Keywords
Assistive technology; Procurement; Health; Disability; Policy
Introduction
Humans and technology
All people use technologies, including assistive devices (AT devices) in order to interact with the world. Imagine climbing to the third floor of a building without steps or a lift, or preparing food without implements. When AT devices fit the person, the environment and the task at hand, a range of outcomes are possible: these usually fall into the categories shown in Table 1.
| Outcome areas |
Examples |
| Maintaining or improving functioning and independence |
A wheeled walker enables ambulatory mobility whilst carrying shopping |
| Facilitating participation |
Accessing a dog-walking oval with a power scooter |
| Enhancing well-being |
Communicating at a family meal with an electronic communication device |
| Protecting, supporting, training, measuring or substituting for body functions, structures and activities |
Managing continence with a range of continence products |
| Preventing avoidable impairments, activity limitations and participation restrictions |
Maximising skin integrity with pressure cushion and mattress |
Table 1: Outcomes of AT.
In the case of people living with disability and/or the effects of ageing, the role of assistive devices becomes even more critical as a thin margin of health and multiple effects of disability mean the ‘capability gap’ between aspirations and achievement is greater. The role of AT as recognised within the National Disability Standards refers to three specific areas relating to the appropriate selection of AT devices. These are: people’s right to be treated fairly, community participation and inclusion, and support to reach desired outcomes.
The AT device market
There are many products and technologies globally available to consumers of AT devices. It may be impossible to select from an entire world of choices, leading to ‘bounded reality’, an economic term used to describe the pragmatic constraints people use to manage the issue of choice [1]. For example, one may choose to select products which are locally available, locally manufactured, or can be purchased via the internet, second-hand, or within a certain price bracket. Other than minimum quality safeguards such as Australian Consumer Law, some regulation from entities such as the Therapeutic Goods Administration and voluntary Australian Standards, free choice means consumers are effectively free to make poor choices. An exploration of what a ‘good’ or a ‘poor’ choice might mean for AT consumers will be explored below, but first, we will consider the situation where consumers cannot afford the AT they need.
In recognition of the high cost burden associated with purchasing assistive devices, governments may step in on the basis that AT, like healthcare, is a ‘merit’ good [2]. Arguments for the role of government in AT supply include the notion of a ‘social contract’, where society has a role to play in the welfare of its members, as well as economic calculations indicating that society will benefit or save money in the long run by subsidising individuals who cannot afford necessary assistive devices, yet need them to decrease care costs, increase independence, avoid downstream costs such as secondary health problems, and of course to flourish and participate in life.
Tensions between full choice and managed provision
Governments may provide resourcing to obtain AT devices. The method of resource allocation then arises. Traditionally, systems have been constructed to manage both the allocation (who is eligible for resourcing) and also the delivery of the AT device. AT service delivery schemes in Australia typically then maintain an intake and an allocation system, and may also store, maintain, refurbish and reallocate devices. Most AT funding schemes were established in the 1970’s and, to varying extents, have ‘legacy’ service features; that is, historical methods of monitoring and control over the range of AT devices funded, and the mechanisms by which resources are expended and AT devices are provided to consumers. Multiple reviews of these funding schemes over recent years, particularly in light of contemporary disability theory [3] human rights developments [4] and high level policy direction [5], open the way for funding schemes to reorient their systems to enable increased choice and control.
What is best practice in AT device acquisition?
A substantial evidence base documents the effectiveness of appropriate AT solutions [6,7] and the significant adverse outcomes and lost participation opportunities if AT devices are not present, under provided, or are inappropriately selected and used [8,9]. There is international agreement [10] regarding seven key steps required to ensure AT devices are well identified and fit for the individuals purpose and environment. Table 2 lists steps as outlined in the international literature, and in column 2, outlines an Australian version of these steps:
| European Statement on AT Service Delivery Steps https://www.atis4all.eu/news/detail.aspx?id=406&tipo=1 |
Australia’s AT best practice steps (ARATA papers) https://www.arata.org.au/download/NDIS/fullbkgndpapers_v10int.pdf page 4-6 |
| Initiative (the first contact with the service delivery system) |
Entry into equipment funding schemes |
| Assessment (evaluation of needs) |
Needs assessment / initial AT assessment and prescription |
| Selection of the assistive solution (defining the individual AT programme) |
Trial (including progressive assessment, fitting, training, review / evaluation) |
| Selection of the equipment (choosing the specific equipment within the AT programme) |
| Authorisation (obtaining funding) |
Approval/ Funding |
| Implementation (delivering the equipment to the user, fitting and training) |
Provision (incl fitting, customising, set-up and training) |
| Management and Follow up (maintenance and periodic verification |
Review (of AT and of human) |
Table 2: AT Best practice steps.
