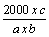Keywords
|
| Nyctanthes arbor-tristis; Authentication; Night jasmine; Quality control |
Introduction
|
| Nyctanthes arbor-tristis Linn belonging to the family Oleaceae, is a mythological plant having high medicinal value in Ayurveda. It is commonly known as harsinghar in Hindi, parijat in Sanskrit and night jasmine in English. It is distributed widely in sub-Himalayan regions and southwards to Godavari and is predominantly native to southern Asia. The geographical distribution of the plant extends from northern Pakistan and southern Nepal through northern India and south east to Thailand and also in other parts of the world [1]. The plants leaves are having immense medicinal value and are thus commercially exploitable. It is popular garden shrub that grows to a height of 3.0- 4.5 m and holds an important role in conditions related to fever and inflammation [2]. Its leaves are rough with hairy surface, opposite, ovate in shape and serrate margin. The decoction of leaves is widely used in Ayurvedic medicine for the treatment of sciatica [3], arthritis [4] and malaria [5] besides this it is also used as tonic and laxative [6,7]. This plant holds a promising role in expelling worms [8], wound healing [9], Immunomodulation [10]. It is also used as anti-helminthic [11,12], anti-amoebic [13], anti-trypanosomal [14] and larvicidal [15]. The phytochemical analysis of the leaves revealed the presence of flavanol glycosides (astragaline and Nicotiflorin) [16], Triterpenoid (Nyctanthic acid and oleanolic acid) [17], iridoid glycosides (arborside A,B,C) [18] and Iridoid glucoside (arborside D) [19]. The different parts of this plant is having different medicinal values but the present research focusses on establishing the quality standards of the leaves of N. arbor-tristis as per the WHO guidelines. |
Material and Methods
|
|
Collection and authentication of crude drug
|
| Young leaves of N. arbor-tristis Linn were collected in the month of august from Jamia Hamdard campus, New Delhi and were authenticated by Dr. Sunita Agarwal, Scientist F, Head of Raw Material Herbarium and Museum (RHMD), National Institute of Science Communication and Information Resources (NISCAIR), Pusa campus, New Delhi, India (Ref. No. NISCAIR/RHMD/consult/2014/2506/85) and was deposited further for future reference. The leaves were washed with water; shade dried followed by drying in oven at 35°C. The drug was then powdered and kept in air tight container at room temperature away from moisture for different standardization procedures. All the chemicals and reagents used were of analytical grade. |
|
Morphological studies
|
| N. arbor-tristis leaves were examined to study all its morphological characteristics. Complete examination of untreated sample of leaves of N. arbor-tristis was carried out under diffused sunlight and artificial source similar to day light [20]. |
|
Powder microscopy
|
| The microscopic examination of powdered leaf material was performed to detect and to establish various peculiar microscopic characters in order to differentiate between the adulterated and the substituted powdered or intact leaves supply. Slides of powdered leaf material was prepared using formalin, glycerine and water (8:1:1 v/v/v) and were thus embedded and seen under motic microscope of B1 series on different magnifications at 10x, 40x, 100x after staining with phloroglucinol and HCl [20]. |
|
Physicochemical screening
|
| The various physicochemical parameters like loss on drying, ash values, extractive values [21], were determined for cold, hot and successive extractions in which 4.0 g of coarse powdered and sieved drug was taken and dissolved in different solvents (Petroleum ether, ethyl acetate, chloroform, acetone and methanol) in increasing polarity. Total acid insoluble and water soluble ash values were determined as per the standard guidelines prescribed by the WHO [20]. |
