Key words
|
| |
| chitosan; sodium diclofenac; microparticles; ionic interaction |
| |
Introduction
|
| |
| Sodium diclofenac is a non-steroidal antiinflammatory drug (NSAID) used to relieve the inflammation, swelling, stiffness, and joint pain associated with rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. The main side effects of the drug (in case of the long-term use) may include: abdominal bleeding, pain or cramps, and swelling, anemia, blood clotting problems, constipation, diarrhea, dizziness, fluid retention, gas, headache, heartburn, indigestion, itching, nausea, peptic ulcers, rash, ringing in the ears, and vomiting. Sodium diclofenac remains un-dissolve in the acidic environment such as the stomach (pH 1 to 3) that leads to delay in the onset of drug effect, but dissolves quickly in fewer alkaline conditions with pH 5-8 (1; 2). On the other hand, sodium diclofenac dissolves quickly in the intestinal alkaline condition so the effect of the long term drug uptake is impossible according to the short biological half life. Thus, total bioavailability of orally used sodium diclofenac has few important challenges. |
| |
| So preparing formulations of diclofenac sodium with an immediate and controlled release property not only can increase competence and reduce the medication side effects (3 ,4). However, delayed in onset of the drug associated with very low biological half-life, causing repeated a day dose several times that has been both cause side effects and reduction in the patient compliance. In order to overcome these problems, a drug type can be design which improve the solubility in the stomach as well as reduce the onset time of the drug effect. These will reduce dosage frequency following the slow controlled release. Therefore, the efforts of many researchers have been concerned to design a controlled release system for sodium diclofenac and other NSAID compounds (3,5, 6, 7). |
| |
| Chitosan is a linear polysaccharide derived from chitin that has been used in different pharmaceutical forms and drug delivery systems ( 8, 9, 10, 11). Chitosan also were used in various membranes in order to simulate the human epidermis and animals such as mice and rabbits ( 12, 13). However, sodium diclofenac not only is a good candidate for the preparation of slow-release formulation, but the chitosan-sodium diclofenac prepared formula has several advantages include lower solubility in the intestine, slow-released form and improve drug absorption. |
| |
| The purpose of this study was to prepare and evaluate the chitosan beads as a controlled drug release system for sodium diclofenac. Another purpose is to investigate the conditions in which the polymer bead is formed, and hence the dependence of the drug releases on bead formation. |
| |
Material and Methods
|
| |
|
Materials
|
| |
| Sodium diclofenac was gift from Hakim pharmaceutical Company (Tehran, Iran). Chitosan with the deacetylation degree of 95% and the molecular weight of 360 kDa was purchased from Fluka (Switzerland). Tween 80, sodium tripolyphosphate; monobasic potassium phosphate and sodium hydroxide were purchased from Merck (Germany). Dialysis bag was purchased from Toba Azema Co, Tehran (Iran). Minitab14 software was used for experimental design and the evaluation of the effect of variables on responses. |
| |
|
Determination of sodium diclofenac
|
| |
| The amount of microparticles loading and release rate of the drug determined using UV spectroscopy at wavelengths of 276 nm. The validity of assay method, linearity, repeatability, accuracy and limit of quantification (LOQ) were calculated. |
| |
|
Preparation of microparticles
|
| |
| Controlled release sodium diclofenac loaded microparticles were consisted of sustained and immediate released particles. In order to study the impact of independent variables on characteristics of microparticles, the full-factorial design with four variables and two levels were used. The percentage of Chitosan (%c), sodium poly phosphate (%s), Tween 80 (%T) and drug-chitosan proportion were selected as independent and drug loading efficiency, the rate of drug release and particle size as dependent variables (table 1). Sixteen formulations were prepared based on full factorial design. Sustained release microparticles prepared based on the method reported by Fattah et al (14). |