While it can be said that more complex (that is, technically advanced and highly adjustable) AT devices carry inherently higher risks, complexity also arises through other factors. The interaction between the person, their environment, the task or outcome area they desire also brings about complexity, regardless of the AT device. This means the above best practice steps are applicable across a wide range of AT devices, from low cost or technologically straightforward, to customised or custom made AT devices. So even provision of the lowest risk AT device can be complex due to the health status of individuals, as well as the interactions between the person, their environment and other AT devices.
AT service delivery in Australia
Australian AT funding schemes currently engage in a range of these best practice steps. Recent critiques however have identified a number of limitations across various schemes:
1. Recent examination of the AT market in Australia suggests that current information asymmetries impact on consumer choice [11].
2. The significant shortfalls in resourcing to meet demand which cause sub-optimal service delivery in terms of range, cost, availability and multiplicity of AT devices [12,13].
3. Provision and review stages are often subject to individual supplier arrangements and are not consistently applied or available [14].
In summary, whatever method of resource allocation is used, the extent of resourcing is often an issue. An ongoing tension played out across many aspects of health and disability policy is the adequacy of resourcing for supports such as products and technologies [15]. One action is to manage demand and AT device costs, but the question then arises: how can this be done in a contemporary disability theory context emphasizing choice and control? Evidently, the ability to spread limited resourcing further is a key factor in being able to deliver good practice in AT service delivery. This brings up the issue of cost management.
Cost containment strategies
A body of literature across policy and economics has identified the challenges of maximising ‘social benefit from the resources available subject to reasonable concerns with justice'(page 82). Approaches include alternative financing [16], the use of wait time strategies [17], and public procurement strategies [18]. While some jurisdictions fare better than others, overall, provision systems are flawed [19,20].
In Australia currently, government programs are encouraged to engage in market based strategies to manage costs [21]. Current economic approaches suggest governments could consolidate buying power rather than operating a large number of programs, and that government procurement programs can purchase equipment at a discount to retail prices with efficiencies from bulk buying [14]. The National Disability Agreement also suggests that States should consider collaborative multi-jurisdictional procurement strategies, thereby reducing red tape requirements in tendering. It is important to note that procurement strategies do not necessarily rest on cost, but rather on “value for money”, considering cost in the context of quality and other value adds such as Standards compliance and supplier service.
Method
This paper uses policy case study methodology to explore what choices and outcomes can be realised within a procurement process. We examine the impact of ‘limiting’ supply through the application of quality measures, compared with current systems which enable choice of brand across approved categories of AT device, recommended by the AT practitioner, usually a therapist, who is prescribing the device. We therefore closely examine one aspect of the procurement process: that is, the clinical and technical AT device audits.
Data is obtained from the procurement process itself (the authors are members of the evaluation team from each state funder (WH, JB, AK), and an occupational therapist and advocacy group member (NL). Data also includes narrative responses from AT suppliers and commentary from other AT stakeholders.
Cases examples: DES and SWEP
This paper considers the actions of the state AT funders in Victoria, Australia (Vic), and in South Australia (SA). These AT service providers, alongside other jurisdictional AT funding programs in Australia, face steadily increasing population and demand projections (REF ABS) and increasing pressure on a capped budget. Together, the Vic funders through the State-wide Equipment Program (SWEP) and SA funders through the Department for Communities and Social Inclusion (DCSI) Equipment Program via Domiciliary Equipment Service (DES) embarked on a strategic procurement plan for low cost /high volume non-customised AT, intending to manage costs whilst continuing to meet their programs intent of:
• ‘providing Victorian people who either have a permanent or long-term disability or are frail aged with subsidised aids, equipment and home and vehicle modifications to enhance their independence and facilitate community participation’.
