The Comparative Evaluation of S100B Protein, Glasgow Coma Scale and Neuro-Depiction over Head Injuries in Childhood
Keywords
Head injury; Craniocerebral injury; Detection trial; Childhood; Glasgow coma scale; S100B; Neuroimaging; Intracranial complication; Prognostic biomarker
Introduction
Background
The main goal during the treatment of a Head Injury (HI) is the early revelation of a dynamic intracranial complication because the primary trauma will be early diagnosed and the secondary trauma will be avoided, preventing in this way the development of intracranial hypertension, hypoxia, hypercapnia and finally hypervolemia. In this philosophy there are difficulties concerning the daily clinical activity leading the doctors to actions of defensive medicine, meaning the implementation of numerous clinical laboratory examinations that can ultimately be considered to be unnecessary.
Within the framework of the early diagnosis of a HI the acquisition of a thorough history is considered to be important, together with the evaluation of the vital points, the clinical examination of each system accordingly and the neurological evaluation. If a multiple injured is the case the continuous evaluation and support of the patient is self-evident, which is conducted simultaneously with the diagnostic approach. Likewise, in a patient with an influenced level of consciousness or with dynamic blood instability, the support of the vital points may be required before the completion of the diagnostic survey. The conduction of the targeted laboratory and imaging investigation comes right after [1,2].
Searching thoroughly the relevant bibliography, the search of prognostic biomarkers, as UCH-L1, GFAP, H-FABP, APP, isoenzyme CK-BB, and the coenzyme NSE is ascertained for more than 60 years. The diagnostic reliability, the low cost, the wide distribution and the simple application are considered significant selection criteria of a prognostic biomarker [3,4].
The aim of the current survey is the alterations of S100B protein as prognostic rate of the childhood head injuries severity. The methodology involved the correlation between the S100B protein alterations, the objective evaluation of the level of consciousness and the findings deriving from the neuroimaging.
Materials and Methods
74 hospitalized patients were selected (45 boys and 29 girls) in the 1st Department of Pediatric Surgery AUTH, in the nine months’ period 1/2017–9/2017 in the ages 3-14 years old (average 7.86 years old), who suffered from pure HI, with no case where either the mechanical support of respiration or the hospitalization in an intensive care unit was ever required. The selection criteria of the patients of the present study was the clear neurological and child psychiatric history, the absence of previous craniocerebral injury and finally the potential of the escorts for an adequate informing about the mechanism of the injury and the symptomatology of the young patient.
Based on the severity of the clinical manifestations the patients were divided into 4 groups. Group A (10/74 witnesses) who didn’t suffer from HI and had a clear neurological history. Group B (9/74 patients) with a headache, with no neurological semiology or alteration of the level of consciousness (Glasgow Coma Scale (GCS): 15/15). Group C (40/74 patients) post traumatic amnesia, headache, vomiting, dizziness and a mild reduction of the level of consciousness (GCS: 13-14/15). Finally, Group D (15/74 patients) with loss of conscience, post traumatic amnesia, headache, vomiting, dizziness, and moderate reduction of the level of consciousness (GCS: <13/15).
The definition of S100B with the ECLIA method took place in 3, 12, 24 and 48 hours after the HI. The ECLIA method is based on the norm of double immunostaining (sandwich). The tubes that were used belonged to the Vacutainer SST Company with separation gel of the Becton Dickinson Company. After the collection and the statistic processing of the data, the definition of sensibility followed, of the specialty and of the positive and negative prognostic value of protein S100B.
The rate 0.191 μg/L was defined as the separation point of S100B, which corresponds to the heist rate of S100B to the patients of Group A. All the patients of Group B, C and D were submitted to neuroimaging with the execution of CT skull and brain CT, 6-8 hours after the injury.
