Here particle size of microspheres decrease with increase the volume of external phase, more surface are available for drug release, so finally drug release was increase.
FT-IR study:
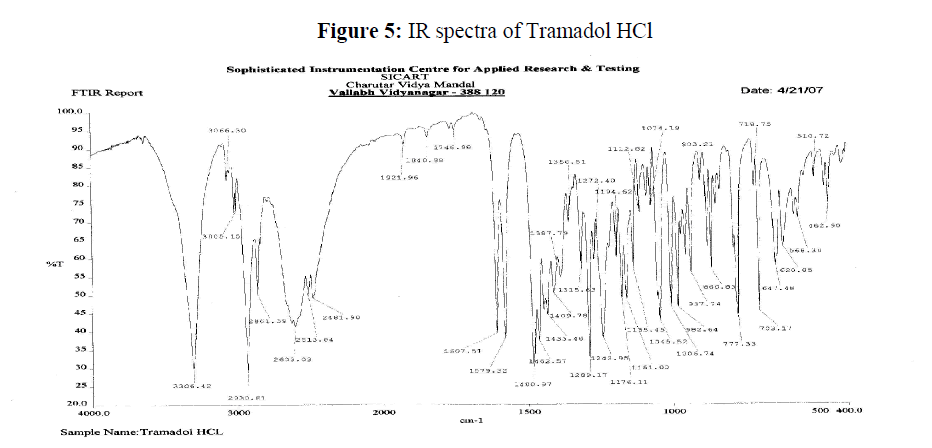
Figure 5: IR spectra of Tramadol HCl
Figure 6: IR spectra of Ethylcellulose
Figure 7: IR of Tramadol microspheres
It was clear that from above IR peaks obtained for different function groups of Tramdol HCl present in microspheres prepared using ethylcellulose as coating material were not much deviated from peak obtained in Std. Tramdol HCl. There for we can conclude that different material were used in preparation of Tramadol HCl microspheres were compatible with tramadol.
4: Conclusion:
For w/o/w multiple emulsion solvent evaporation method two variables such as drug: polymer ratio(X1) and volume of External phase (X2) had significant effect on percentage yield, percentage encapsulation efficiency, particle size and percentage drug release. The ethyl cellulose microspheres containing Tramadol HCl showed initially bursting effect followed by sustained release.
Conflict of Interest: None
Source of Support: Nil
5409
References
- Tripathiís K.D Essential of medical pharmacology, 3 rd edition, Jaypee Brothers medical Publishers (p) Ltd.
- Goodman and Gilmans the pharmacological basis of therapeutics; 10th edition ; McGraw- Hill publishers , medical publishing division; p-1069
- Barars F.S.K essential of pharmacotherapeutics. ; 3rd edition; S.Chand and company Ltd, New Delhi Publishers.; 2000; pp-442;
- Salman M.A., Sahin A., Oge K. Tramadol Encapsulated into PHB micropshres: in vitro release and epidural analgesic effects in rats. Acta Anaesthesiol Scand, 2003; 47:1006-1012
- Tiwari Sandip. B., Murthy T.K.,Mehta P.K. Chowadhry P.B., Controlled release formulation of Tramadol Hydrochloride using Hydrophilic and Hydrophobic matrix system, AAPS PharmaScitech, 2003; 4(3) :1-6
- Vyas S.P. and Khar R.K. ìTargeted and controlled drug deliveryî, CBS publishers and Distributors, New Delhi 417-457.
- Llin W.J , Yang C.Y., TSA S.Y.: In vitro Modified release of acyclovir from ethyl cellulose microspheres, Journal of microencapsualtion, 2001; 18(5):559-565
- Liao S, Hill JF, Nayk RK. Pharmacokinetics of tramadol fol-lowing single and multiple oral doses in man. Pharm Res. 1992;9: 308-315.
- Tegeder I, Lotsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinetics. 1999;37: 17-40.
- Parikh RH, Parikh JR, Dubey RR, Soni HN, Kapadia KN. Poly(D,L-Lactide-Co- Glycolide) Microspheres Containing 5-Fluorouracil: Optimization of Process Parameters. AAPS PharmSciTech. 2003; 4(2): 13
- Lecaroz C, Carlos Gamazo, Renedo M.J.; Biodegradable micro and nanoparticles as long term delivery vehicles for gentamicin, Journal of microencapsualtion, 2006, 23(7), 782-792.
- DyerJ.R.,ìApplicationofAbsorptionoforganic compound ì 2003,22 Published by Ashok K. Gosh, New Delhi