• ‘the provision of equipment, home modification services, … to eligible people who have permanent disabilities or impaired functional capacity, to live safely and independently in the community in South Australia.’
As adjoining States, Vic and SA share similar program intent, AT device types and client base, and had both undergone recent program reforms. A shared cross-jurisdictional commitment to best practice and recognition of the opportunity to leverage complementary expertise, as well as awareness of the value of bulk purchasing led to the decision to collaborate. Based on these principles, in 2013-2014 SWEP and DES committed to jointly tender for low cost, high volume non-customised AT.
Non-customised equipment makes up approximately 33% (≈$4.5 M) of the annual aids and equipment expenditure across both jurisdictions, consisting of 93 different item types. These items were selected based on analysis of program spend over the past 3 years on high volume, low cost, non-customised equipment.
Key AT device categories successfully contracted within this tender include bathing and toileting equipment, beds and mattresses, chairs, hoists, portable ramps, mobility aids, pressure cushions, basic manual wheelchairs and scooters.
The procurement process is outlined in Appendix 1, and the expectations of the process in Table 3 below:
| Clinical benefits |
Service quality benefits |
Fiscal / sustainability benefits |
• AT device meets specifications and is fit for purpose
• Suitable for range of clients / situations
• AT device pre-evaluated for quality and durability
• Ease of adjustment and use
• Compatibility and interaction with other items
• Increased AT device ‘re-issuability’
• Training and user instructions for clients
• Reduced clinical assessment and selection time |
• Reduce waiting times
• Supplier capability
• Warranty and spare parts • availability
• Compliance with relevant standards
• Regulatory mandatory • criteria met
• AT device quality and • ability to repair
• Delivery arrangements including delivery time-frames, installation and education
• Access for trial |
• Price
• Commitment of orders and • volumes
• Ability to refurbish and reissue
• Increased AT device longevity
• Ease of storage, cleaning and • maintenance
• Expanded client group within • a fixed budget
• Reduced wait times for clients • for access to funding and • delivery of AT |
Table 3: Expectations from the low cost, high volume non-customized AT procurement process.
Device audits
AT devices tendered by shortlisted suppliers underwent a two stage clinical and technical evaluation process, with an initial assessment against a mandatory quality standard criterion. All devices to which a quality standard applies were required to be compliant with the relevant standards. Depending on the device this ranged from Australian Standards, international standards, foreign national standards or standard set by a body of manufacturers. Only those devices that met this criterion progressed to the desk top and then hands on evaluation process.
The evaluation team for this audit in addition to Vic and SA procurement representatives consisted of clinical representatives from both Vic and SA including DES staff, SWEP clinical advisors and expert prescribers, DCSI Equipment Program prescribers, technical representatives including a rehabilitation engineer and warehouse representatives.
The AT devices initially underwent a desktop evaluation by the team to ensure that they met the minimum specifications described and therefore were suitable and fit for purpose. This included features meeting required specifications, and durability by virtue of construction and materials. Those AT devices which had passed the desk top evaluation underwent further hands on quality audit by the team.
The clinical team assessed each AT device using predetermined criteria for scoring to determine how well the product met the described specifications. These included criteria such as ensuring the products were easy to adjust and use by both clients and carers, suitable to use with a range of clients or situations, were safe to use, and had the ability to be refurbished and reissued. For example, mobile shower chairs were assessed for safe transfers and use by carers, ensuring appropriate use in intended environments such as clearance over toilet and use in shower, seats not providing any pressure points, features such as armrests and legrests easy to use and adjust, other options or accessories available.
The technical team assessed the durability of the products including the materials, and the ability to maintain and repair. This assessment was imperative to ensure that products chosen were high quality for client safety and longevity and could be easily repaired in the client home ensuring minimal time out of service.
Warehouse staff also ensured that products could be easily stored, cleaned and safely transported and installed within the required environments.
Results
In terms of numbers, Figure 1 outlines the numbers of tenders submitted (39), items tendered (93), suppliers shortlisted (18 for desktop audit, then 15 for hands on evaluation and value for money evaluation), items evaluated via desktop audit (824) and subsequent hands on evaluation (548), and finally items evaluated on value for money criteria (473). This resulted in 10 contracted suppliers for 86 products in total.