|
Loss on drying
|
| The powdered drug sample (10 g) was taken without preliminary drying and was placed on a tarred evaporating dish and dried at 105°C for six hours and weighed. The drying was continued until two successive reading matches each other or the difference between two successive weighing after drying for 30 minutes in a desiccator, showed not more than 0.01 g difference [22]. |
|
Fluorescence analysis
|
| Many drugs shows unique phenomena of fluorescence when the cut surface or the powder is exposed to UV light and helps in their identification. The powder of leaf was prepared after passing it through mesh 40 (Unique- standard test sieve) and its fluorescence character was studied both in daylight and in UV light (254 and 366 nm)using different solvents like sodium hydroxide, picric acid, acetic acid, hydrochloric acid, nitric acid, iodine and ferric chloride etc [23]. |
|
Phytochemical screening
|
| The phytochemical evaluation of the drug was carried out as per the standard method. Previously dried powdered material (5.0 g) was taken and extracted in a soxhlet apparatus with petroleum ether, chloroform, methanol and water successively. The extracts were evaporated to dryness under vacuum. These extract were used for the analysis of different phytoconstituents viz. alkaloids, carbohydrates, phenolic, flavonoids, proteins, amino acids, saponins, mucilage and resin etc. present in it [24,25]. |
|
Powdered drug reaction with different reagentss
|
| The powdered drug was treated separately with different acids and reagents like picric acid, hydrochloric acid, nitric acid, iodine, ferric chloride and sodium hydroxide. The colours shown by drug with different reagents were recorded under the normal daylight as well as under the microscope [26]. |
|
Determination of pH
|
|
pH 1% solution:
|
| An accurately weighed 1.0 g of powdered drug was dissolved in 100 ml of distilled water. It is then filtered and the pH of the filtrate was recorded with a standardized glass electrode [22]. |
|
pH 10% solution:
|
| An accurately weighed 10 gm of powdered drug was dissolved in 100 ml of distilled water. It is then filtered and the pH of the filtrate was recorded using pH meter [27]. |
|
Determination of fat content
|
| A weighed quantity of powdered sample (3.0 g) was extracted with anhydrous ether in a continuous extraction apparatus for six hours. The extract was filtered in a clean, dry and weighed flask. Then, the extraction flask was rinsed with small quantity of ether, filtered and added to the weighed flask. The solvent is evaporated and dried in an oven to a constant weight at 105°C [28]. |
|
Determination of resin content
|
| The accurately weighed drug sample (5.0 g) was rapidly refluxed with acetone (3×200 ml) for six hours to exhaust the drug for resin content. The excess solvent was removed by distillation on water bath. The residue so obtained was then suspended in distilled water and transferred to a separating funnel, the suspension was repeatedly extracted with ether (2×200 mL) in order to extract out all the resin content completely. The ether extract was cooled out and dried over anhydrous sodium sulphate and excess ether was removed by heating on water bath. It was then transferred to a weighed beaker and thus the final weight was noted down [28]. |
|
Determination of foaming index
|
| About 1.0 g of plant material was reduced to a coarse powder and it was weighed accurately and transferred to a 500 mL conical flask containing 100 mL of boiling water. It was boiled moderately for 30 minutes, cooled and filtered into a100 ml volumetric flask and sufficient distilled water was added to dilute the volume. |
