Key words
|
| |
| Proniosomes, non-ionic surfactant, penetration, entrapment efficiency, vesicles. |
| |
Introduction
|
| |
| The skin that covers throughout the body severs as a platform for drug delivery, but the stratum corneum the outer layer act as an obstacle for the delivery of drug through the skin [3]. Several technologies was developed to bypass or modulate this barrier, thereby delivers definite amount of drug at a defined rate to the dermal microcirculation; which includes physical, chemical and carrier approaches [4]. Thereby, making it a more prominent carrier than the oral delivery as it circumvents the variables that influence gastrointestinal absorption, overcome the hepatic metabolism and delivers the drug at controlled and constant rate thereby reducing the side effects [5]. |
| |
| Colloidal carrier have distinct advantages over conventional drug delivery as it act as drug containing reservoirs, modification of the particle composition or surface can adjusts the release rate to the target site. Even though, these carriers produce some problem with the industrial production and clinical application, are likely to play an increasingly important role in drug delivery[6, 7]. Among the various colloid carriers, liposome and niosomes can encapsulate both hydrophilic and lipophilic drugs. The hydrophilic drug is encapsulated inside the vesicles whereas the lipophilic drug is partitioned between the hydrophilic domains. Liposomes are produced by the self-assembly of phospholipids in aqueous phase to form bilayer which may be spherical unilamellar or multilamellar vesicles. They were considered to as an efficient carrier to transdermal drug delivery as it can loosen the stratum corneum and hence help in the penetration of drug. Though they produce potent action in pharmaceutics as drug delivery, still produces significant problems. That is, liposome may undergo some problems like degradation by hydrolysis in aqueous solution, sedimentation and aggregation on storage and cannot sterilize for clinical use. So, circumvent these problems, dry a powder form of liposome known as proliposome was introduced. They were considered superior to liposome as it can tolerate sterilization and are much more stable physicochemically. Still, chemical instability like oxidation of phospholipids was not avoided. These pave the way to the discovery of non-ionic surfactant vesicles known as niosomes. Niosomes first reported in the seventies in the field of cosmetics and now used in drug targeting. In both these colloidal carrier, phospholipids and non-ionic surfactant used act as penetration enhancer and hence can overcome the barrier of transdermal drug delivery. Although the niosomes, overcame the problem associated with chemical stability on storage, has some physical problems like aggregation, fusion, leakage of drug from the vesicles and hydrolysis of drug on storage were produced [8-11]. |
| |
| To minimize physical instability of niosomes such as aggregation, fusion and leaking and provided additional convenience in transportation, distribution, storage and dosing, proniosomes was discovered [12]. Proniosomes are dry formulations of surfactant-coated carrier, which when needed, rehydrated by brief agitation in hot water. These are considered superior drug delivery system because of low cost, greater stability, non-toxic, biocompatible, biodegradable and non-immunogenic, as it is nonionic in nature [13-15]. The proniosomes tailored according to the situation by modifying the structure. Proniosomes as a gel are considered efficient carrier for transdermal drug delivery, as it can encapsulate large variety of drug and will be converted to niosomes in-situ, which can loosens the stratum corneum, easy transfer of drug through it and hence overcoming the barrier. |
| |
|
Structural Components of Proniosomes Gel Surfactant
|
| |
| Non-ionic surfactants are used; they considered the important structural component. They act as Vesicle Forming Agents. The nature of vesicles formed depends upon HLB value in addition, phase transition temperature [15]. |
| |
| HLB value is a good indicator to predict the vesicle formation and entrapment efficiency. HLB number in between 4 and 8 is compatible with vesicle formation. Another important parameter is the phase transition temperature, higher TºC are more likely in the ordered gel form forming less leaky bilayer, thus having higher entrapment efficiency, while surfactants of lower T° C are more likely in the less ordered liquid form [15, 17, 21]. |