Results
The correlation of the HI mechanism to the study group is shown in Table 1. The correspondence of the S100B rates, per survey group with the findings of the neuroimaging is presented in Table 2 while the range of S100B per group is presented in Supplementary Figure 1. The various fluctuations of S100B in 3/9 patients of group B and in 4/40 patients of group C who exhibited subdural hematoma are presented in Supplementary Figures 2 and 3 accordingly. The duration of the hospitalization of the patients was from 2 to 5 days. No loss of patient or severe disability occurred. 7/64 patients with intracranial complication were transferred to neurosurgical clinic and they were operated because of subdural hematoma. The patients received specific direction while leaving and they were rechecked in regular medical offices after 14 days and after a trimester. In the context of this re-evaluation we didn’t notice in any patient either an indication of disability or complication or a remaining of the symptoms.
| Injury mechanism |
Total
n (%) |
Group Α
witnesses |
Group Β |
Group C |
Group D |
| Fall from the same height |
26 (41%) |
- |
1/26 |
18/26 |
7/26 |
| Fall from height |
16 (25%) |
- |
2/16 |
10/16 |
4/16 |
| Collision to a stable object |
4 (6%) |
- |
0/4 |
2/4 |
2/4 |
| Fall from bike |
11 (17%) |
- |
1/11 |
8/11 |
2/11 |
| Hit from a moving object |
5 (8%) |
- |
5/5 |
0/5 |
0/5 |
| Beating |
2 (3%) |
- |
0/2 |
0/2 |
2/2 |
Table 1: The correlation of the HI mechanism to the study group.
| Group |
S100B range rates in hours |
Neuroimaging Results |
| 3 |
12 |
24 |
48 |
| Α |
0.07-0.19 μg/L
AVG
0.1433 μg/L |
0.06-0.18 μg/L
AVG
0.1436 μg/L |
0.06-0.18 μg/L
AVG
0.1448 μg/L |
0.07-0.18 μg/L
AVG
0.1413 μg/L |
Absence of intracranial complications |
| Β |
0.08-1.68 μg/L
AVG
0.339 μg/L |
0.07-1.70 μg/L
AVG
0.327 μg/L |
0.04-1.35 μg/L
AVG
0.269 μg/L |
0.04-1.02 μg/L
AVG
0.208 μg/L |
Absence of intracranial complication: 6/9
Subdural hematoma: 3/9 |
| C |
0.05-0.35 μg/L
AVG
0.147 μg/L |
0.05-0.34 μg/L
AVG
0.134 μg/L |
0.03-0.27 μg/L
AVG
0.114 μg/L |
0.03-0.21 μg/L
AVG
0.090 μg/L |
Absence of endocranial complication: 36/40
Subdural hematoma: 4/40 |
| D |
0.11-0.34 μg/L
AVG
0.198 μg/L |
0.09-0.33 μg/L
AVG
0.191 μg/L |
0.07-0.30 μg/L
AVG
0.167 μg/L |
0.05-0.25 μg/L
AVG
0.136 μg/L |
Absence of endocranial complications |
Table 2: The correspondence of S100B rates, per survey group, with the findings of the neuroimaging.
Statistical analysis
For the statistical processing of the results the method of symmetric normalization were used [5,6]. The analysis of correspondences took place for the pair of variables GSS- S100B within 3, 12, 24 and 48 hours, while for the analysis of correspondences (simple and multiple) the appropriate categorization of variables was used.
Initially this precondition was evaluated with the check of independence χ2 (chi-square test) for the variables GCS and S100B (3, 12, 24 and 48 hours afterwards) [7,8].
Statistically what was found was an important difference between the 4 groups to the measurement of S100B after 3 hours (F (3,67)=3.140, p=0.031<0.05), after 12 hours (F (3,67)=3.080, p=0.033<0.05), after 24 hours (F(3,67)=3.207, p=0.029<0.05) and after 48 hours F(3,67)=3.354, p=0.024<0.05).
In Table 3 the results of the dependence between GCS and the rates of protein S100B are exposed. From the results what is evident is that statistically there is an important dependence between GCS and the level of S100B after 3 hours (χ2 (12)=26.936, p=0.008<0.05) and the level of S100B after 24 hours (χ2 (12)=22.607, p=0.031<0.05) while there is no statistically important dependence between the category of GCS and the level of S100B after 12 hours (χ2 (12)=17.528, p=0.131>0.05) and the level of S100B after 48 hours (χ2 (12)=18.458, p=0.102>0.05) (Table 3).
