Mini Review - (2024) Volume 0, Issue 0
Prevalence and Incidence of Infectious Neuropathies in a Population with Neuropathic Pain and Presenting With Complex Regional Pain Syndrome
Jose J. Monsivais1* and
Erica Kozorosky2
1Hand and Microsurgery Center of El Paso Gateway West, Suite El Paso, Texas, USA
2Burell College of Osteopathic Medicine Arrowhead Drive Las Cruces, New Mexico, USA
*Correspondence:
Jose J. Monsivais, Hand and Microsurgery Center of El Paso Gateway West,
Suite El Paso, Texas,
USA,
Email:
Received: 28-May-2024, Manuscript No. IPJNN-24-14911;
Editor assigned: 30-May-2024, Pre QC No. IPJNN-24-14911(PQ);
Reviewed: 13-Jun-2024, QC No. IPJNN-24-14911;
Revised: 20-Jun-2024, Manuscript No. IPJNN-24-14911 (R);
Published:
27-Jun-2024, DOI: 10.4172/2171-6625.15.S10.003
Abstract
Purpose: The purpose of this study was to investigate the prevalence and incidence of antecedent and current infections with neurotropic viruses in a patient population with chronic neuropathic pain and presenting with Complex Regional Pain Syndrome (CRPS). The diagnosis was based on the International Association for the Study of Pain criteria.
Methods: This study investigated a pool of 409 patients who presented over a 10 year period with neuropathic pain and a history of one of the following diagnoses: Carpal tunnel syndrome, cubital tunnel syndrome, brachial plexopathy, or radiculopathy, or had received a nerve block, chemodenervation, or a brachial plexus block for relief of neuropathic symptoms and presented with acute symptoms suggestive of CRPS. Evaluations included physical assessments, antibody titer screening, inflammatory markers, skin biopsy results, and electro diagnostic studies when indicated.
Results: A total of 74 patients out of 409 neuropathic pain patients (prevalence 18.1%) had at least one positive titer of the following: Cytomegalovirus (CMV), Epstein-Barr Virus (EBV), Herpes simplex virus 1 and 2 (HSV), Parvovirus, Varicella- Zoster (VZV), or COVID-19. The most commonly associated pathogen found in this study was HSV-1 (n=22, 5.37%), followed by HSV-2, EBV, CMV, and Parvo (all n=8, 1.95%), VZV (n=7, 1.71%), and COVID (n=6, 1.47%). A total of 8 out of 74 patients (incidence 10.8%) met criteria for acute infectious neuropathy and required antiviral therapy with successful resolution of symptoms for over one year. The median nerve was most frequently involved.
Conclusions: If infectious neuropathies are not considered, there is the risk of using CRPS as the only diagnosis, thereby increasing morbidity, chronicity, and in some cases ongoing nerve damage. Patient screening for infectious neuropathies should include neurotropic viral screening as part of the routine workup. In our series, a prevalence of 18.1% was a significant frequency that triggered a more comprehensive workup. These conditions are treatable and a satisfactory response is highly probable.
Keywords
Complex regional pain syndrome; Neuralgia; Neuropathic pain;
Immune system; Nerve compression syndromes; Virus disease
Background
In a small percentage of patients with neuropathic pain and
exacerbation of symptoms suggestive of Complex Regional
Pain Syndrome (CRPS), the lead author noted decades ago that
an infectious neuropathy was occasionally present in these
situations. Specific treatment of the infectious neuropathy
resulted in improvement or remission in a short time period. This prompted an investigation into a larger population of patients
with neuropathic pain who developed CRPS signs and symptoms
to determine the prevalence of infectious neuropathies in this
population. Diagnosis of CRPS was based on the international
Association for the Study of Pain accepted criteria for CRPS [
1]
Introduction
The mechanisms underlying CRPS are poorly understood, but multi-modal interactions that include tissue trauma, abnormal pain processing, autonomic imbalance, and immune system alteration are hypothesized [2]. While tissue trauma resulting from nerve compressions has long been recognized as a pre-disposing factor of CRPS, immune system alterations from viral infections and decreased fiber density are less well-recognized [3]. Evidence suggests viral infections are linked to neuropathic pain syndromes, and alterations in skin innervation due to decreased fiber density may be associated with CRPS [4,5]. However, standard screening criteria for CRPS do not include viral screening or skin biopsies [6]. The purpose of this study was to investigate the prevalence and incidence of antecedent and current infections with neurotropic viruses in a patient population with chronic neuropathic pain and presenting with CRPS symptoms.
Methods
p>This study was a retrospective review with a prospective
component of a pool of 409 patients who presented with
neuropathic pain and were documented to have had one of
the following criteria: Carpal tunnel syndrome, cubital tunnel
syndrome, brachial plexopathy, radiculopathy, or had received
a nerve block, chemodenervation, or a brachial plexus block
for relief of neuropathic symptoms and presented with acute
symptoms suggestive of CRPS.
This study was approved under Burrell IRB 0093_2022. The
patients were evaluated using multiple diagnostic indicators.