| |
|
Cholesterol
|
| |
| Cholesterol acts as “vesicular cement” in the molecular space that formed by the aggregation of monomer to form the bilayer [15]. Thereby increasing the rigidity decreases the permeability drug through the membrane and hence improves the entrapment efficiency. However, beyond certain concentration cholesterol will compete with the drug for the space within the bilayer, thereby excluding drug and can disrupt the regular linear structure of vesicular membrane. In addition to this, it can also act stabilizing agent [15, 21]. |
| |
|
Phosphatidyl choline
|
| |
| PC acts as stabilizing agent as well as penetration enhancer. The commonly used PC is lecithin i.e. soya lecithin, egg lecithin. Hydrogenated lecithin will enhance the rigidifying effect of cholesterol and formation of less leaky vesicles, hence more advantageous than unhydrogenated lecithin [19, 20]. The reason behind this is the presence of double bonds in the unhydrogenated phosphatidylcholine permit the chains to bend (undergo conformational rotations to give cis/trans conformations), causing the adjacent molecule not to be tightly close to the bent phosphatidylcholine molecule when they assembled to form the niosomal membrane; thereby, the membrane become more permeable [15, 18]. |
| |
|
Solvents
|
| |
| The solvent can act as penetration enhancer and in turn affect the vesicular size formation. Solvents commonly used are alcohols, mainly, ethanol, propanol, butanol, isopropanol. Researchers have reported that ethanol showed larger vesicular size due to the slow phase separation as it has greater solubility in water, whereas due to the branching of isopropanol it showed smaller vesicular size [16-17]. |
| |
| In addition, reports suggest that the drug penetration is maximal for isopropanol due to the reason that the branched structure will act as co-surfactant and might loosen the bilayer packing resulting into the increased release of drug [16-17]. |
| |
|
Carriers
|
| |
| The carriers used in the preparation should be safe and non-toxic, free flowing, poor solubility in the loaded mixture solution and good water solubility for ease of hydration [23]. The carrier when used in the proniosomes preparation permitted flexibility in the ratio of surfactant and other components that incorporated. In addition to this, it increases the surface area and hence efficient loading. Commonly used carriers are maltodextrin, sorbitol monopalmitate, lactose monohydrate, spray dried lactose, glucose monohydrate and sucrose stearates. Of these carriers sorbitol, glucose monohydrate and lactose monohydrate is difficult to coat with the loading mixture solution due to their solubility in this solution and upon application; the samples became viscous slurries. Whereas, when maltodextrin used as the carrier in the proniosomes preparation, it permitted flexibility in the ratio of surfactant and other components, which is incorporated [24]. Hence, it considered as an efficient carrier for proniosomal formation. |
| |
|
Solulan
|
| |
| Solulan added to the formulation to prevent the aggregation due to steric hindrance[32]. |
| |
|
Stearylamine
|
| |
| Steraylamine added to the formulation to increase the entrapment efficiency [23-24]. This could be attributing [24]: |
| |
| (a) The strong electrostatic forces involved in the interaction of the negatively charged drug with the positively charge inducer stearylamine. |
| |
| (b) The repulsion between likely charged vesicles which would minimize aggregations and hence, improving the nebulization efficiency percentages. |
| |
|
Oleic acid
|
| |
| The oleic acid added to the formulation to induce the negative charge to the vesicles [25]. Hence, cause reduction in zeta potential and vesicular size. |
| |
|
Preparation of Proniosomal Gel
|
| |
| The principle behind the preparation of proniosomal gel is that it forms sol phase at high temperature (60ºC) for the complete dissolution of surfactant and it will not form micelle, as the solvent used is very small. When small amount of water is added to the above mixture W/O micro emulsion is formed, in this aqueous phase is bound by continuous phase using surfactant at the interphase. On cooling, it will decrease the solubility of the surfactant and cholesterol in the solvent and lowers solvent-gelator affinities as their limited solvent system. The formed gel is lamellar micellar model [26]. |