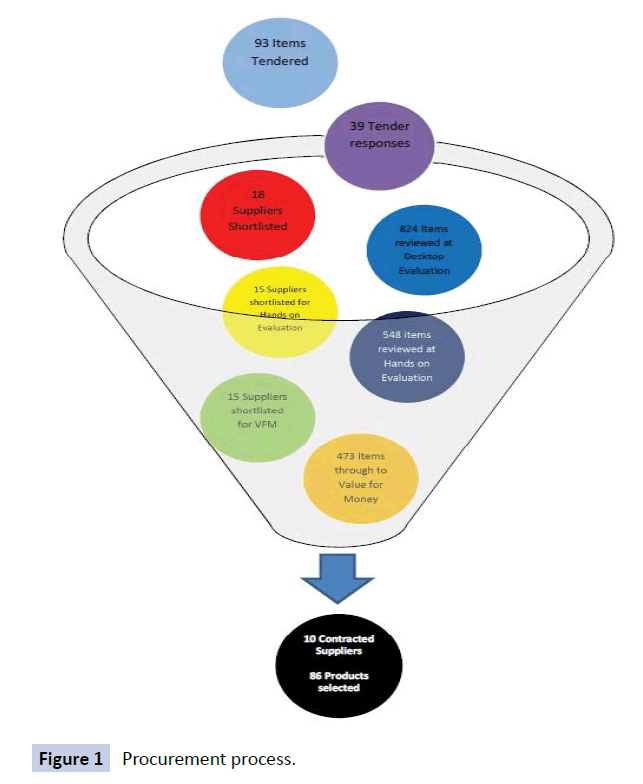
Figure 1: Procurement process.
From the perspective of SWEP and DES, a wide range of successful outcomes occurred. Table 4 outlines the expectations of the tender process and the range of projected and actual outcomes from the point of view of the AT funding bodies involved.
| Expectations of tender process |
Outcomes against expectation areas |
| Fiscal / sustainability outcomes |
• Price
• Anticipated increase of up to 30% in AT items provided within a fixed budget
• Commitment of orders and volumes
• Ability to refurbish and reissue
• Increased AT device longevity
• Ease of storage, cleaning and maintenance |
• Doubled projected savings applied directly to subsidy, ensuring more AT able to be provided for the same total subsidy commitment
• Single supplier contracts for over 80 items resulting in prescribers no longer needing to seek quotes for these items and suppliers preparing quotes for items they have no guarantee of being awarded order for.
• Commitment to training for contracted repair agent
• AT device quality assured
• Agreed warranty and access to spare parts
• Contractual requirements ensuring locked in pricing and protection against currency fluctuations
|
| Service quality outcomes |
• Reduced wait times
• Warranty and spare parts availability
• Delivery arrangements including delivery timeframes, assembly, installation and education
• Compliance with relevant standards
• Access for trial
|
• Supplier KPI’s
• Contractual arrangements
• Agreed delivery and set up
• Guaranteed availability of products
• Certified compliance of products against relevant Standards
• Guaranteed quality of products
• Commitment from suppliers/funders |
| Clinical outcomes: clients |
• Meets client needs
• Training and user instructions
• AT device meets specifications
• Suitable and fit for purpose
• Reliability of equipment assured
• Compatibility and interaction with other items
• Ease of adjustment and use
• AT device quality and durability
|
• AT device performance assured through guaranteed quality of products
• Capacity of device to be fitted to meet unique needs of individual clients
• Fewer delays for supply and repairs
• No longer required to contribute ‘gap funding’ for cost of AT device, or amount required significantly reduced
• Reduce waiting times for both access to funding and delivery of AT
|
| Outcomes for Clinicians |
|
• Capacity to assess AT device quality
• Reduced administrative burden
• Reduced reliance on supplier marketing
|
• No need to evaluate item quality (Guaranteed quality of products)
• Saving time for clinicians
• No quotes
• Statewide contractual obligations/KPIs
• Catalogue developed with all contracted suppliers and their subcontractors noted
• Guaranteed access to contract items for trial |
| Outcomes for Funders |
|
| |
• Value for money for funders
• Significant discounting for volume purchases
• Improved quality of AT device lowers risks and reduces repair costs
• Simplified ordering systems
• Single invoice management
• Reassurance that spare parts will be on hand
• KPI management, relationship management and administrative support
Known re-issue stock |
| Outcomes for AT suppliers |
|
| |
There are 2 types of suppliers affected:
• the successful contractors who receive predictable volumes, can take advantage of their own bulk purchasing, increase their market share and potentially access an even greater pool if there is spill-over to other states;
• the unsuccessful suppliers who lose market share because they were either unsuccessful or didn’t participate |
Table 4: Outcomes of the low cost, high volume non-customized AT procurement process.