| |
| Sodium diclofenac dispersed in water and pH was set on 6. Chitosan dissolved in 1% of acid acetic solution, pH adjusted on 5.2 then 1% of Tween 80 was added. Defined amount of drug solution was added dropwise to chitosan solution to providing desirable drug-chitosan proportion. Then a certain volume of chitosan-drug compound (5 ml) was added drop by drop with stirring into 10 ml of sodium polyphosphate solution. This mixture sonicated for 5 minutes and stored 24 hrs in refrigerator temperature. Microparticles were recovered by centrifugation at 4000 rpm for 5 min and dried at 50?C for 24 hrs. |
| |
| The immediate particles were produced by poly vinyl prolidone (PVP) and manitol with equal proportion as carrier. PVP and manitol poured into container containing methanol until completely dispersed. Then the solvent was evaporated and stored in desiccators for two days and pulverized by passing through 60-mesh sieve (15). Different controlled release formulations were obtained by mixing sustained and immediate release particles with various ratios. |
| |
|
Drug entrapment efficiency
|
| |
| The percentage of encapsulated sodium diclofenac was determined by spectrophotometric determination at 276 nm after centrifugation of the aqueous dispersion. The amount of free drug was detected in the supernatant and the amount of encapsulated drug was calculated as a result of the initial drug minus the free drug. Amount of drug loading evaluated by two parameters; loading capacity (LC) and loading efficacy (LE) (16): |
| |
| LE= [(total amount of diclofenac) – (free diclofenac)]/ total amount of diclofenac |
| |
| LC= [(total amount of diclofenac) – (free diclofenac)]/ weight of microparticles |
| |
|
Morphological characterization
|
| |
| Surface morphology and stability of microparticles were studied by SEM (VP-1455, LEO, Germany). Microparticles were dried and coated by gold palladium. |
| |
|
Particle size analysis
|
| |
| The mean particle diameter and polydispersity index (PDI) of microparticles were calculated in water by laser light diffractometry, using Malvern MasterSizer SM 2000K (Malvern Instruments, UK). The samples were stirred and followed by sonication for 2 min for resuspending microparticles in water. |
| |
|
In vitro release study
|
| |
| Release studies for sixteen sustained formulation were performed in the simulated small intestine condition by pH 7.4. The release profile characterized using a six panel USP dissolution apparatus with 900 ml of medium with pH 7.4 and a temperature of 37?C. Furthermore, 5 ml of dissolution medium were withdrawn and replaced with fresh dissolution medium. Absorption of samples taken from centrifuge and separate clear supernatant solution and the appropriate dilution, by spectrophotometer at wavelength nm 276 against the control (dissolution test formulations were prepared without the drug). For immediate release particles, dissolution tests were performed same as sustained release microparticles in pH 2 and 7.4 for 2 hrs (15). |
| |
Results and Discussion
|
| |
|
Validity of drug measurement method
|
| |
| The relationship between the light absorption and concentration was significant (R2 = 0.994, P<0.001). Repeated surveys accountability in measurement methods within and between days for sodium diclofenac is represents the desired repeatability of measurement method on different days and caused nearly the same operation as well as error free results. The difference between real numbers and the estimated concentrations was about 5% that indicates the closeness of the estimated values to real results. |
| |
|
Encapsulation Efficiency
|
| |