| |
|
Coacervation method
|
| |
| The method is that a precise amount of drug and surfactant: Alcohols (1:1) taken in a clean and dry, wide mouth small glass tube. After mixing all the ingredients, the open end of the glass tube covered with a lid to prevent loss of solvent from it and warmed on the water bath at 60-70°C for about 5 minutes, until the surfactant dissolves completely. The aqueous solution added and warm on a water bath until a clear solution formed which when cooled to convert to gel. The final ratio of surfactant: alcohol: aqueous phase is 5:5:4 w/w/w [17, 18, 27]. |
| |
|
Factor Affecting Entrapment Efficiency and Size of Vesicles
|
| |
| Entrapment efficiency is the measure of solute retention [28]. High entrapment efficiency means a less time and less effort needed to remove the unentrapped drug. Entrapment efficiency and vesicular size are important parameters to predict the stability of the dispersion [29]. If the prepared formulation remains the unchanged vesicular size and entrapment efficiency even after storage, hence the formulation considered stable. |
| |
|
Non-ionic Surfactant
|
| |
| The non-ionic surfactant used act as vesicle forming agent, the amount and its nature will affect the entrapment and vesicular size. |
| |
| A. Nature of non-ionic surfactant |
| |
| The entrapment efficiency and vesicular size depends upon the HLB value, chemical nature and phase transition temperature. The surfactant with high or low HLB value has high entrapment efficiency with some approaching 100%. In addition to HLB, the chemical structure of the surfactant i.e., the alkyl chain length will also affect the entrapment, which is directly proportional to the phase transition temperature. The higher the alkyl chain higher will be the entrapment efficiency; as it is having high phase transition temperature thereby it is more likely to form orderly gel form and hence less leaky [30]. While surfactant with lower phase transition temperature are likely to form less orderly liquid. The increase in leakiness means it has less entrapment efficiency. |
| |
| This is evident from the report that, Sorbitan mono oleate (span 20, span 80) and Stearate Sucrose Ester shows higher entrapment efficiency, as their alkyl chain is longer thereby higher phase transition temperature [31]. Whereas, Tween 20 and 80 have higher HLB, value and hence showed lower entrapment compared to span series. While Tween 80 has comparatively higher efficiency than Tween 20, because of higher phase transition temperature due to longer alkyl chain [15]. |
| |
| B. Amount of surfactant |
| |
| The increase in surfactant amount will increase the entrapment efficiency because the surfactant being the vesicle forming agent [15]. |
| |
|
Cholesterol
|
| |
| Cholesterol that acts as cementing agent, when its concentration is increased which will considerably decrease the entrapment efficiency. It will retard the afflux profile of entrapped drug. The reason behind decrease in the entrapment efficiency due to increase in cholesterol is that with higher amounts of cholesterol may compete with the drug for packing space within the bilayer, hence excluding the drug as the amphiphiles assemble into the vesicles [32]. |
| |
| However, the effect of cholesterol will vary the entrapment efficiency according to the surfactant used. This is evident from the report that, increase in cholesterol content will vary the entrapment efficiency according to the surfactant used. In case of Brij 52(HLB- 5.3) has insignificant effect on the entrapment efficiency as the concentration of cholesterol is increased, but Brij 76 the HLB value is 12.6 indicating low hydrocarbon chain volume in comparison with hydrophilic surface area. Increased cholesterol content will increase the lipophilic character and hence the entrapment efficiency. Whereas, span series the entrapment efficiency was increased with cholesterol to some extent further increase lead to the decreased entrapment efficiency. This can explained to be due to the following fact that a small increase in cholesterol increases bilayer hydrophobicity and stability, thus the permeability is decreases as it efficiently traps the drug in the bilayer as vesicles are formed. However, the cholesterol may compete with the drug as the concentration is beyond certain limit [32-33]. |