| S100B |
GCS |
p-value |
| 12 |
13 |
14 |
15 |
| n |
% |
n |
% |
n |
% |
n |
% |
| 3 hours |
1 |
0 |
0.00% |
4 |
19.00% |
8 |
42.10% |
2 |
22.20% |
0.008 |
| 2 |
3 |
20.00% |
7 |
33.30% |
5 |
26.30% |
1 |
11.10% |
| 3 |
6 |
40.00% |
4 |
19.00% |
6 |
31.60% |
3 |
33.30% |
| 4 |
3 |
20.00% |
3 |
14.30% |
0 |
0.00% |
0 |
0.00% |
| 5 |
2 |
13.30% |
1 |
4.80% |
0 |
0.00% |
1 |
11.10% |
| 12 hours |
1 |
0 |
0.00% |
0 |
0.00% |
3 |
15.80% |
0 |
0.00% |
0.131 |
| 2 |
1 |
6.70% |
5 |
23.80% |
6 |
31.60% |
3 |
33.30% |
| 3 |
3 |
20.00% |
7 |
33.30% |
4 |
21.10% |
1 |
11.10% |
| 4 |
2 |
13.30% |
1 |
4.80% |
0 |
0.00% |
2 |
22.20% |
| 5 |
1 |
6.70% |
1 |
4.80% |
0 |
0.00% |
1 |
11.10% |
| 24 hours |
1 |
0 |
0.00% |
1 |
4.80% |
4 |
21.10% |
1 |
11.10% |
0.031 |
| 2 |
1 |
6.70% |
6 |
28.60% |
5 |
26.30% |
2 |
22.20% |
| 3 |
7 |
46.70% |
5 |
23.80% |
8 |
42.10% |
2 |
22.20% |
| 4 |
2 |
13.30% |
1 |
4.80% |
0 |
0.00% |
0 |
0.00% |
| 5 |
1 |
6.70% |
0 |
0.00% |
0 |
0.00% |
1 |
11.10% |
| 48 hours |
1 |
0 |
0.00% |
4 |
19.00% |
7 |
36.80% |
2 |
22.20% |
0.102 |
| 2 |
6 |
40.00% |
7 |
33.30% |
4 |
21.10% |
2 |
22.20% |
| 3 |
4 |
26.70% |
5 |
23.80% |
8 |
42.10% |
2 |
22.20% |
| 4 |
1 |
6.70% |
0 |
0.00% |
0 |
0.00% |
0 |
0.00% |
| 5 |
0 |
0.00% |
0 |
0.00% |
0 |
0.00% |
1 |
11.10% |
Table 3: Results of χ2 dependence between GCS and of protein S100B values. Due to the low frequency of GCS in every category linear by linear association approach was utilized.
In Table 4 the results of the measurement of S100B are exposed after 3, 12, 24 and 48 hours between the 4 groups and the level of the observed importance of the variation analysis is noted (ANOVA) [9].
| Group |
Statistics |
3 hours |
12 hours |
24 hours |
48 hours |
| A |
Average |
0.147 |
0.15 |
0.149 |
0.142 |
| Typical variation |
0.047 |
0.049 |
0.045 |
0.043 |
| Minimum |
0.071 |
6.90% |
0.073 |
7.20% |
| Maximum |
0.191 |
21.40% |
0.194 |
19.20% |
| Β |
Average |
0.373 |
36.60% |
0.300 |
23.10% |
| Typical variation |
0.58 |
59.30% |
0.472 |
35.30% |
| Minimum |
0.084 |
7.60% |
0.043 |
4.10% |
| Maximum |
1.68 |
170.20% |
1.358 |
102.10% |
| C |
Average |
0.148 |
13.50% |
0.111 |
8.90% |
| Typical variation |
0.069 |
7.00% |
0.060 |
5.20% |
| Minimum |
0.051 |
4.40% |
0.028 |
1.30% |
| Maximum |
0.355 |
34.30% |
0.276 |
21.20% |
| D |
Average |
0.204 |
19.30% |
0.173 |
14.40% |
| Typical variation |
0.064 |
6.40% |
0.066 |
5.60% |
| Minimum |
0.129 |
12.20% |
0.102 |
8.80% |
| Maximum |
0.349 |
33.20% |
0.301 |
25.60% |
| p-value |
0.031 |
3.30% |
0.029 |
2.40% |
Table 4: Descriptive measurement results of S100B.