| LE and LC of different sustained release microparticles are shown in table2. The correlation between LE and percentage of sodium polyphosphate was direct and significant (P= 0.000), and percentage of Tween 80 (P= 0.000) was indirect and significant (P=0.006) correlation with drugchitosan proportion. This finding suggested physical mechanism for sodium diclofenac loading into chitosan microparticles. Chitosan is a polysaccharide with positive charge but sodium polyphosphate and sodium diclofenac in used pH demonstrate negative charges. In this state in ionic competition, increase in amount of sodium tripolyphosphate to cause reduction in drug loading but this was not observed. These results indicated physical mechanism as main mechanism for drug loading. This finding is contrary with results that suggested ionic interaction for sodium diclofenac loading in chitosan microparticles (17). |
| |
| Piyakulawat et al (6) reported loading efficiency for sodium diclofenac - chitosan/carrageenan beads between 88-96% by that is higher than reported in present study. |
| |
| On the other hand the correlation between independent variables and LC was not significant because the weight of microparticles were in wide range for all formulations. This finding indicated variation in microparticles collection. |
| |
|
Drug release
|
| |
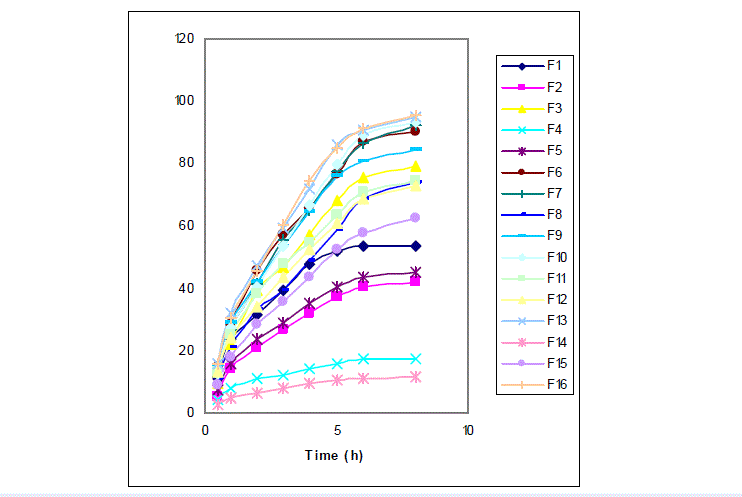
| The result of drug dissolution in case of the sustained release particles is presented in table 3 as percentage at different time courses. Figure 1 illustrated the release profile. The findings show that only the correlation between tween 80 and drug release after 1 and 8 hours was significant (P = 0.04 and P= 0.035 respectively). Percentage of drug released after 8 hours was in the range between 12 % from 95 %, which were observed in formulations 16 and 14, respectively. Based on the information obtained in this research increase in the tween 80 by increasing the affinity of drug to chitosan reduced the released drug after 1 and 8 hours. Due to the nature of the chitosan and low hydrophilicity of sodium diclofenac, seems tween 80 increase the drugs loading and reduce the release percentage by reducing the surface tension and increase hydrophilicity. |
| |
| On the other hand sodium polyphosphate percentage, although increased loading, but failed in affecting the percentage and speed of drug release. Sodium polyphosphate increases the LE through increasing the physical loading. Furthermore, because sodium diclofenac has no electrical interaction with chitosan, so the interaction between sodium phosphate and chitosan has no effect on the drug release rate. In site of the importance of electrical interactions between drug and chitosan, due to ion competition, with increasing concentration of sodium polyphosphate, release of drug from the polymer network should accelerate but such behavior was not observed. Drug release pattern that was observed from controlled release formulation was similar to sustained release particle, but higher amount of drug released after 1 and 8 hours was observed in case of controlled release formulation. Manjunatha et al reported 50 – 90 % of sodium diclofenac released from chitosan microparticles (15) which was in consistency with the result of drug release pattern in present study. |
| |
|
Particle size distribution and morphology
|
| |
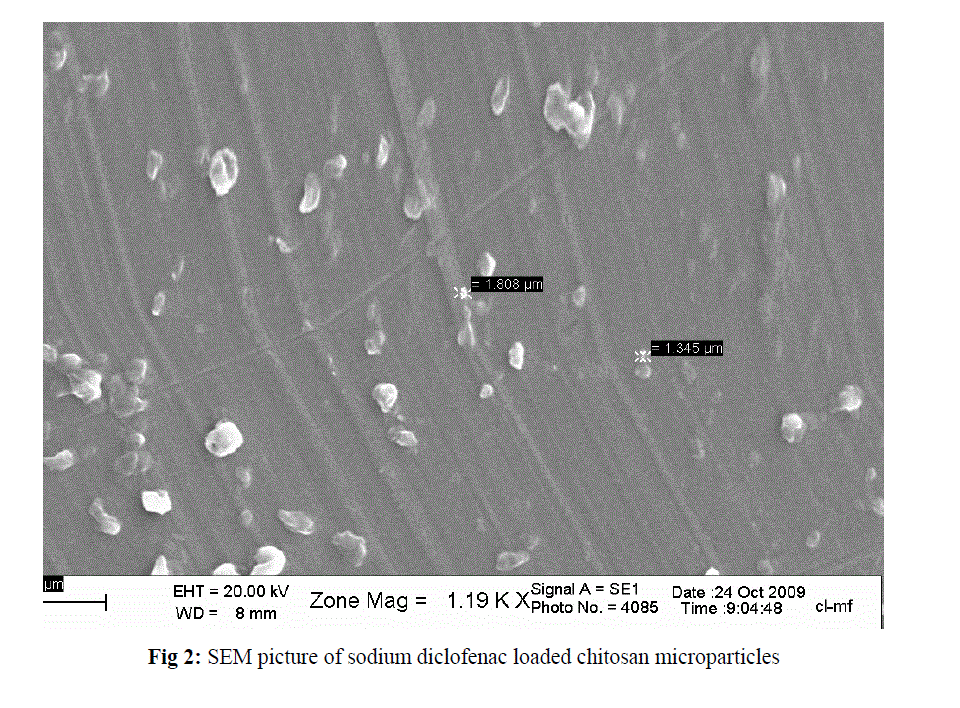
| The mean of particle size for sodium diclofenac loaded chitosan microparticles was found in the range of 4.8 (formulation 4) and 9.29 (formulation 12) µm with monodisperse type. The relation between drug-chitosan proportion and particle size was direct and significant (P= 0.000). The morphology of chitosan microparticles as shown in Fig 2 indicates particles aggregation with irregular shape. |
| |
Conclusion
|
| |
| The sodium diclofenac loaded chitosan microparticles were prepared by ionic cross-linking with sodium tripolyphosphate successfully. Loading efficiency of sustained release microparticles to be touched by percentage of cross-linking agent, Tween 80 and drug-chitosan proportion. Chitosan microparticles provided suitable sustained release pattern in the manner that with increasing in percentage of Tween 80, the released amount reduced. |
| |
Acknowledgment
|
| |
| This paper is issued from Pharm D thesis and financial support was provided by Ahvaz Jundishapur University of Medical Sciences. |
| |
Conflict of Interest
|
| |
| NONE |
| |
Source of Support
|
| |
| NIL |
| |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