Discussion
Perspectives of AT supplier
From the perspective of AT suppliers, the results of the procurement process have influenced the AT marketplace in Victoria and South Australia. Considering these changes from the perspective of the AT best practice steps (Table 2) is proposed as the most objective way to separate actual practice issues from more general responses to change. AT supplier concerns focus on the best practice steps of trial, provision and implementation:
‘Towards the end of 2014 several major AT tenders were announced in Victoria, South Australia and Queensland. Although a great deal of thought and discussion goes into the preparation of the tenders, it is only after the projects have been operating for some months that we get to see the real-world impacts on AT outcomes for consumers... Early indications are that there are some real challenges for trials of AT packages due to the fact that many suppliers are only on contract for a specific basket of items and not their full range. If you are contracted to supply only the wheelchair, there is no incentive to trial other essential items (cushions, backrests, etc.) that are needed as part of the whole AT solution. We are also being told that allied health practitioners in some rural towns are finding it impossible to obtain trial items as the local supplier is not on contract. Such issues demonstrate that providing AT is not a simple process and the pursuit of savings can add costs and frustration elsewhere’ [22].
In response and in order to mitigate issues with access to AT for trial, both SWEP and DES have implemented imprest stores of the contracted items across the states to ensure prescribers have ready access to the items.
From the perspective of AT consumers
It is beyond the scope of this article to evaluate overall consumer satisfaction, although this is a key outcome area worthy of future investigation and part of ongoing quality reviews. The consumer involved on the panel reports, “The process was really good and a lot of work was done by the staff to get a great outcome. I really enjoyed being part of the evaluation team and helping contribute to make decisions’.
From the perspective of AT prescribers
Significant anecdotal feedback from occupational therapists, physiotherapists and other prescribers notes a range of impacts of the new order. The presence of a finite list of devices in some ways simplifies life for prescribers. The workshop audit assures quality on a range of practical aspects often beyond the time capacity of therapists to ascertain for themselves. Beyond this assurance of minimum quality standards, therapists must evaluate specific features against the individual needs of clients and it is with this aspect of the procurement process that many prescribers report challenges. Further processes are required to request and clinically justify devices which are not ‘on the procurement list’. While this is certainly an option, it remains to be seen whether prescribing practice is in any way skewed with strong incentives to recommend the easier option: that which is on the list. Otherwise, key critiques include the unintended effects of splitting related items, for example the supplier of hoists is separate to the supplier of slings or the supplier of a wheelchair may be different to the supplier of a pressure cushion . Finally, prescribers note the difficulties in arranging trials of equipment where previously ‘local’ suppliers are not contracted to provide items, and where other planned trial options such as using the reissue pool, are procedurally difficult. From a best practice perspective these issues affect the ability to trial and to implement full solutions.
Choice within procurement systems
A range of quality outcome gains are found to justify a procurement process which limits full market choice. The procurement process presented in this article developed appropriate specifications, and measured and benchmarked supplier capability and assessment of AT devices against quality based parameters such as durability, standards compliance, and value for money. Prior to the audit process, this role has fallen to the assessing health professional. It is unlikely that the majority of health professionals have either the time or resources to conduct such a rigorous product evaluation, and through this process assurance can be given that a robust pool of AT devices is now on offer.
It is recognised that the ‘funnel’ impact of the procurement process creates a shorter list of options than otherwise exists. That is, the procurement process (with extensive organisational compliance aspects) could eliminate AT devices which might otherwise be fit for purpose, yet were not evaluated. In order to manage this narrowing of choice, future processes should pursue other methodologies, for example allowing selection of equipment which meets the quality parameters to be purchased through organisations which have demonstrated their capacity to meet indicative KPIs.