| The decoction was poured into 10 stoppered test tubes in successive portion of 1.0, 2.0, 3.0 ml and so on up to 10 mL and the final volume was adjusted with distilled water up to 10 mL. The tubes were stoppered and shaken in a lengthwise motion for 15 seconds (two shakes per second) then it was allowed to stand for 15 minutes and the height of foam was then measured [20]. |
|
Determination of swelling index
|
| The prescribed quantity of leaf material (3.0 g was reduced to a required fineness, weighed accurately and was taken into a 25 mL glass stoppered measuring cylinder. Twenty five ml of distilled water was added into it and the mixture was shaken thoroughly in every 10 minutes for one hour. It was allowed to stand for three hours at room temperature. The mean value of individual readings were taken and calculated with respect to 1.0 g of plant material [20]. |
|
Determination of bitterness value
|
| The bitter properties of herbal material are determined by comparing the threshold bitter concentration of an extract of the materials with that of a dilute solution of quinine hydrochloride. The bitterness value is expressed in unit equivalent to the bitterness of a solution containing 1.0 g of quinine hydrochloride in 2000 mL. After rinsing the mouth with safe drinking-water, 10 mL of the most dilute solution was used to swirl it in the mouth mainly near the base of the tongue for 30 seconds. If the bitter sensation is no longer felt in the mouth after 30 seconds, spit out the solution and wait for one minute to ascertain whether this is due to delayed sensitivity. Then rinsed with safe drinking-water. The next highest concentration should not be tasted until at least 10 minutes have passed [20]. The threshold bitter concentration is the lowest concentration at which a material continues to provoke a bitter sensation after 30 seconds. All solutions and the safe drinking-water for mouth washing should be at 20−25°C. The bitterness was calculated using the following formula.. |
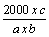 |
| Where a = concentration of the stock solution (mg/mL) |
| b = volume of the plant drug with the threshold bitter concentration |
| c = quantity of quinine hydrochloride (in mg) in the tube with the threshold bitter concentration |
|
HPTLC fingerprinting
|
|
Sample preparation:
|
| The methanolic extract of leaf sample was prepared by direct extraction method in which shade dried leaves of N. arbor-tristis were macerated in methanol overnight, sonicated and filtered. The filtrate was evaporated to dryness on water bath. Thus, the methanolic extract formed was stored and used for TLC fingerprinting profiling. For this purpose 5 mg of dried methanolic extract was taken and dissolved in 1 ml of methanol to make the concentration of 5 mg/ml of extract sample. Similarly for standard, 0.5 mg of standard Astragaline (procured from Sigma Aldrich, 95% pure) was taken and dissolved in 1 ml of methanol to make the concentration of 0.5 mg/ml. |
|
HPTLC instrumentation and sample application:
|
| The HPTLC fingerprints of the extract of drug were established by developing the solvent systems for their separation by thin layer chromatography. The solvent system, in which maximum and well resolved spots were found, was selected for HPTLC. The samples and standard were applied as 8.0 μL and 5.0 μl respectively and the band length was kept to 6.0 mm and distance between tracks was 13 mm on precoated silica gel 60 F254 plates (E. Merck, 0.20 mm thickness) using Linomat V (HPTLC sample applicator). After sample application, the plates were developed up to 80 mm in twin trough glass chamber saturated with the respective solvent system. The chromatograms were scanned at 255 nm followed by spectral analysis. Reprostar Chromatography Documentation System was used for taking photograph of the HPTLC plates. |
Results and Discussion