| |
|
Stearyl amine
|
| |
| Stearylamine when incorporated in the formulation will increase the entrapment efficiency as it produces strong electrostatic interaction between the negative charge drug and positive charged inducer stearylamine [34]. |
| |
|
Mechanism of Action
|
| |
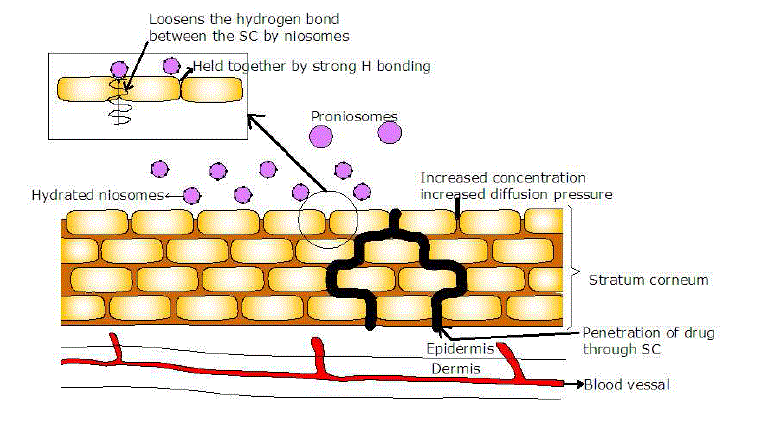
| The exact mechanism of penetration of drug in the vesicles through the skin are not yet explored, but the penetration will depends on the nature and type of the drug used, vesicles formed and hydration temperature for the conversion of proniosomes to niosomes. The lipids used in the preparation of proniosomes, act as carrier that will form depot at the site of action and hence sustains the action. The rate-limiting step in the penetration of drug through the transdermal drug delivery is the lipid (ceramides) part of stratum corneum, which packed tightly as bilayer by hydrogen bonding. The hydrogen bonding will strengthen and stabilize the lipid bilayer and as a result will impart the barrier property of stratum corneum[34-32]. |
| |
| Proniosomes will hydrate to niosomes when applied to skin. On to the skin surface, the niosomes formed adsorbs fuses and loosens the ceramides by competitively breaking the hydrogen bond network leading to high thermodynamic activity at the interface. This will increase the increases the concentration gradient and hence increases the diffusion pressure for the driving of drug through the stratum corneum. |
| |
|
Factors Influencing the Penetration of Drug Through The Skin
|
| |
| The proniosomes when applied to skin, the hydration of proniosomes the drug from the niosomal vesicles will release and penetrate through the skin[34]. The entrapment efficiency and release of drug from the formulation are inversely related. The more the drug retained in the vesicle, slower is the release profile. Penetration of drug through stratum corneum is influenced by non-ionic surfactant, lecithin, cholesterol, solvents. |
| |
|
Non-ionic surfactant
|
| |
| The principle component non-ionic surfactant in proniosomes will interact with the tightly bound hydrogen bonding in the stratum corneum and loosens it, thereby penetrates in to the cell[34]. The penetration ability of surfactant through the skin will depend upon the transition temperature and HLB value. |
| |
| The surfactant with higher the transition temperature will penetrate slowly through the skin. The reason behind this is it needs higher temperature to form disordered liquid crystalline state and to completely fluidizes and hence less permeable. Whereas the contrary happen in case of surfactant will low transition temperature and hence the permeation increases. This is evident from the study of sorbitan ester that span 40 and span 60 have high transition temperature and hence low permeation at 37°C than span 20 and span 80 as it has low transition temperature, which forms disordered liquid crystalline state and completely fluid[26]. |
| |
| The HLB value of surfactant also influence the permeation of drug, high HLB value results in reduction in the surface free energy which allows to form vesicles of larger size and hence small size is exposed to dissolution medium and skin. Nevertheless, span 20 will have high HLB value and vesicles of larger size but faster release obtained because of low transition temperature. In case of span 80 containing formulation, the transdermal flux is more due to the reason that it has low transition temperature and in addition to that vesicular size is small, which produces rapid penetration of drug[17, 39]. |
| |
|
Lecithin
|
| |
| Incorporation of lecithin into the proniosomal formulation will enhance the penetration of drug, as these will form vesicles of smaller size and hence increase hydrophobicity. Among the lecithin, soya lecithin produces penetration enhanced than egg lecithin because soya lecithin contains unsaturated fatty acid like oleic and linoleic acid. This will produce enhanced penetration, as the double bond in the unsaturated fatty acid will cause the chain to bend by providing the conformational rotation to give cis/trans conformation, thereby increased permeability, as the molecules are not tightly packed. Whereas, egg lecithin contains saturated fatty acid and produces less penetration as it forms inclusion of hydrogenated lecithin produces the bilayer molecule to get more rigid and less permeable[15, 40, 41]. |