Evaluations included physical assessments, antibody titer
screening, inflammatory markers, skin biopsy results, and
electrodiagnostic studies when indicated. The physical
assessment included findings of hyperhidrosis, allodynia,
vasomotor instability, herpetiform rash, or erythematous rash
with dermatomal distribution. Antibody titers (IgG & IgM) were
screened for Herpes Simplex Virus 1 and 2 (HSV 1&2), Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Parvovirus, Varicella-
Zoster (VZV) and COVID-19. Inflammatory markers (Erythrocyte
Sedimentation Rate (ESR), C-Reactive Protein (CRP), and white
blood cell count) were evaluated as evidence of acute viral
activity in association with signs and symptoms of CRPS. Skin
biopsy results were evaluated for decreased fiber density.
Electrodiagnostic studies were used to confirm the diagnosis.
Power analysis
A total of 66 participants were needed, with 22 in each of three
independent groups to adequately power the study for a large
effect size. The effect size was defined as f=0.4, an alpha value
of 0.05, and a beta value of 0.20. The power calculation was
performed using G*Power. Frequency and percentage statistics
were performed to establish measures of prevalence and
incidence.
Results
A total of 74 patients out of 409 neuropathic pain patients
(prevalence18.1%) had at least one positive titer of the following:
Cytomegalovirus (CMV), Epstein-Barr Virus (EBV), Herpes
Simplex Virus 1 and 2 (HSV), Parvovirus, Varicella-Zoster (VZV), or
COVID-19. The most commonly associated pathogen found in this
study was HSV-1 (n=22, 5.37%), followed by HSV-2, EBV, CMV, and
Parvo (all n=8, 1.95%), VZV (n=7, 1.71%), and COVID (n=6, 1.47%).
A total of 8 out of 74 patients (incidence 10.8%) met criteria for
acute infectious neuropathy (positive inflammatory markers
(sedimentation rate and C-reactive protein), rash, skin biopsy and
positive titers IgG and IgM).
The majority of those who met the criteria were given antiviral
therapy for 4-6 weeks with successful resolution of symptoms for
over one year. A small number of patients required longer courses
of therapy (up to one year). Median nerve involvement was the
highest prevalence at 44.1%. Of note, the median nerve is the most
frequently involved nerve in CRPS. Following the median nerve
prevalence were the ulnar nerve (16.0%), cervical radiculopathy
(7.7%), brachial plexopathy (4.0%), radial neuropathy (3.0%),
lumbar radiculopathy (1.8%), tibial neuropathy (1.5%), common
peroneal neuropathy (0.8%), sural neuropathy (0.5%). The order
of prevalence of nerve involvement in this study replicates that
reported in the literature (Tables 1-3) [7].
| Infectious agent |
HSV-1 |
HSV-2 |
EBV |
CMV |
Paro |
VZV |
COVID |
| IgG |
22 |
8 |
8 |
8 |
8 |
7 |
6 |
| IgM |
3 |
1 |
1 |
|
|
|
2 |
Table 1: Positive Viral Titers.
| |
Physical findings |
Inflammatory marker |
Decreased fiber density |
| |
IgG |
IgM |
IgG |
IgM |
IgG |
IgM |
| HSV-1 |
5 |
1 |
5 |
1 |
1 |
|
| HSV-2 |
|
|
2 |
|
1 |
|
| EBV |
|
|
|
|
1 |
1 |
| CMV |
3 |
3 |
|
|
|
|
| Paro |
1 |
|
1 |
|
1 |
|
| VZV |
2 |
|
1 |
|
1 |
|
| COVID-19 |
1 |
|
1 |
|
|
|
| Totals |
12 |
4 |
10 |
1 |
5 |
1 |
| Section totals |
16 |
11 |
6 |
Table 2: Virus Association with Other Findings.
| Infectious agent |
HSV-1 |
HSV-2 |
EBV |
CMV |
Parvo |
VZV |
COVID |
| Combined IgG & IgM |
4.20% |
1.50% |
1.50% |
1.30% |
1.30% |
1.20% |
1.30% |
Table 3: Prevalence of Infectious Agents.
Discussion
While knowledge about the evaluation and management
of chronic neuropathic pain has greatly improved in the last
decade, multiple knowledge gaps exist and a subset of patients
do not respond to conventional therapies. Considering an
infectious neuropathy opens up additional treatment options.
For example, some of the conditions are responsive to specific
antiviral antibiotic therapy. Some patients may require longterm
suppressive therapy (up to a year) to prevent recurrences.
Effective medications in this study were acyclovir, valacyclovir, and
valganciclovir. In this patient population, the more pronounced
rashes were associated with HSV-2. When this population is
compared with available prevalence percentages for HSV-1 and
HSV-2 in the general population, this population has lower rates
of both (Figures 1 and 2) [8].
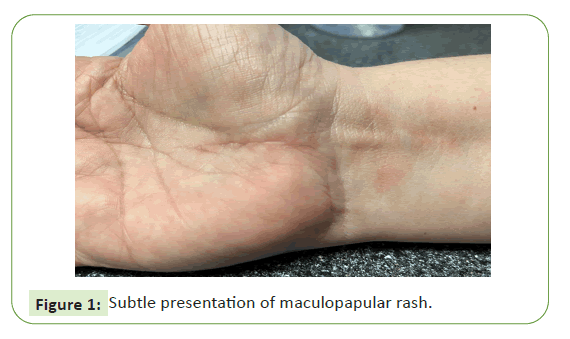
Figure 1: Subtle presentation of maculopapular rash.