|
|
Morphological characters
|
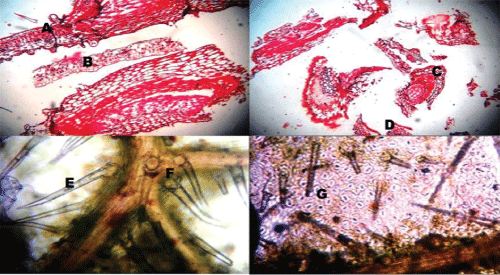
| The morphological characters were observed on the basis of visual analysis (Figure 1). |
| Colour: Light to dark green |
| Odour: Indistinct |
| Taste: Bitter and astringent |
| Leaf: Simple, 5-14 cm long, 2.5-7.5 cm wide, ovate, acute to acuminate; both surfaces rough, scabrous, densely pubescent; lower with appressed hairs |
| Margin: Entire or distinctly toothedBase: Round to somewhat cuneate |
| Venation: Reticulate, lateral vein 3-6 pairs, more conspicuous on lower side. |
|
Microscopy of the leaf
|
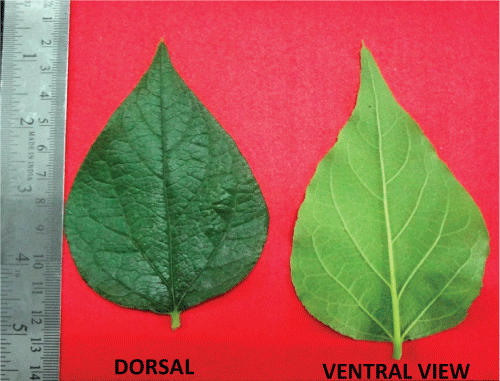
| The transverse section of leaf (Figure 2) passing through the midrib convexly projects on lower side, and slightly grooved with a shallow central elevation on upper side. U-shaped meristele was found, the arms of which reach almost up to the palisade tissue of lamina, which lies in the midrib, leaving wide parenchymatous cortex at lower side and lignified pitted parenchymatous zone in the centre of an arc of xylem. Simple and compound starch grains and occasionally few lignified fibres were present in the pericyclic region. Few layers of collenchyma were laid under both the epidermis, the upper layer located adjacent at the opening of U-shaped xylem. The lower epidermis of midrib bears large number of multiseriate papillose projections. It bears simple covering unicellular trichomes of various sizes containing cystolith at the base. Glandular trichomes with unicellular stalk and bicellular head filled with dark brown content were also present. |
| The cells of upper epidermis of lamina were thick walled somewhat straight and devoid of stomata. The lamina adjacent to the midrib showed two rows of epidermis. The cells of lower epidermis are smaller in size than that of upper one with sinous walls at places, cuticle striated and transverse by numerous anomocytic stomata. Both the epidermii are papillose and bear, short and long unicellular trichomes with pointed apex, and multi-cellular spherical head or bicellular head, sessile glandular trichomes with four celled head are also present. Underneath the upper epidermis lie two rows of palisade cells, followed by 7-9 rows of spongy parenchyma traversed by vascular strands and encircled by parenchymatous cells. |
|
Powder microscopy
|
| Powder microscopy of N. arbor-tristis (Figure 3) showed the fragments of upper epidermis with straight or slightly wavy cells and devoid of stomata. Walls of upper epidermis are found with straight or slightly wavy cells and devoid of stomata. Unlike this, walls of lower epidermis are distinctly sinuous and contain many anomocytic stomata, adjacent epidermal cells of which are with distinctly striated cuticle, simple, covering, warty, thick walled trichomes of various sizes, embedded with cystolith at the base; glandular trichomes are with unicellular stalk, bicellular head and sessile trichomes with 4-celled head. Fragments of lamina showing epidermis and rows of palisade cells, Fragments of lamina showing epidermis and rows of palisade cells. |
|
Physicochemical analysis
|
|
Extractive values:
|
| The extractive values were studied on dried leaf powder as mentioned in methodology. It indicates the approximate measures of the chemical constituent in plant. All the values were taken in triplicate and the mean average was taken (Table 1). |
|
Ash value:
|
| The total ash values, acid insoluble ash value and water soluble ash value were found to be 14.3% w/w, 4.7% w/w, 10.5% w/w respectively. Ash value is useful in determining authenticity and purity of drug and also these values are important quantitative standards. |
|
Loss on drying:
|
| The loss on drying was found to be 19.1% w/w and is done to determine the moisture as well as volatile component in the crude drug. |
|