| |
|
Cholesterol
|
| |
| Increase in cholesterol, one of the common additives for preparing the proniosomes, results in increased micro viscosity of the membrane leading to more rigid bilayer. It has an ability to cement the leaking space in a bilayer membrane, which results in more intact bilayer and hence decreases the permeability [15, 21]. |
| |
|
Proniosomal Assisted Delivery of Therapeutic Moiety as Transdermal Delivery
|
| |
| Although, the advent of niosomes into the pharmaceutical arena hailed from the cosmetic industry, but it was only recently that the transdermal delivery was taken into consideration. Literatures reveal that proniosomes/niosomes can easily penetrate the stratum corneum due the presence of non-ionic surfactants. Furthermore, they can be used to controlled percutaneous drug delivery vesicles. Hence, the unique and promising for the release of drugs using proniosomes renders it an attractive candidate as an enhancer to administer drugs throughout the skin. The advantage of proniosomal gel is that the system can be directly formulated into a transdermal patch without dispersing vesicles into the polymer matrix; the various advantages are tabulated in table 1. The various pharmacological moieties that have been used in the transdermal drug delivery are NSAIDs, antihypertensive, hypolipidaemic, contraceptive, hormones and so on. These are emphasized in table 1, which summarizes the research on Proniosomal gel as transdermal administration. |
| |
|
Contraceptive agents
|
| |
| B.Vora et al.[17] developed proniosomal based transdermal drug delivery of levonorgesterol and extensively evaluated it both in vitro and in vivo. Studies were carried out to find out various parameters like vesicular size, entrapment efficiency and found that the vesicular size and entrapment efficiency depend on the non-ionic surfactant, alcohol and various component used. In the present study, it found that entrapment efficiency was almost 100% and niosomes formed from it exhibited high entrapment efficiency. In addition, they found that in-vitro permeation studies also depends upon the various ingredient used in the formulation. Invivo studies of transdermal delivery using proniosomes of drug was compared with ointment with washable base, and found that proniosomal gel was having greater interference with corpus lutea than the washable ointment. |
| |
|
Hormones
|
| |
| J.-Y. Fang et al. [18] examined the feasibility of proniosomes as a transdermal delivery system for estradiol. The study revealed that encapsulation efficiency of proniosomes was nearly 100% and the permeation of estradiol with span 40 and span 60 was higher through the skin. In addition to this, cholesterol in the vesicular bilayer did not significantly affect the entrapment efficiency and penetration. Hence, the study concluded that type and content of non-ionic surfactant is the important parameter that affects efficiency of transdermal estradiol delivery. |
| |
|
Antihistamine drugs
|
| |
| J.Varshosaz et al.[19] studied the influence of different processing and formulation variables on Chlorpheniramine Maleate proniosomes as transdermal drug delivery using sorbitan monopalimate. The formulation was evaluated the effect of composition, type of surfactant and alcohol on the drug loading, rate of hydration, vesicle size, polydispersity, entrapment efficiency and drug release. The study revealed that the proniosomes containing span 40 along with lecithin controlled the drug release continuously. |
| |
|
Antinflammatory drugs
|
| |
| Ibrahim A. Alsarra et al.[36] carried out research to study a noninvasive alternative route for the delivery of ketorolac using proniosomes to overcome the pharmacokinetic problems of drug like short halflife. The proniosomal gel was formulated using various surfactants like Span 20 and Tween 40 and lecithin and cholesterol. The study revealed that prepared proniosomal gel of ketrolac showed good permeation and have high entrapment efficiency. Span 20 containing proniosomal gel have enhanced skin flux through the skin than the Tween 40 proniosomes. Hence, the study concluded that proniosome act as promising carrier for the delivery of ketorolac due to their simple production and facile up. |