Conclusion
The primary purpose of AT funders such as DCSI and SWEP is to provide efficient and effective AT device service delivery for eligible individuals. In the pursuit of value for money, governments may utilize competitive tendering processes. This paper reports on outcomes of a tender intended to ensure the provision of high quality equipment delivered in a timely way, fitted with the appropriate level of expertise, and representing the best value for money.
In terms of outcomes from the perspective of state funders, this procurement strategy has ensured that more equipment can be provided to more clients within the same budget. The robust evaluation process has ensured that the AT devices selected have been certified under relevant standards, meet the specifications required by 85% of the client group and have been secured at the best possible price. SWEP and DES staffs, as well as undertaking extensive roadshows to inform the sector regarding the procurement process and outcomes, remain engaged with the sector to ascertain the systemic impacts and to respond where possible in a way which positively facilitates appropriate uptake.
Outcomes from the perspective of AT prescribers, AT consumers and AT suppliers are less fully explored but indicative data suggests a range of impacts and outcomes under the new procurement regime. Occupational therapists need to draw on their professional reasoning to determine whether choice, restricted to a subset of quality items, affords acceptable individual solutions is a reasonable pragmatic constraint.
In situations of fiscal constraint significant cost savings leading to increased service are an unarguable benefit of procurement. Aspects of choice support for innovation, sustainability of the supply chain given changed purchasing arrangements and other sector influences are however systemic issues impacted on by procurement arrangements. These and other impacts must be formally reviewed and considered in future procurement processes.
8935
References
- Mooney G, Wheatsheaf H (1992) Economics, medicine and health care (2nd edn.), Hertfordshire, UK.
- Mooney G, Scotton R (1998) Economics and Australian health policy. St Leonards, Australia.
- Kristiansen K, Vehmas S, Shakespeare T (2009) Arguing About Disability - Philosophical Perspectives. Oxon: Routledge.
- Megret F (2008) The Disabilities Convention: Towards an Holistic Concept of Rights. The Int J of Hum Rights 12: 2.
- Federici S, Scherer M, Borsci S (2014) Technology and Disability 26:27-38.
- Cook A, Polgar J (2008) Cook & Hussey's Assistive Technologies: Principles and Practice(3rd edn.) St. Louis: Mosby.
- Roelands M, VanOost P, Stevens V, Depoorter A, Buysse A (2004) Clinical practice guidelines to improve shared decision-making about assistive device use in home care: a pilot intervention study. Patient Education and Counseling 55:252-264.
- Wessels R, Djicks B, Soede M, Gelderblom GJ, Witte LD (2003) Non-use of provided assistive technology devices, a literature overview. Tech and disab15.
- AAATE (2012) Service Delivery Systems for Assistive Technology in Europe: Position Paper. EASTIN.
- Queensland Competition Authority (2014) Price Disparities for Disability Aids and Equipment.
- Layton N, Wilson E, Colgan S, Moodie M, Carter R (2010) The Equipping Inclusion Studies: Assistive Technology Use and Outcomes in Victoria. Deakin University.
- K.P.M.G. (2006) Final Report of the Review of the Aids and Equipment Program. Human Services.
- Associates JP (2013) Research for the National Disability Agreement: Aids and Equipment Reform, Final Report. Canberra: Disability Policy and Research Working Group.
- Layton N, Wilson E (2010) Doing disability policy better: learning from research and policy change activities for The Equipping Inclusion Studies. Deakin University.
- Wallace JF, Knorr KH (1996) Loan financing for assistive technology: strategies for development, current programs and recommendations for the future. Tech and Disab5:255-265.
- Kreindler S (2010) Policy strategies to reduce waits for elective care: a synthesis of international evidence. Br Med Bull 95:7-32.
- Astbrink G, Tibben W (2013) The role of public procurement in improving accessibility to ICT. Telecomm J Aus 63.
- Wallace J (2011) Assistive technology funding in the United States. Neurorehabilitation28: 295-302.
- Summers M (2011) Ripe for reform: aids and equipment policy. Health Iss pp: 32-34.
- Commonwealth of Australia (2011) National Disability Strategy 2010-2020.