We can observe a statistically important difference between the 4 groups in the measurement of S100B after 3 hours (F(3,67)=3.140, p=0.031<0.05), after 12 hours (F(3,67)=3.080, p=0.033<0.05) after 24 hours (F(3,67)=3.207, p=0.029<0.05) and after 48 hours (F(3,67)=3.354, p=0.024<0.05).
From the checking of minimum important difference (LSD) it came out that after 3 hours the witnesses (Group A) have statistically significant lower measurement of S100B compared to the patients of Group B (p=0.014<0.05). The patients of Group B had higher measurement of S100B compared to those of Group C (p=0.004<0.05) and compared to those of Group D (p=0.048<0.05).
After 12 hours the witnesses (Group A) have statistically significant lower measurement of S100B compared to the patients of Group B (p=0.021<0.05), while the patients of Group B had higher S100B compared to the patients of Group C (p=0.004<0.05) and Group D (p=0.047<0.05) accordingly.
After 24 hours statistically came out that the witnesses (Group A) have significantly lower measurement of S100B compared to the patients of Group B (p=0.043<0.05), while the patients of Group B have higher measurement of S100B compared to the patients of Group C (p=0.004<0.05).
Finally, after 48 hours the patients of Group B had higher measurement of S100B compared to the patients of Group C (p=0.004<0.05).
Evaluating altogether the pre mentioned remarks concerning the higher rates of S100B in Group B, we consider that they must not be evaluated as statistically important due to the primary disadvantage of the inadequate number of its members (Group B) compared to the other groups. In order to document this thinking, I have mentioned the observed proportionality for the increasing tendency of the rates of S100B, moving from Group A to Group B, excluding however Group B.
The evaluation of the diagnostic value of S100B came right after using as a cut off separation point the rate 0.191 μg/L that corresponds to the highest rate of witnesses Group A (seemingly healthy people) (Table 5).
| |
S100B value |
Computed tomography |
| Pathological |
Normal |
| S100B (3 hours) |
Positivea |
12 |
2 |
| Negativeb |
0 |
5000.00% |
| S100B (12 hours) |
Positivea |
11 |
200.00% |
| Negativeb |
1 |
5000.00% |
| S100B (24 hours) |
Positivea |
10 |
0.00% |
| Negativeb |
2 |
5200.00% |
| S100B (48 hours) |
Positivea |
8 |
0.00% |
| Negativeb |
4 |
5200.00% |
| a: maximum normal value (>0.191μg/L); b: Within natural limits (<0.191 μg/L) |
Table 5: Correlation of S100B values with a cut-off point at 0.191 μg/L and of the neuroimaging findings.
In Table 6, 4 indicators of diagnostic and pre-diagnostic reliability of S100B are exposed concerning the sensitivity, the specialization, the negative diagnostic value and the positive diagnostic value.
| S100B |
Sensitivity |
Specialization |
Positive diagnostic value |
Negative diagnostic value |
| 3 hours |
100% |
96.15% |
85.71% |
100% |
| 12 hours |
91.67% |
96.15% |
84.62% |
98.04% |
| 24 hours |
83.33% |
100% |
100.00% |
96.30% |
| 48 hours |
58.33% |
100% |
100.00% |
91.23% |
Table 6: S100B diagnostic value parameters in regard to time elapsed from HI.
In Figure 1 the graph of the change within the time of the parameters of the diagnostic value of S100B is exposed.
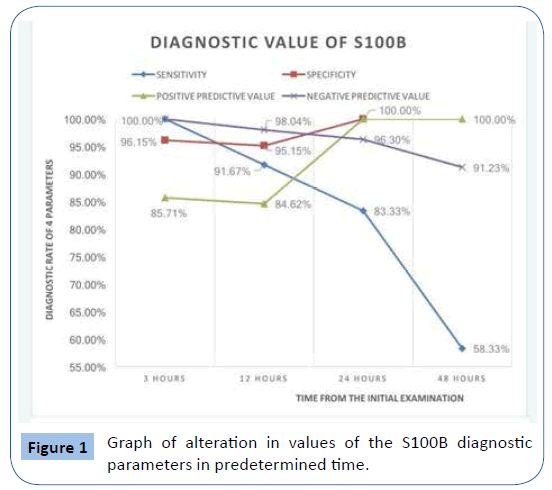
Figure 1: Graph of alteration in values of the S100B diagnostic parameters in predetermined time.
Discussion
In the current survey, 64 patients with head injury and 10 patients “witnesses” participated, who were admitted to our clinic and were classified into 4 groups, Group A, witnesses and groups B-D based on the criterion of the increasing severity of the of the clinical manifestations.
The serum levels of the protein S100B were measured in 3, 12, 24 and 48 hours after the injury. S100B has a short semi-period of life; therefore, the strictness in the compliance with the timetables of the research protocol is of great importance [10]. After the collection and the statistic processing of the data the definition of the sensitivity, of the specialty, of the positive and negative prognostic value of protein S100B followed.
As a cut off separation point of S100B the rate 0.191 μg/L was defined, which corresponds with the highest rate of S100B of the patients of Group A.
Anczykowski et al., noted non-traceable levels of S100B to normal people using immunoassays of chemiluminescence (ECLIA method) in a Cobas 6000 analyst (Roche Diagnostics) [11].
Poli de Figueiredo et al., mentioned as an average condition of the levels of protein S100B in 21 healthy volunteers the rate of 0.04 μg/L (40 mg/ml) using heterogeneous immunoassay [12]. Borg et al. defined as a normal rate of reference of protein S100B in the serum the rate 0.021 μg/L, based on the ELISA method [13].
In a prospective study, Rhine et al., studied the bio rates UCH-L1 and GFAP in 25 children of the age of 11-16 years old with head injury of moderate severity within 6 hours after the injury. For the safe comparison of these measurements they studied a second group of 20 children-witnesses who were admitted to the emergencies with clear injury of the limbs. They noted that the levels of both bio rates were significantly increased in the primary stage (p<0.01) and that the specific bio rates could not be utilized in prognostic level as far as the appearance of post-concussion syndrome is concerned [14].
Papa et al., studied the prognostic diagnostic value of UCH-L1 to 196 children and youngsters with mild or moderate severity head injury. They compared the levels of the bio rate with the findings of the CT scan. The levels of the bio rate were evaluated simultaneously with those of the group of 60 witnesses. As a cut off limit the 0.18 μg/L it was noted 100% sensitivity, 47% specialty and 100% negative prognostic value. Surely the writers consider that an additional survey is demanding in order for the prognostic value to be estimated in children with head injury of mild severity [15].
Walder et al., proceeded in the survey of the prognostic reliability of H-FABP compared with the S100B in 49 patients with head injury of high severity. The main goal of this survey was the prognostic evaluation of a future disability and the survival of the patients. Based on these parameters they concluded that the increased levels of H-FABP within 48 hours after the injury were related to the mortality (75% sensitivity and 93% specialty) [16].
Olivecrona et al., studied comparatively the intracranial pressure the capillary pressure of the brain cortex and of the NSE in 48 patients, of the age of 15-70 years old with craniocerebral injury of high severity. They concluded that the rates of NSE within 72 hours had the highest prognostic value concerning the patients’ mortality (p=0.0045). Utilizing the clinical and imaging data (Morris-Marshall Score) of NSE and the S100B the prognostic mortality estimation was improved (p=0.0008) [17]. Carr et al., studied the prognostic reliability of CK-BB in 64 soldiers with head injury of moderate severity. They positively noted as for CK-BB in 4/64 patients who were testified that suffered from intracranial complication. Furthermore 1/60 patients with negativeness as for CK-BB suffered from intracranial complication. The writers concluded that CK-BB had specialty 97% and sensitivity 11% concerning the early diagnosis of an intracranial complication in patients with HI of moderate severity. The disadvantages of the study are the small sample of the patients’ and the possibility of CK-BB’s degradation while being transferred to the laboratory. To sum up they do not consider that the specific bio rate can be usable in the clinical practice [18].
The correlation of the APP with the intracranial complication has been noted based on experimental models as it has also been noted the positiveness of the immuno-histochemical staining in order for them to distinguish an ischemic (negative) from a traumatic convention in the brain neuronal axons [19]. Based on Zetterberg et al., there is not still the possibility of APP’s utility as a biomarker in the peripheral blood [20].
The patients of groups B-D were submitted to CT scan of the brain where it was noted correspondence of the normal rates of S100B (MO=0.139 μg/L) with negative indications of intracranial complication. The results of the specific study show that protein S100B has extremely high specialty, positive and negative diagnostic value in all times, while it has very high sensitivity after 3 and 12 hours. On the contrary, the sensitivity is noticeably diminished after 24 and 48 hours. More analytically we can observe that protein S100B has 100% specialty after 24 and 48 hours and 96.15% specialty after 3 and 12 hours. This makes it really useful for the positive diagnosis of a situation (for the verification of the positive situation), since from the trials with high specialty a positive result verifies the diagnosis. In addition, S100B values at 3 and 12 hours from ΗΙ are exploitable for exclusion of intracranial complication.
In the present study the results of the use of protein S100B as a prognostic rate were encouraging. The contribution to the diagnosis and to the monitoring of the young patients either of mild or moderate severity head injury was of critical importance.
Ruan et al., concluded that the use of S100B as a tracing tool of a mild or moderate severity head injury instead of the implementation of a CT scan reduced the price and the exposition of the young patient to radiation was avoided [21].
Testified the prognostic correlation of the increased rates of S100B and of the intracranial complication in patients with HI [22-24]. They truly consider that S100B could be utilized as a prognostic biomarker in the clinical praxis.
In a clinical survey of Anderson et al., it was concluded that in 459 healthy people, and 17 multi-injured, with no testified head injury, the levels of protein S100B were <0.032 μg/L AV. 0.010 μg/L. [25]. Their observation converges with the relevant of our study since the rates of S100B were within the fluctuation of the relevant rates of group A. Townend et al., preceded in a post analysis of 18 clinical studies concerning the prognostic value of protein S100B in head injuries. They therefore concluded that the high level of S100B (>0.25 μg/L), with a cut off limit 0.142 μg/L during the initial evaluation of patients with head injury and with low level of consciousness, was more frequently related to some kind of disability. However, it doesn’t seem to exist any correlation between S100B and the neuropsychological condition of the patients. They finally concluded that patients with high levels of S100B in the initial evaluation (>2.5 μg/L) can represent a high risk group of some kind of disability after a head injury [26].
Savola et al., concluded to the same results, since that noted that the levels of protein S100B with cut off 0.13 μg/L 6 hours after the HI, were correlated proportionately to the severity of the intracranial complication in 224 patients (0.98 μg/L AV) who suffered from moderate or heavy severity head injuries. The patients with head injuries had significantly higher rate of S100B (0.17 μg/L) compared to the patients with light severity HI or absence of trauma (0.07 μg/L, p<0.001). The levels of S100B were related also to the severity of the brain damage (p<0.001) with the highest rates to be apparent in patients with moderate or severe brain damage (1.27 μg/L). Finally, in cases of high severity HI, the levels of S100B were proportionately increased (0.35 μg/L) [27]. This conclusion converges to the results of the present study.
In a meta-analysis of Unden and Rommer in 12 clinical studies that included 2466 adult patients with head injuries of moderate severity, after they correlated the levels of protein S100B with the corresponding imaging findings of the brain’s CT scan, they noted its high prognostic value with high sensitivity (75%-100%) and lower specialty (28%-77%). In the total of patients only 6 showed low rate of protein S100B with pathological CT scan. The cut-off of all operations was 0.10 μg/L [28]. Their results converge with the relevant of our study.
In a prospective multi-central study Biberthaler et al., searched for the concentrations of protein S100B (cut off 0.10 μg/L) in 1309 patients with head injuries of moderate severity, aiming to the specification of an additional brain CT scan and S100B was measured during the admission, a procedure which we followed in the protocol of the present study. In 93/1309 (7.10%) it was noted intracranial complication based on the findings of the CT scan. They concluded that the sensitivity of S100B was 99% (96%-100%) and the specialty was 30% (29%-31%) [29]. Results of this study do not converge with the present study as far as the specialty of S100B is concerned. We believe that the separation of the patients of the present study into 3 different groups (B-D), opposed to the total evaluation of the 1309 patients of the multicentral study was critical for this differentiation.
In a multi-central study of Manzano et al., that included 73 pediatric patients with HI of moderate severity, the CT scan findings of the brain were correlated with the rate of S100B (cut off 0.14 μg/L) in the first 6 hours after the injury. In essence this methodology corresponds to the first measurements based on the protocol of the present study. Based on the results in patients older than 2 years old it was noted sensitivity 100% (95% CI, 81%-100%) and specialty 37% (95% CI, 30%-37%). The differentiation as for the results of the present study concerning the low specialty, we believe that lies on the one hand on the meticulous categorization we performed and on the other hand on the higher cut off in our incidents (0.191 μg/L compared to 0.14 μg/L) [30].
Based on the pre mentioned notices concerning the prognostic value of protein S100B, Calcagnile et al., used the specific biomarker as a basic criterion for the organization of treatment strategy in young patient who is submitted to the ED with a HI of moderate severity. Young patients with HI of moderate severity and early rate of protein S100B<0.10 μg/L are neither submitted to CT scan of the brain nor hospitalized preventively for observation [31]. Therefore, it is apparent the tendency of ownership of the high prognostic value of S100B in patients with HI of moderate severity in the clinical praxis.
The specific treatment strategy finds us in absolute accordance not only as for the part of the diagnostic accuracy of S100B is concerned but also as for the necessity of its exploitation in the clinical praxis.
We believe that our study exhibits the following limitations:
• The insufficient number of the members belonging to group B compared to the other groups. Therefore, we didn't evaluate as statistically significant the higher levels of S100B of group B opposed to those of groups C and D.
• It does not include patients who were treated in an outpatient basis or were managed in an Intensive Care Unit.
• It does not include patients with comorbidity.
• No comparison was made of S100B with another biomarker.
The withdrawal of the pre mentioned limitations in a forthcoming study will constitute an improved version of our manuscript.
Conclusions
• The early searching of protein S100B during the initial evaluation of a child with a HI can constitute a significant diagnostic test for the possibility of the coexistence or not of an intracranial complication; based on the results of the present study it was concluded sensitivity 100%, specialty 96.15%, positive prognostic value 85.71% and negative prognostic value 100%.
• We believe that the measurement of protein S100B must constitute a tracing testing that will be performed systematically to children who are submitted to the ED because of HI. The rate of protein S100B is of critical importance for the origination of treatment strategy in child with a HI.
• The normal rate of protein S100B during the early evaluation of a child with HI, with no indication of a co-existing intracranial complication, could constitute a catalyst for the limitation of imaging testing undertaking and reduction of hospital admissions.
• In the context of the comparative evaluation of protein S100B rates at the specified time, appears to be increased percentage of the specialty and of the positive prognostic value, while the sensitivity and the negative prognostic value tend to be diminished.
• The measurement of protein S100B could be repeated before discharge from hospital, because it could essentially constitute as a documentation of the improving clinical condition.
24447
References
- Sarioglu FC, Sahin H, Pekcevik Y, Sarioglu O, Oztekin O (2018) Pediatric head trauma: An extensive review on imaging requisites and unique imaging findings. Eur J Trauma Emerg Surg 44: 351-368.
- Guilliams K, Wainwright MS (2016) Pathophysiology and management of moderate and severe traumatic brain injury in children. J Child Neurol 31: 35-45.
- Agoston DV, Shutes-David A, Peskind ER (2017) Biofluid biomarkers of traumatic brain injury. Brain Inj 31: 1195-1203.
- Yokobori S, Hosein K, Burks S, Sharma I, Gajavelli S, et al. (2013) Biomarkers for the clinical differential diagnosis in traumatic brain injury-a systematic review. CNS Neurosci Ther 19: 556-565.
- Goodman LA (1981) Association models and canonical correlation in the analysis of cross-classifications having ordered categories. J Am Stat Assoc 76: 320-334.
- Greenacre MJ (2007) Correspondence analysis in practice. 2nd Edition, Chapman and Hall/CRC, New York, USA.
- Corder GW, Foreman DI (2014) Non-parametric statistics: A step-by-step approach. 2nd Edition, Wiley, New York, USA.
- Fisher RA (1922) On the interpretation of chi square from contingency tables, and the calculation of P. J Royal Stat Socie 85: 87-94.
- Maxwell SE, Delaney HD (2004) Designing experiments and analyzing data: A model comparison perspective. 2nd Edition, Mahwah, New Jersey, USA.
- Jackson RG, Sales KM, Samra GS, Strunin L (2000) Extra cranial sources of S100B. Br J Anaesth 86: 601.
- Anczykowski G, Kaczmarek J, Jankowski R, Guzniczak P (2012) The reference level of serum S100B protein for poor prognosis in patients with intracranial extracerebral hematoma. EJIFCC 5:66-78.
- Poli de Figueiredo LF, Biberthaer P, Simao FC, Hause C, Mutschler W, et al. (2006) Measurement of S100B for risk classification of victims sustaining minor head injuries-First pilot study in Brazil. Clinics 61: 41-46.
- Borg K, Bonomo J, Jauch EC, Kupchak P, Stanton EB, et al. (2012) Serum levels of biochemical markers of traumatic brain injury. ISRN Emergence Medicine 1: 11.
- Rhine T, Babcock L, Zhang N, Leach J, Wade SL (2016) Are UCH-L1 and GFAP promising biomarkers for children with mild traumatic brain injury? Brain Inj 30: 1231-1238.
- Papa L, Mittal MK, Ramirez J, Silvestri S, Giordano P, et al. (2017) Neuronal biomarker ubiquitin C-Terminal hydrolase detects traumatic intracranial lesions on computed tomography in children and youth with mild traumatic brain injury. J Neurotrauma 4: 2132-2140.
- Walder B, Robin X, Rebetez MM, Copin JC, Gasche Y, et al. (2013) The prognostic significance of the serum biomarker heart-fatty acidic binding protein in comparison with s100b in severe traumatic brain injury. J Neurotrauma 30: 1631-1637.
- Olivecrona Z, Bobinski L, Koskinen LO (2015) Association of ICP, CPP, CT findings and S-100B and NSE in severe traumatic head injury. Prognostic value of the biomarkers. Brain Inj 29: 446-454.
- Carr ME Jr, Masullo LN, Brown JK, Lewis PC (2009) Creatine kinase BB isoenzyme blood levels in trauma patients with suspected mild traumatic brain injury. Mil Med 174: 622-625.
- Hayashi T, Ago K, Nakamae T, Higo E, Ogata M (2015) Two different immunostaining patterns of beta-Amyloid Precursor Protein (APP) may distinguish traumatic from nontraumatic axonal injury. Int J Legal Med 29: 1085-1090.
- Zetterberg H, Blennow K, Hanse E (2010) Amyloid beta and APP as biomarkers for Alzheimer's disease. Exp Gerontol 45: 23-29.
- Ruan S, Noyes K, Bazarian JJ (2009) The economic impact of S100B as a pre-head screening test on emergency department management of adult patients with mild traumatic brain injury. J Neurotrau 26: 1655-1664.
- Bechtel K, Frasure S, Marshall C, Dziura J, Simpson C (2009) Relationship of serum S100B levels and intracranial injury in children with closed head trauma. Pediatr 124: e 697-704.
- Hallén M, Karlsson M, Carlhed R, Hallgren T, Bergenheim M (2010) S-100B in serum and urine after traumatic head injury in children. J Trauma 69: 284-289.
- Nylén K, Ost M, Csajbok LZ, Nilsson I, Hall C, et al. (2008) Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir 50: 221-227.
- Anderson RE, Hansson LO, Nilsson O, Diglai-Merzoug R, Settergren G (2001) High S100B levels for trauma patients without head injuries. Neurosurgery 48: 1255-1258.
- Townend W, Ingebrigtsen T (2006) Head injury outcome prediction: A role for protein S-100B? Injury 37: 1098- 1108.
- Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemeia O, et al. (2004) Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J Trauma 6: 1229-1234.
- Unden J, Rommer B (2010) Can low serum levels of S100B predict normal CT findings after minor head injury in adults? An evidence-based review and meta-analysis. J Head Trauma Rehabil 25: 228-240.
- Biberthaler P, Pfeifer U, Kroetz M, Mussack T, Kanz KG (2006) Serum S100B concentration provides additional information for the indication of computed tomography in patients after minor head injury: A prospective multicentre study. Shock 25: 446-453.
- Manzano S, Holzinger IB, Kellenberger CJ, Lacroix L, Klima-Lange D, et al. (2016) Diagnostic performance of S100B protein serum measurement in detecting intracranial injury in children with mild head trauma. Emerg Med J 33: 42-46.
- Calcagnile O, Unden L, Unden J (2012) Clinical validation of S100B use in management of mild head injury. BioMed Central Emerg Med 12: 13.