| |
| Ajay B Solanki [37] et al. performed research on ketoprofen loaded proniosomal gel with an aim to increase the half-life of the drug and to overcome the adverse events. In the present study, the proniosomal gel was prepared by slurry method and optimized formulation was compared with the plain gel to evaluate the permeation of drug through the formulation. Hence, the study concluded that the proniosomal gel showed enhanced penetration and in addition to this overcame the adverse events, as it was transdermal delivery. |
| |
| Chandra et al.[38] studied the feasibility of the Piroxicam loaded proniosomal gel and also to study the ability of lipid vesicles to deliver drug through the skin. In the present study, the proniosomal gel was compared to conventional niosomes and plain gel of carbopol for the penetration through the skin. Hence, the study concluded that proniosomal loaded showed high entrapment efficiency and thereby increased penetration as the surfactant deliver the drug through the skin. |
| |
|
Antihypertensive Drug
|
| |
| A.Azeem et al,[35] carried out the research with an aim to explore the mechanism of the penetration of drug through the skin. The study showed the stratum corneum is the rate limiting step and when the proniosomes were applied to the skin, it get hydrated and converts to niosomes. The hydrated niosomes will break the hydrogen bond network leading to high thermodynamic activity at the interface. This will increase the increases the concentration gradient and hence increases the diffusion pressure for the driving of drug through the stratum corneum. |
| |
| Reena Thakur et al,[39] developed a proniosomal gel of losartan potassium and studied were carried out to evaluate it’s the pharmacokinetic parameter. In the present study, proniosomal gel of Losartan potassium using different surfactant was formulated and evaluated. The optimized formulation used to evaluate the bioavailability of the formulated product with the marketed product and revealed that significant greater amount of drug reached the systemic circulation than the marketed formulation. |
| |
| The composition of proniosomes has same composition as that of niosomes, and proniosomes that is applied will get converted to niosomes when applied to skin. Unfortunately, there is not enough research conducted to investigate toxicity of niosomes. Researchers measured proliferation of keratinocytes in one of the topical niosomes formulations. The effect of surfactant type on toxicity was investigated. It was determined that the ester type surfactants are less toxic than ether type surfactants. This may be due to enzymatic degradation of ester bounds. In general, the physical form of niosomes did not influence their toxicity as evident in a study comparing the formulations prepared in the form of liquid crystals and gels[45]. |
| |
Conclusion
|
| |
| Obviously, the field of proniosomal drug delivery is still in the infancy and increasing gradually during the past decades, and we expect that this trend will continue to increase further. Proniosomes have proven useful for the delivery of anti-hypertensive, anti-cancer and anti-inflammatory agents. The literatures have also suggested that niosomes has proven to target certain areas of the mammalian anatomy and exploited as diagnostic imaging agents, hence future studies based on these should conducted in proniosomes to exploit it in targeted and diagnostic imaging |
| |
| Researchers have also suggested that hydrophobic drugs and macromolecules encapsulated in niosomes are more stable than low molecular weight drugs and hence the transdermal drug delivery using proniosomes extended to the field of proteins and other large molecules. In addition to this, the transdermal drug delivery using proniosomal carrier can be studied for certain drugs antibacterial and antifungal drugs , it is certain that pharmacokinetic problems of these drugs can be overcome by loading in the proniosomes carrier and also can be used to study the feasibility of the carrier .Hence, the proniosomes in transdermal drug deliveries are should to exploit more for more pronounced efficiency and permeability. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |