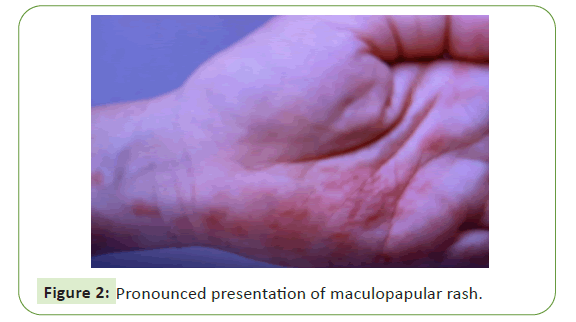
Figure 2: Pronounced presentation of maculopapular rash.
The patient presented at one month post-op from open carpal
tunnel release with an acute episode of pain after an uneventful
post-op course. She had positive viral titers and elevated
inflammatory markers (ESR and CRP) and was treated with a sixweek
course of acyclovir until the viral titers and inflammatory
markers were negative.
There was no recurrence after two years. The patient presented
with an exacerbation of pain during non-operative treatment
for ulnar nerve entrapment at the elbow. The diagnosis was
supported by clinical and electrodiagnostic studies. She had
positive viral titers and was treated with an eight-week course
of acyclovir until viral titers and inflammatory markers (ESR and
CRP) were negative. There was no recurrence after one year, and
the neuropathy has remained stable.
Erythrocyte sedimentation rate, C-reactive protein, white count,
elevated IgM and presence of a rash were used to determine the
acuity of the infectious process and confirmation of the process
with a positive skin biopsy.
Conclusion
The importance of considering the diagnosis of infectious
neuropathy in patients who present with CRPS and who do not
respond to conventional therapies opens the possibility of offering
other pathways of intervention. If infectious neuropathies are not
considered, there is the risk of using CRPS as the only diagnosis,
thereby increasing morbidity, chronicity, and perhaps ongoing
nerve damage based on the findings by skin biopsy of decreased
fiber density.
Patient screening for infectious neuropathies should include
neurotropic viral screening as part of the routine workup. In
our series, a prevalence of 18.1% and incidence of 10.8% was
a significant frequency that triggered a more comprehensive
workup. These conditions are treatable and a satisfactory
response is highly probable. In patients with neuropathic pain
who present with exacerbation of pain and symptoms suggestive
of CRPS, infectious neuropathy must be considered. Some of
the conditions are responsive to specific antiviral antibiotic
therapy. If infectious neuropathies are not considered, there is the
risk of missing the opportunity to provide definitive treatment for
infectious neuropathies and perhaps prevent ongoing fiber damage.
References
- Harden RN, McCabe CS, Goebel A, Massey M, Suvar T, et al. (2022) Complex regional pain syndrome: Practical diagnostic and treatment guidelines, 5th Edition. Pain Med 23:S1-S53.
[Crossref] [Google scholar] [PubMed]
- Russo M, Georgius P, Santarelli DM (2018) A new hypothesis for the pathophysiology of complex regional pain syndrome. Med Hypotheses 119:41-53.
[Crossref] [Google scholar] [PubMed]
- Monsivais JJ, Baker J, Monsivais D (1993) The association of peripheral nerve compression and reflex sympathetic dystrophy. J Hand Surg Br 18:337-338.
[Crossref] [Google scholar] [PubMed]
- Widyadharma IPE, Dewi PR, Wijayanti IAS, Utami DKI (2020) Pain related viral infections: A literature review. Egypt J Neurol Psychiatr Neurosurg 56:105.
[Crossref] [Google scholar] [PubMed]
- Kharkar S, Venkatesh YS, Grothusen JR, Rojas L, Schwartzman RJ (2012) Skin biopsy in complex regional pain syndrome: Case series and literature review. Pain Physician 15:255-266.
[Google scholar] [PubMed]
- Mesaroli G, Hundert A, Birnie KA, Campbell F, Stinson J (2021) Screening and diagnostic tools for complex regional pain syndrome: A systematic review. Pain 162:1295-1304.
[Crossref] [Google scholar] [PubMed]
- Gallo AC, Codispoti VT (2010) Complex regional pain syndrome type II associated with lumbosacral plexopathy: A case report. Pain Med 11:1834-1836.
[Crossref] [Google scholar] [PubMed]
- McQuillan G, Moran KD, Flagg EW, Ram PR (2018) Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief 304.
[PubMed]
Citation: Monsivais JJ, Kozorosky E (2024)
Prevalence and Incidence of Infectious
Neuropathies in a Population with
Neuropathic Pain and Presenting With
Complex Regional Pain Syndrome. J Neurol
Neurosci Vol. 15 No.S10:003
Copyright: https://www.imedpub.com/journal-of-neurology-and-neuroscience-advertising-111.html