Fluorescence analysis:
|
| The powdered leaves of N. arbor-tristis (mesh size 40) was examined under day light and UV light. The observation was recorded and given in Table 2. |
|
Phytochemical screening
|
| The phytochemical screening was carried out on different extracts of N. arbor-tristis (Petroleum ether, ethyl acetate, chloroform, acetone, alcohol, hydro alcohol, aqueous given in Table 3. The petroleum ether extract showed the presence of fats, lipids and terpenoids. The ethyl acetate extract was found to contain saponins, glycosides, flavonoids and terpenoids which are poorly present in it. The chloroform extract contains steroid and flavonoids. The alcoholic extract of leaf confirmed the presence of flavonoids and glycosides in it. Glycosides were also strongly found in hydro alcoholic extract too. The aqueous extract confirmed the presence of carbohydrate, glycosides, flavonoids and saponins in it. |
|
Powdered drug reaction with different reagents
|
| The powdered drug reaction with different reagents was carried in normal light and is given in the Table 4. The various reagents used are Iodine, ethanol, ferric chloride, KOH, NaOH etc. |
|
Determination of pH
|
| The mean pH value of 1% solution and 10% solution was found to be 6.20 and 4.95 respectively. |
|
Determination of fat content
|
| The mean percent of fat content in the sample was found to be 1.64%. |
|
Determination of resin content
|
| The mean resinous content was found to be 4.9%. |
|
Determination of foaming index
|
| The height of the foam in every test tube was less than 1 cm, so the foaming index was less than 100. |
|
Determination of swelling index
|
| The swelling index was found to be less than 100 which indicate that its swelling index was in normal range. |
|
Determination of bitterness value
|
| Bitters are the medicinal plant material that have a strong bitter taste and are employed therapeutically, mostly as appetizing agents. Their bitterness stimulates secretion in the gastrointestinal tract, especially of gastric juice. |
| Bitterness value was found to be 2.89 |
|
HPTLC fingerprinting
|
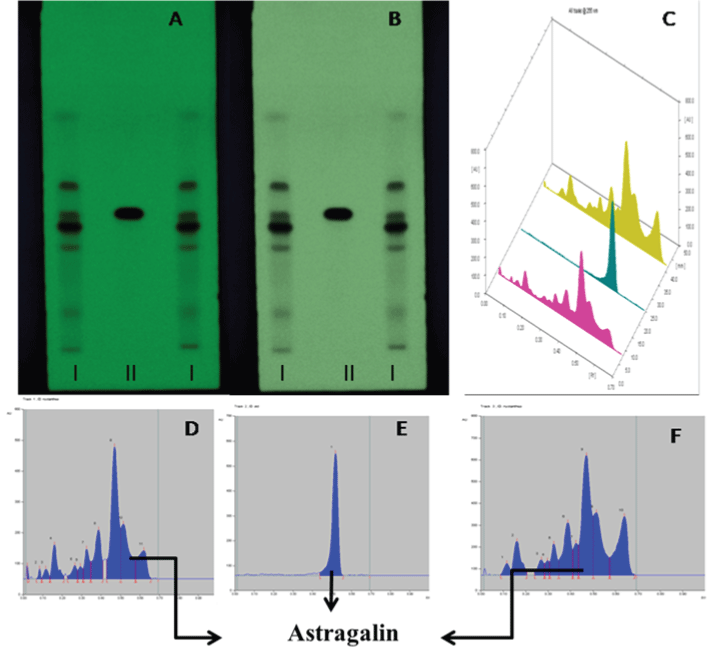
| Different solvent systems were tried by hit and trial method for the different extracts. Satisfactory separation of constituents was obtained in solvent system ethyl acetate: methanol: water (100:16.5:13.5, v/v/v). The samples were applied and chromatograms were developed in solvent. The chromatograms then scanned at wavelength 265 nm. The HPTLC fingerprints of methanol extract were showing presence of Astragaline (Kaempferol-3-o glucoside) at Rf value 0.54 (Figure 4 and Table 5). |
|
Pesticide residues
|
| The pesticides residues were found to be in normal range. |
|
Heavy metal content
|
| Heavy metal analysis of drugs showed that lead, cadmium, mercury and arsenic are nil in N. arbor-tristis leaves. |
Conclusion
|
| Standardization of herbal drugs or establishing quality standards of herbal drugs is current need of the hour to raise the standard of herbal or polyherbal formulations available in the market. In the present study N. arbor-tristis leaves were evaluated for physicochemical and phytochemical analysis as per the WHO guidelines, which was never done before for this plant. This helps in laying down all the quality control parameters of this drug, which serves as a standard monograph, this in turn help to maintain the quality of several formulations by maintaining its purity and authenticity. Thus, this information can be useful in distinguishing and determining type of chemical compounds present in the leaf and set as a platform for the future researches. |
Acknowledgement
|
| The authors are grateful to the Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi for providing valuable support. |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|