Abstract
Cancer is uncontrolled mitosis where reactive oxygen species (ROS) act as one of the mito- gens. Although elevated ROS concentrations are one of the key points in the development and spread of cancer, exceeding the threshold limit becomes lethal to cancer cells. Due to an independent anti-oxidant system and metabolic reprogramming, cancer cells can withstand high ROS concentrations (e.g., the Warburg effect). The ability of cancer cells to control ROS levels is an intricate strength and a crucial factor for effective cancer treatment. Multiple cancer therapies, like radiotherapy and chemotherapy, rely on ROS accretion to induce cancer cell death but they simultaneously they damage normal cells. These therapies intended to block this adaptation should be expected to kill cancer cells. Alteration in the nature of cancer cells by ROS-generating molecules and making them more selective for apoptosis can be the practical mode of treatment for malignancies. Due to their ROS-enhancing properties and anticipated changes in multiple signaling pathways, a number of natural product compounds and repurposed medicines have recently been investigated for the management of cancer. In this review, we describe the specific role of ROS in tumorigenesis and clinically tested repurposed ROS-generating drugs and their metabolism for cancer therapy.
Keywords
Reactive oxygen species; Metabolic reprogramming; Warburg effect; Drug repurposing; Cancer therapy; Apoptosis
Introduction
Cancer has become a devastating disease worldwide. A total of 8.8 million people died from cancer in 2015, and it is expected that 13 million will die worldwide by 2030 [1]. Cancer has a progressive way in humans due to environmental pollution, junk food, and the sedentary lifestyle of humans, and it can develop in any part of the body. Men are more susceptible to developing lung, prostate, colorectal, gastrointestinal, and liver cancer than women, who are more likely to develop breast, ovarian, endometrial, lung, cervix, and stomach cancer [2]. Cancer has various hallmarks, such as hyperproliferation, angiogenesis, insensitivity to antigrowth factors, apoptosis resistance, migration, weak immune system, inflammation, and genome instability [3]. Although modern cancer treatments have significantly improved patients’ quality of life, many cannot survive because of cancer complexity, delayed diagnosis, drug resistance, and multiple unknown reasons [4]. At an early stage, most cancers respond positively to conventional therapy and later become insensitive to drugs. Reactive oxygen species (ROS) are pleiotropic molecules or free radicals produced by multiple complex mechanisms. One of the mechanisms is the incomplete oxidative phosphorylation that occurs during biomolecule breakdown, particularly in the electron transport chain [5]. In a state of homeostasis, natural enzymatic systems nullify these highly reactive molecules or even antioxidant substances to shield the cells from the harmful effects of ROS by scavenging them. Oxidative stress, a condition of a cell characterized by the oxidation of vital molecules, can be caused by ROS overproduction, malfunctions in the scavenging mechanisms, or even an inadequate supply of antioxidants [6]. Previously, ROS were viewed as mere byproducts of respiration from the electron transport chain of mitochondria. They randomly interact with cellular targets like intracellular lipids, proteins, and DNA can cause damaging effects on these biomolecules [7].
ROS acts as a double-edged sword in both pathological processes like neurodegenerative diseases, carcinogenesis, and even cancer heterogeneity [8], and in some physiologic processes where hydrogen peroxide is required for cytokine, insulin, growth factor, AP-1, and NF-kB signaling. In addition to cancer cells, non-cancer cells also contribute to the development of tumors [9]. Nonneoplastic tu-mor-associated cells, including cancer-associated fibroblasts (CAFs), endothelial cells, immune cells, adipocytes, and pericytes, create and accumulate large levels of ROS [10]. Many CAFs can be seen in some solid tumors, including pancreatic, prostate, and breast cancer, instead of the few CAFs typically present in renal and brain cancers [10 ]. However, all of these distinct cells (cancerous or not) associated with peritumoral neoangiogenesis form the heterogenic and desmoplastic tissue and are observed in several solid tumors. Desmoplasia fosters tumor proliferation by generating a hypoxic and acidic environment [11]. A mechanical stretch could convert fibroblast to myofibroblast, contributing to cancer stemness and drug resistance [12]. As a result of oxidative stress, which is powered by a high degree of mitochondrial ROS generation, this altered microenvironment is nourished by a hypoxic and acidic milieu. Figure 1 demonstrates how ROS are produced and how normal cells can transform into tumors.
Figure 1: (A) Different ways of ROS production in a cell. The general representation of ROS generation by cellular enzymes and the electron transport chain are represented in Figure 1. The chief sources of intracellular ROS include the mitochondrial ETC and NADPH oxidases. SOD1 and SOD2 can convert O2¯ into H2O2; then H2O2 can be converted into H2O. Meanwhile, H2O2 can also be converted into •OH, HOCl, and HOBr by Fe2+ and myeloperoxidase, respectively. NO is responsible for the conversion of O2¯ into ONOO¯ and •OH. (B) Types of cells in tumorigenesis. Diagram of the potential cells of origin of CAFs, including resident fibroblasts, endothelial cells, epithelial cells, mesothelial cells, mesenchymal stem cells, and adipocytes with probable mechanisms..
In this review, we aim to describe the chemistry of ROS, which is homeostatic in non-cancerous and cancerous cells and causes ROS-mediated cancer cell death via extrinsic and intrinsic apoptosis. The multitasking nature of ROS in autophagy, necrotic, and ferroptosis cell death is delineated. We also shed light on exploring ROS in cancer treatment via radiotherapy, photodynamic therapy, and chemotherapeutic drugs. Finally, we mention some of the ROS-generating drugs undergoing clinical trials for cancer treatment.
Chemistry of ROS
ROS can be both radicals and nonradicals derived from oxygen (O2). Superoxide (O2•¯) and the hy-droxyl radical (•OH) are radicals, while hydrogen peroxide (H2O2) and singlet oxygen (1O2) are non-radicals. The oxygen diatom’s electronic configuration is [2He4]2s42p8, with the first ten electrons in the bonding orbitals and two unpaired electrons in a π anti-bonding orbital. When an electron is re-moved from O2, a superoxide cation radical (O2•+) is formed. In contrast, adding a single electron produces the superoxide anion radical (O2•¯) [13]. The anionic radical (O2•¯) cannot diffuse quickly throughout the cell and is unreactive toward electron-rich substrates. Superoxide (O2•¯) converts to hydrogen peroxide (H2O2) by superoxide dismutase (SOD) [14]. H2O2 is recognized as the key redox signaling agent and produces various intracellular ROS in contrast to superoxide. Hydrogen peroxide is widely distributed within cells and reacts with thiol groups with low pKa or proteins containing transition metals (e.g., [Fe–S] clusters). In fact, [Fe-S] clusters are the primary cellular target of (O2•¯) mediated toxicity due to electrostatic attraction. The transition metal-catalyzed reduction in H2O2 produces a highly reactive hydroxyl radical (•OH) [15] via the Fenton reaction without an antioxidant protection system. Thus, excessive O2•¯/ H2O2 production causes a cascade of radical chain reactions that damage important biomolecules and promote oxidative stress. ROS are also exogenously generated in organisms by X-ray, UV radiation, pollutants, xenobiotics, biotransformation of dietary chemicals, some diet components, such as transient metal ions, and inflammatory reactions that occur during normal cellular metabolism [16]. The imbalance in pro-oxidant/ antioxidant causes oxidative stress, which results in the oxidation of cellular components, the alteration of cytoplasmic/nuclear signal transduction pathways, the modulation of gene and protein expression, and changes in the activities of DNA and RNA polymerases [17].
Regulation of ROS Production
ROS can emerge from a variety of intracellular sources. The most important are mitochondria, NADPH oxidases, and other enzymes. In most cell types, mitochondria are considered the primary source of intracellular ROS. More specifically, the electron transport chain complexes I and III, which pump protons out of the inner mitochondrial membrane, are responsible for most superoxide production within the mitochondria [18].
In addition to an H+-translocating ATP synthetic complex, oxidative phosphorylation in mitochondria involves four electrontransporting complexes. NADH–ubiquinone oxidoreductase (complex I) and ubiquinol–cytochrome c oxidoreductase (complex III) produce the majority of superoxide radicals [19]. Mammalian complex I is a protein assembly comprising thirty-four subunits encoded by nuclear DNA and seven subunits encoded by mitochondrial DNA (mtDNA). A series of evolutionarily conserved electron carriers conduct electrons derived from the initial oxidation of NADH through several iron–sulfur centers to eventually reduce ubiquinone by a mechanism that pumps protons from the mitochondrial matrix to the outside of the mitochondrial membrane. This complex produces sem-iquinones, which have been identified as potential electron donors for converting O2 into O2¯, though mechanisms that are not fully understood [20]. Nonetheless, there are two possible sites at the end of the cofactor chain where oxygen could access electrons: the flavin moiety and the quinine-binding site. Complex III, in turn, reduces equivalents generated in complexes I and II contained in ubiquinol and transfers them to the final electron acceptor cytochrome c via reactions with cytochrome b, the Rieske iron– sulfur protein, and cytochrome c1 [21]. Two semiquinone species are produced during complex III’s mechanism operation. The Q-cycle mechanism proposed for the ubiquinol cytochrome c reductase’s function begins with ubiquinol donating one electron to the Rieske iron–sulfur protein, producing a semiquinone near the outer face of the inner membrane, which then reduces the first cytochrome b heme (b1). The second cytochrome b heme (bH), which is located closer to the matrix side of the membrane, accepts one electron from the first heme and reduces ubiquinone to form ubisemiquinone, which is then reduced by another electron to form ubiquinol [22].
Because the structure and functional mechanisms of complex I are still unknown, inhibiting it is a good way to study it. More than 60 different family compounds inhibit this complex [23], with rote-none being the most commonly used to inhibit ROS formation during reverse electron transfer and induce it during forward electron transfer. The reverse electron transfer from succinate to NAD+ pro-duces more superoxide than the forward electron transfer [24]. According to the complex I inhibitory technique, the iron-sulfur cluster may be the location of an electron leak, in which oxygen ends up catching an electron and producing superoxides [23]. Complex I structural modifications are critical in the ROS production mechanism. Mutations in mtDNA, whether constitutive or caused by mtDNA damage, can result in these modifications, which can lead to a variety of pathogenesis and cell aging [25]. A decrease in electron transfer activity, which increases ROS production, is one of the consequences of a domain change in the respiratory complexes, establishing a vicious cycle of oxidative stress and energetic decline. This mitochondrial metabolism dysregulation is thought to be at the root of age-related degenerative diseases and cancer [26].
Another important source of ROS is NADPH oxidase (NOX), a multisubunit enzyme that catalyzes O2•− production by reducing O2 with NADPH as the electron donor. NOX-produced ROS are regarded as normal and necessary. They are involved in microbial killing, and superoxide produced by NOX2 caus-es phagocyte respiratory bursts. The second function is related to cell signaling regulation. NOX-generated ROS can specifically and reversibly react with proteins, changing their activity, locali-zation, and half-life [27].
ROS Homeostasis in Non-Cancerous Cells
Natural enzymatic systems, like glutathione and nicotinamide adenine dinucleotide phosphate, help non-cancerous cells maintain their levels of ROS. ROS function as signaling molecules/ secondary messengers at the basal level, regulating several biological processes important for maintaining the physiologic function, such as cellular proliferation and differentiation, inflammation associated with innate and adaptive immunity, and tissue maintenance and aging [28]. Because of their high reactivity, ROS tend to participate in redox reactions, thus playing an essential role in a cell’s redox balance. As a result, several homeostatic mechanisms have evolved to balance the steadystate production and elimination of oxidants; this balance is referred to as redox homeostasis [27]. Non-cancerous cells limit ROS accumulation through scavenging enzymes (e.g., superoxide dismutases, catalase, glutathione peroxidases), inducible antioxidant expression (e.g., NAD(P)H: quinone oxidoreductase 1 [NQO1]), and the peroxiredoxin, thioredoxin, and glutathione/ glutaredoxin systems.
ROS Homeostasis in Cancerous Cells
In cancer cells, steady-state levels of intracellular and extracellular ROS are generally higher than in non-cancerous cells. High metabolic rates, mitochondrial DNA mutations, oncogenic lesions, and hy-poxic tumor microenvironments (TMEs) have been proposed as endogenous mechanisms [29]. First, rapidly multiplying cell’s high energy and nutrient demands cause hypermetabolism, increasing in-tracellular and extracellular ROS production in cancer cells’ mitochondria, endoplasmic reticulum, and membranes. Second, mitochondrial DNA mutations can cause defects in respiratory complexes along the electron transport chain, resulting in an increase in ROS production. Third, oncogenic mutations disrupt key intracellular signaling pathways that influence metabolism and protein translation, resulting in ROS accumulation [30]. Oncogenic K-Ras-transformed pancreatic cancer cells, for example, have been shown to stimulate ROS production via a signaling cascade activating NADPH oxidase 1 (NOX1). On the other hand, some tumor suppressors, such as BRCA1, p53, and SIRT3, promote antioxidant function in the cell, and their cancer-associated loss increases ROS accumulation. Finally, hypoxia associated with the TME of solid tumors has been shown to cause ROS production, possibly via effects on cytochrome oxidase [31].
ROS-Mediated Cancer Cell Death
High levels of ROS, as previously stated, play an important role in cancer therapy by activating various cell death types such as apoptosis, autophagy, ferroptosis, and necrotic cell death [32].
ROS-activated extrinsic and intrinsic apoptosis
The most common type of cell death is apoptosis, which includes death receptor-dependent apoptosis (also known as extrinsic apoptosis) and mitochondrial-dependent apoptosis (known as intrinsic apoptosis). The TNF (tumor necrosis factor) and the Fas pathway are two major extrinsic apoptosis pathways. TNFinduced ROS may also activate ASK1 and subsequent ASK1- mediated extrinsic apopto-sis-signaling cascades [32,33]. The TNF-related apoptosis-inducing ligand (TRAIL) can cause cell apoptosis via the TNF pathway, as shown in Figure 2. ROS have been shown to suppress post-transcriptional c-FLIP (the endogenous TRAIL inhibitor) expression, facilitating extrinsic apopto-sis. NAC (N-acetyl-L-cysteine), a ROS scavenger, effectively restores c-FLIP expression and inhibits ex-trinsic apoptosis [34]. ROS are required for extrinsic apoptosis induced by the Fas ligand (FasL) by en-hancing ubiquitination-mediated FLIP degradation. Furthermore, studies have found that ROS plays an important role in regulating the expression of functional FasL, which can bind the Fas receptor on tumor cells and eventually induce tumor lysis [35]. Exogenous oxidants and redox-active substances, such as MAP kinase activators, chemotherapeutic agents, and H2O2, could regulate FasL expression, implying that ROS control FasL expression. The majority of antitumor ROS inducers have been shown to exert anticancer activity through a ROS-dependent mitochondrial apoptotic pathway [36]. Super-oxide anions cause rapid and massive Cyto-c release from the mitochondrion, causing cell apoptosis via VDAC-dependent permeabilization of the mitochondrial membrane [37]. Furthermore, the oxida-tion of adenine nucleotide translocase (ANT) by ROS is linked to the regulation of the opening of the mitochondrial permeability transition pore (MPTP). Oxidative modification of pro-caspase-9 at Cys403 causes auto cleavage and activation by interacting with Apaf-1 [37]. Furthermore, ROS have been shown to regulate the activity of Bcl-2 family proteins, which are the dominant regulators of the mitochondrial apoptotic pathway. The main mechanisms are as follows: (1) H2O2 can directly oxidize Bcl-2 at Cys158 and Cys229, reducing its anti-apoptotic activity, and (2) O2•¯ can inhibit Bax and Bad ubiquitination while promoting Bcl-2 ubiquitination [38].
Figure 2: The role of ROS in cancer cells: ROS drives mitogenic signaling cascades. Increased ROS levels contribute to sustained cell survival and proliferation through many pathways including PI3K/AKT, MAPK/ERK1/2, and PKD. High levels of ROS, resulting from abnormal cellular metabolism and inflammation, promote tumor proliferation, vascularization, and metastasis, while an excessive amount of ROS is likely to induce senescence and/or apoptosis.
Dual effect of autophagy by ROS
In mammalian cells, autophagy is a natural and regulated selfdegradative process in which unneces-sary or dysfunctional cytoplasmic components are degraded in the lysosome. Numerous anticancer drugs have been shown to induce autophagy [39]. Depending on the circumstances, autophagy either promotes or inhibits drug-induced cell death. ROS have been shown to induce autophagy by regulat-ing various signaling pathways. Notably, the redox regulation of autophagy is linked to ROS exposure levels, duration, and cell type. ROS-induced autophagy also has a dual effect on cells. To begin, cells can protect themselves by inducing autophagy in order to reduce oxidative stress. Excess ROS, on the other hand, can promote autophagic cell death [40].
A serine/threonine kinase, the mammalian target of rapamycin (mTOR), inhibits the autophagic pro-cess. H2O2 inhibits mTOR activity and induces autophagy in breast cancer cells by activating the ATM/LKB1/AMPK/TSC2 pathway [41]. Furthermore, H2O2- induced BNIP3 expression suppresses mTOR activity and initiates autophagy in C6 glioma cells. Mitochondrial-derived H2O2 induced autophagic cell death in malignant glioma cells by sanguinarine treatment. Beclin-1, a key autophagy-related protein, is involved in autophagy initiation. ROS have been shown to increase the levels of beclin-1 [42]. Another autophagyassociated protein, ATG4, has been shown to be an ROS target. Under starvation conditions, H2O2 oxidizes and inactivates ATG4, promoting LC-3 lipidation and thus inducing autophagy.
ROS-mediated necrotic cell death
Necrotic cell death, also known as necroptosis or necrosis, was once thought to be an uncontrolled type of cell death characterized by organelle swelling and membrane rupture [43]. However, mount-ing evidence suggests that necrosis, like apoptosis, involves complex molecular circuitry. RIP1 and RIP3 have been identified as two critical regulators during necroptosis execution. Some evidence in-dicates that ROS, as RIP1 and RIP3 modulators, play a role in necroptosis. Mitochondrial ROS regulate RIP1 by promoting autophosphorylation and causing oxidation, which results in the formation of intermolecular disulfide bonds [44]. Following that, phosphorylated RIP1 promotes RIP3 recruitment into the necrosome, resulting in necroptosis. Furthermore, mitochondrial ROS mediates calcium-induced RIP1/RIP3 complex formation and necroptosis in human colon cancer cells. Although there is evidence that ROS can regulate RIP1 and RIP3, the type of ROS involved in RIP1- or RIP3-induced necroptosis is unknown. RIP1 and RIP3, on the other hand, have been identified as driving forces for ROS production. It has been demonstrated that RIP1 and RIP3 contribute to the production of intracellular ROS in shikonin-treated glioma cells [45]. Artesunate-induced ROS is also dependent on RIP1 in renal carcinoma Caki cells. However, the mechanism of ROS induced by RIP1 or RIP3 is unknown. By directly binding to these proteins, RIP1 and RIP3 may increase the activity of several metabolic enzymes such as glutamate– ammonia ligase, glutamate dehydrogenase 1, and gly-cogen phosphorylase. These metabolic enzymes catalyze the generation of mitochondrial ROS [45,46].
Ferroptosis: cell death by lipid ROS
Ferroptosis, also known as ferroptotic cell death, is a novel type of cell death that is iron-dependent [47]. Ferroptosis is involved in the formation of iron-dependent ROS and the oxidation of lipids [48]. By suppressing glutathione (GSH)-dependent antioxidant defenses, some compounds and antioxidants cause cell ferroptosis. System xc-cysteine/glutamate is a cell surface heterodimeric amino acid antiporter. Glutathione synthesis requires cysteine import via system xc. Glutathione activates GPX4 to prevent lipid ROS production [48]. Sorafenib, sulfasalazine, and erastin cause ferroptosis by directly inhibiting system xc and preventing cystine uptake. SLC1A5 transports glutamine into the cell, where it is metabolized to α-ketoglutarate. Then, α-ketoglutarate helps to form oxidizable membrane lipids and subsequent lipid ROS. Many anticancer drugs have been shown to kill cancer cells by utilizing ferrous iron to generate radicals and cause ferroptosis. For example, the antimalarial drug artesunate has been repurposed as a ferroptosis inducer to exert anticancer effects [49]. The most promising agent against cancer stem cells, salinomycin, has been shown to inhibit iron translocation, elicit an iron-depletion response, and induce ferroptosis.
ROS: Antioxidant Balance in Cancer as a Therapeutic Approach
The importance of ROS in cancer progression provides a compelling biological rationale for targeting tumor redox balance as an anticancer treatment strategy [50]. A unique sensitivity of cancer cells to ROS, antioxidant equilibrium strengthens the case. However, as cancer cell populations adapt and evolve over time, radiation and chemotherapy can result in the emergence of treatment-resistant clones capable of producing high levels of antioxidant proteins [51]. The key question is how to target these resistant clones for therapeutic benefit. There is evidence that therapies aimed at disrupting tumor redox balance can be effective against cancer cells that are resistant to conventional radiation or chemotherapy. Preclinical research has shown that therapies that target tumor redox balance, ei-ther by increasing ROS or disrupting antioxidant defense mechanisms, have activity in radia-tion-resistant or chemoresistant cell lines [52]. As a result, novel therapies that target redox homeo-stasis have the potential to transform our ability to kill cancer cells [53]. Although resistant clones may have increased antioxidant capacity, this only raises the ROS threshold required to cause cell death, rather than eliminating the cytotoxic threshold. When enough ROS are produced, they scav-enge upregulated antioxidant proteins until enough ROS accumulate to become cytotoxic. Several treatments are being tested with the goal of increasing ROS above the cytotoxic threshold [54]. One example is napabucasin, an investigational small molecule bioactivated by NQO1 that uses NQO1 an-tioxidant upregulation as a therapeutic target in cancer cells. In vitro, cancer cell studies in multiple solid tumor cell lines (e.g., colon) have revealed that napabucasin bioactivation results in the produc-tion of cytotoxic levels of ROS in an NQO1-dependent manner, causing DNA damage and cell death as well as affecting cell signaling pathways [55]. Because increased NQO1 expression is linked to in-creased ROS levels in cancer cells and NQO1-positive cancer cells are sensitive to napabucasin [55], this agent is expected to have selective activity in cancer cells. Furthermore, NQO1-positive cancer cells were found to promote STAT3 phosphorylation (pSTAT3) in the TME, implying that pSTAT3 levels may serve as a biomarker of tumor redox balance and napabucasin susceptibility. In a phase 3 study, napabucasin was tested on patients with previously treated metastatic colorectal cancer (NCT02753127). High-dose vitamin C is another ROS-mediated investigational anticancer treatment. When pharmacological doses of vitamin C are oxidized, they produce high levels of ROS hydrogen peroxide and can deplete glutathione, the most common antioxidant found in noncancerous and cancerous cells [56].
High-dose vitamin C is another ROS-mediated investigational anticancer treatment. When pharma-cological doses of vitamin C are oxidized, they produce high levels of ROS hydrogen peroxide and can deplete glutathione, the most common antioxidant found in both non-cancerous and cancer cells [57]. In vitro, vitamin C-produced hydrogen peroxide was found to selectively kill cancer cells due to the lower metabolic clearance of hydrogen peroxide by cancer cells compared to non-cancerous cells, which may be due to disrupted intracellular iron metabolism [58]. Decreased catalase activity correlated with cancer cell sensitivity to hydrogen peroxide, suggesting that catalase levels may serve as a marker of susceptibility to vitamin C therapy. Vitamin C’s cytotoxic mechanism is linked to its depletion of antioxidant molecules in metabolically altered cancer cells, resulting in ROS accumulation, DNA damage, and decreased adenosine triphosphate (ATP) levels [59]. Because ATP is required for the proper functioning of drug efflux pumps, ATP depletion via ROS accumulation may be a useful strategy for overcoming drug resistance. One study found that using nanoparticles to generate ROS reduced efflux pump function and overcame multidrug resistance in cancer cells. Other preclinical studies support vitamin C’s potential role in treatment-resistant cancer. One study found that vitamin C preferentially eradicated cancer cells with high expressions of sodium-dependent vitamin C transporter 2 and known resistance to chemotherapy in cell lines and patientderived xenograft models of hepatocellular carcinoma [60].
ROS and Anti-Neoplastic Therapies
ROS, as previously discussed, can have opposing cellular effects, promoting either cell proliferation and tumor progression or cell death and tumor regression. ROS are a “two-edged sword” in that they act not only as disease inducers/sustainers but also as therapeutic weapons in cancer [61]. Cancer cells evolve under specific endogenous and exogenous oxidative conditions, becoming highly differ-entiated based on cell type and tumor evolution stage. Tumors adapt to harsh environments by developing powerful antioxidant mechanisms and even using endogenous ROS for proliferation. Despite cancer cells’ inherent resistance to ROS-induced cell death, therapies that induce an oxidative burst in tumors proved effective [62].
Novel therapeutic anti-neoplastic strategies based on ROS formation and/or antioxidant mechanism modulations seek to exploit differences between normal and cancer cells. Unfortunately, existing an-ticancer therapies have negative effects on normal tissues, which are partially triggered by ROS, lim-iting the applicable dosage and anti-tumor activity [63].
ROS-mediated processes in radiotherapy
Radiotherapy is a clear example of anti-neoplastic treatment whose mechanism is primarily based on ROS by combining the properties of an extremely efficient DNA damaging agent with a high spatial focus on the tumor. Ionizing radiation is one of the most effective tools in cancer therapy. Radiotherapy is limited due to the high carcinogenic potential of ionizing radiation [64], as evidenced by an increased cancer risk in atomic bomb survivors in Japan. Tumor cells, however, have been shown to be more sensitive to radiotherapy than normal cells, as radiation causes preferential mitotic cell death in rapidly dividing cells, which are then destroyed by necrosis [65]. A negative side effect of radiotherapy is the harmful inflammatory response caused by necrosis.
Ionizing radiation-induced damages
Radiotherapy causes cell damage through the direct ionization of DNA and other cellular targets, as well as indirect effects mediated by ROS produced during radiation-induced water hydrolysis [66]. Furthermore, when cells are exposed to ionizing radiation, they produce ROS and other toxic radicals. Radiotherapy causes nuclear DNA damage, such as single- and double-strand breaks, which causes cell cycle arrest. The recruitment of DNA repair enzymes protects normal cells from radiation injury while decreasing therapy efficacy [67]. Ionizing radiation can also severely damage mitochondrial DNA, altering the expression of various genes that encode critical proteins in the oxidative phosphorylation system, resulting in ROS production and ATP synthesis impairment [68]. This mechanism explains the elevated ROS levels associated with radiation-induced genomic instability [69]. It is a long-term, delayed effect of ionizing radiation that occurs even in unirradiated progenies of irradiated cells. As previously mentioned, the superoxide anion-mediated bystander effect is when non-irradiated cells exhibit a phenotype typically associated with radiation exposure [69].
Cellular protection mechanisms against ionizing radiation
Radiation type/dose, cellular proliferation status, antioxidant efficacy, and repair mechanisms all influ-ence cellular response to radiotherapy. Thus, Mn-SOD appears to play a critical role in protecting normal cells from radiation-induced ROS injury at the mitochondrial level [70]. Furthermore, antioxidant therapy can prevent both short- and long-term radiotherapyinduced cell injury [71]. Antioxidants may simultaneously induce radioresistance in cancer cells, reducing radiotherapy efficacy. Recent research supports the benefits of specific antioxidants that selectively induce apoptosis in cancer cells but not normal cells by increasing oxidative stress or synchronizing tumor cells to a radiosensitive phase of the cell cycle [71,72]. The opportunities lie in identifying radio sensitizers, which act differently in cancer and normal cells. The induction of HO-1 by p53 is associated with a rescue mechanism against the deleterious effects of radiotherapy in bystander healthy tissue. HO-1 inhibits apoptosis via heam degradation products, such as the potent antioxidant bilirubin. Autophagy has been shown to protect both normal and cancer cells from the harmful effects of ionizing radiation by removing damaged cellular elements [73]. Low-level radiation below antineoplastic doses may be beneficial or, at the very least, neutral to health. Chronic low-level radiation exposure, according to the radiation hormesis theory, protects cells from radiation injury, apparently resulting in improved health. This hypothesis is supported primarily by in vitro tests and a few epidemiological studies, all of which are still questionable due to limited data and short-term radiation exposure. Although radiation hormesis has been rejected by national and international radiation regulatory councils, the distinct effects of substances and radiation at low and high doses remain an exciting area of research that requires adequate experimental models and the collection of reliable data [74].
ROS-mediated processes in photodynamic therapy
Photodynamic therapy (PDT) is a relatively new treatment for a variety of cancers as well as non-malignant diseases [75]. PDT is divided into two steps. (1) A non-toxic photosensitizer is selec-tively taken up by tumor cells. (2) The photosensitizer is then locally excited by irradiation with mon-ochromatic light of the appropriate wavelength and undergoes a series of photooxidation reactions [76]. A large amount of ROS is produced, which causes tumor cell death. As a result, if the photosen-sitizer is not cytotoxic on its own, PDT is a highly tumor-targeted therapy with few side effects. Singlet oxygen is thought to underpin the ROSmediated therapeutic effect of PDT, while other ROS produced concurrently appear to be less involved [77]. Light excited the photosensitizing molecule to an excited singlet state, which may then undergo inter-system crossing, resulting in a relatively longlasting triplet state. This triplet state can either exchange an electron or a hydrogen atom with neighboring molecules (type I photochemical reaction) or transfer energy to molecular oxygen (type II photochemical reaction). Singlet oxygen is a reactive oxygen species that interacts with proteins, nucleic acids, and lipids. Because singlet oxygen has a short lifetime within the cell and can migrate in tissues less than 20nm after formation, the induced injury is localized near the singlet oxygen production site [78]. The biological effects of PDT are highly dependent on tumor cell type and evolution stage, molecular oxygen availability, and cancer cell-intrinsic oxidative stress. In cancer cells exposed to PDT, various ROS-mediated signaling cascades are activated, which results in adaptive or cell death responses. In contrast to radiotherapy and chemotherapy, which cause a slow host response, the stress and damage caused by ROS in PDT-treated tumors cause a rapid and massive release of danger signals (oxidative stress, release of intracellular components), which are quickly recognized by the host as an acute localized insult [79]. PDT causes a molecular interaction of cell death pathways, balancing apoptosis, necrosis, and autophagy. Apoptosis is triggered if the photosensitizer is found in mitochondria. When photosensitizers bind to the plasma membrane, singlet oxygen causes tumor cell necrosis [80]. PolyADP-ribose polymerase regulates the transition from apoptosis to necrosis in PDT-treated tumors and the associated inflammatory response. A lot of effort is being put into developing new photosensitizers right now. Synthetic variants are preferred because they allow for a clear relationship between photosensitizer and biological effects. Modified porphyrins, chlorins, bacteriochlorins, phthalocyanines, naphthalocyanines, pheophorbides, and purpurins, as well as metabolic precursors, such as aminolevulinic acid, have been developed as the “second generation” of photosensitizers [81]. They are intended to penetrate deeper into cancer tissues due to improved photophysical properties (higher activation wavelengths) and be more efficient at producing singlet oxygen. Other properties of photosensitizers, such as aggregation, ionic charge, solubility, and partition between aqueous and lipid phases, play an important role in PDT by enhancing photosensitizer uptake by cancer cells, tumor selectivity, and short-term retention in cells and tissues. Despite the disadvantages of photosensitization and light accessibility at the tumor site, PDT remains a promising antineoplastic treatment due to its high tumor-targeting action and low side effects [82].
ROS and chemotherapy
The majority of anti-neoplastic agents (antifolates, nucleoside and nucleotide analogs, vinca alkaloids, taxanes, etoposide, and camptothecins) have well-established mechanisms of action that do not involve free radical intermediates or free radical generation [83]. Meanwhile, it is widely accepted that anthracyclins, most alkylating agents, and platinum coordination complexes exert anti-tumor activity or toxic side effects by causing oxidative stress. The hepatic microsomal monooxygenase system, xanthine oxidase, Fenton and Haber–Weiss reactions, and the mitochondrial electron transport chain are all involved in drug-induced oxidative stress [84]. Drug-induced oxidative stress superimposes on the intrinsic one in cancer cells, resulting in potent ROS-mediated cytotoxic processes that preferentially kill tumor cells or inhibit their proliferation. Novel therapeutic strategies capitalize on oxidative stress and specific redox reactions. Acridine-based anticancer drugs that bind to DNA via intercalation and either donate or accept electrons from DNA showed promising results. As a result, these drugs participate in long-range electron transfer reactions and have anti-neoplastic activity [85]. We will then focus on specific issues concerning ROSgenerating anti-neoplastic chemotherapies and their interactions with cellular redox balance modulators.
Endogenous antioxidants as pro-oxidants in tumors treated with chemotherapeutics
It has been demonstrated in vitro that anti-neoplastic therapies may benefit from the co-administration of specific antioxidants capable of improving anti-tumor activity or reducing side effects [86]. The therapeutic approach of combining oxidative stress-inducing agents with specific an-tioxidants is gaining popularity and validity in cancer treatment, but the interference of endogenous antioxidants with chemotherapy remains a point of contention. Because of its antioxidant activity, it was found that thioredoxin can confer resistance to ROS-generating anticancer drugs on cancer cells. In contrast, Ravi et al. (2005) [87] demonstrated that thioredoxin can enhance daunomycin cytotoxi-city in human breast carcinoma MCF-7 cells, revealing surprising pro-apoptotic and even pro-oxidant activities of thioredoxin in cancer cells. Daunomycin exerts anti-tumor effects by undergoing redox cycling, which causes ROS generation and the formation of semiquinone radicals, both of which cause DNA damage and tumor cell apoptosis [88]. Thioredoxin has been shown to increase daunomycin cytotoxicity through two mechanisms: (1) increased drug-induced superoxide radical generation via the NADPH-oxidase pathway, which requires thioredoxin overexpression; and (2) thioredoxins provide reducing equivalents for the drug’s redox cycling toward the DNA intercalating semiquinone radical. Thioredoxin action may become pro-apoptotic and pro-oxidant in cancer cells treated with specific chemotherapeutics, according to these findings. Meanwhile, even when treated with anti-neoplastic agents, thioredoxin retains its antioxidant and anti-apoptotic properties in normal cells [89]. The nature of the oxidative burst (superoxide anion versus hydrogen peroxide), critical amounts of ROS, the cellular intrinsic oxidative status, apoptotic machinery, and the action mechanism of chemotherapeutics on the one hand, and the action mechanism of chemotherapeutics on the other, may dictate the action of the thioredoxin system as pro- or antioxidant and pro- or anti-apoptotic.
Synergy between ros-inducing chemotherapies and exogenous antioxidants
Arsenic trioxide is being used to treat multiple myeloma. Arsenic trioxide inhibits the promyelocytic leukemia-retinoic acid receptor alpha gene, which is the product of the APL chromosomal transloca-tion [90]. Furthermore, by interfering with the mitochondrial electron transport chain, arsenic trioxide causes a significant accumulation of ROS and oxidative stress in leukemia cells. The intracellular redox status appears to be important in predicting a tumor cell’s response to arsenic trioxide, which accounts for the drug’s good therapeutic efficacy in APL but not other cancers [91]. Redox regulatory factors that are commonly regarded as antioxidants have been shown to enhance arsenic trioxide’s anti-neoplastic activity by exerting pro-apoptotic and pro-oxidant effects. Thus, ascorbic acid can increase the cytotoxicity of arsenic trioxide by producing hydrogen peroxide, which raises the oxidative stress induced by arsenic trioxide above a critical threshold in multiple myeloma [92]. Electron para-magnetic resonance (EPR) tests show that Trolox can also be changed by arsenic trioxide producing a phenoxyl radical that may be carcinogenic. Arsenic trioxide extends the life of this dangerous radical by inhibiting Coenzyme Q reductase. Trolox, unlike other ROS-producing chemotherapeutic drugs like doxorubicin, cisplatin, and etoposide, does not increase apoptosis [93]. Trolox protects in vitro pe-ripheral blood mononuclear cells from arsenic trioxide’s toxic attack. As a result, Trolox has the ad-vantage of increasing the killing of specific tumor cells treated with arsenic trioxide while maintaining anti-tumor immune defense mechanisms. Despite being designed as an antioxidant, Trolox acts as a pro-oxidant and pro-apoptotic agent in certain cancer cells while retaining its protective antioxidant effects in normal cells [94].
ROS-generated repurposed drugs for cancer treatment
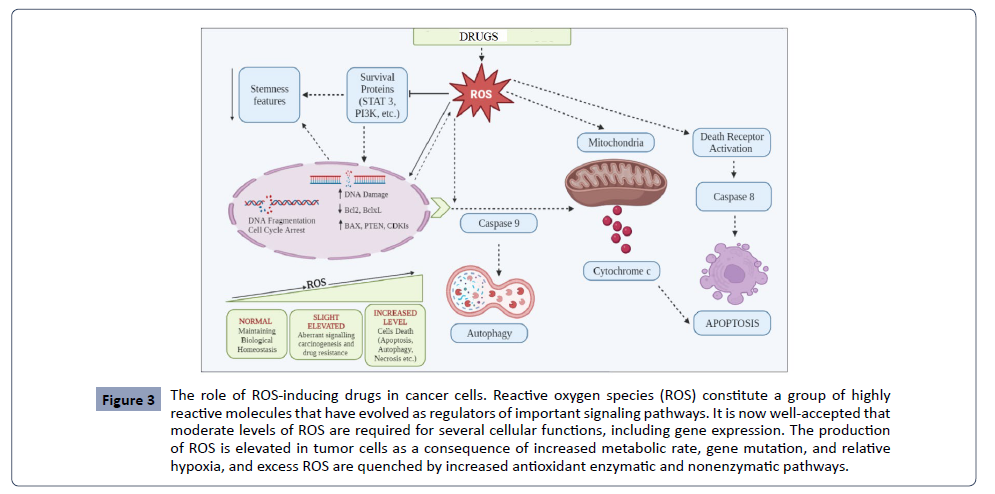
The drug during metabolism interact with the mastermind cytochrome P450 (CYP450) enzyme in phase I. CYP contains heme moieties, which help with oxidizing lipophilic drugs and converting them into hydrophilic molecules (Figure 3). Catalytic oxidation of a drug by CYP causes a loss of electrons from the drug and makes it in active drug metabolite. The second phase of drug metabolism proceeds through glucourodination, which glucouronosyltransferase (UGT) catalyzes. During this process, glucuronic acid binds to the substrates’s functional group via covalent linkage. The drug detoxification process combines several enzyme activities like glutathione-s-transferase and NADPH, quinone oxidoreductases to reduce oxidative stress. ROS are the byproducts of a drug’s metabolism [95]. Understanding the role of the paradoxical nature of ROS toward normal cells and cancer cells, several ROS-inducer drugs, such as antibacterial, anthelmintic, antimalarial, cardiovascular, and antipsychotic, have been repurposed for the treatment of different types of cancers [96]. Most of them have been tested for clinical trials as anticancer drugs. These drugs either interfere with the intracellular antioxidant system of tumor cells or reshuffle their metabolic pathways using ROS as signaling molecules for killing cancer cells [96].
Figure 3: The role of ROS-inducing drugs in cancer cells. Reactive oxygen species (ROS) constitute a group of highly reactive molecules that have evolved as regulators of important signaling pathways. It is now well-accepted that moderate levels of ROS are required for several cellular functions, including gene expression. The production of ROS is elevated in tumor cells as a consequence of increased metabolic rate, gene mutation, and relative hypoxia, and excess ROS are quenched by increased antioxidant enzymatic and nonenzymatic pathways.
Antibacterial
Antibiotics are known to treat microbial infections. In the recent repurposing approach of drugs to treat other ailments, some of the antibiotics are tested for cancer management [97]. This is because they may target DNA non-specifically, like doxorubicin, daunorubicin, idarubicin, etc. Secondly, the intestinal microbiome may modulate the anticancer immune system [98]. Thirdly, mitochondria, sim-ilar to prokaryotic cells, are essential for energy production and apoptosis control. One of the mechanisms of ROS inducing molecules to treat cancer by mitochondrial dysfunction in cancer cells is re-ported by most researchers [99].
Doxorubicin (DOX): Doxorubicin is a broad-spectrum antibiotic obtained from the streptomyces peucetius microorganism. It comes under the anthracycline group of therapeutic agents. It has been widely redirected to treat breast cancer [100]. Pharmacologically, it works by three different mechanisms: (i) by inserting into a DNA strand and inhibiting DNA replication and (ii) by acting as an enzyme topoisomerase II enzyme inhibitor II, which is necessary for DNA replication, by generative oxidative stress in cancer cells through the free radical formation. In every aspect, it is known to kill tumor cells. The msetabolic pathway of doxorubicin initiates ROS production. The phase I/II clinical trial of DOX has been completed (NCT02888665) with Pembrolizumab [101]. In the presence of NADH, its quinone moiety converts into semiquinone through a one-electron reduction mechanism in the mitochondrial electron transport chain system by complex 1. The generated semiquinone moiety reacts with molecular oxygen and forms superoxide radicals. Dox transforms back to its original state of quinone. This cyclic mechanism generates a massive amount of superoxide radicals, which produce a variety of ROS/ RNS species [102]. Some endogenous enzymes, such as reductase and nitric oxide synthase, help in the catalytic redox cycle of dox. Several enzymes like cytochrome P450, aldo/keto reductase, and carbonyl reductase play crucial roles in the metabolism of dox. Five metabolites in which dox transforms are DOXol, DOXsemiquinone, DOX-hydroxyaglycone, Dox-deoxyaglycone, and Doxol-aglycone. Some of the metabolites induce cardiotoxicity. Therefore, efforts have been made to perform a targeted delivery of dox with different dendrimer nanocarrier systems to enhance its efficacy and reduce toxicity [103].
Tigecycline: Tigecycline is an FDA-approved broad-spectrum antibiotic for multidrug-resistant microbial infections. It belongs to the glycylcycline class and is structurally similar to tetracycline antibiotics [104]. Repurposing this drug for cancer treatment is due to it elevating ROS levels in a dose-dependent manner in malignant lung cells, which alters mitochondrial membrane potential and adenosine triphosphate (ATP) levels. Simultaneously, the antibiotic’s mechanism of action is also documented to interfere with signaling pathways and targets such as PI3/AKT, AMPK-mediated mTOR, MYC, HIFs, and Wnt/β- signaling pathways to curb cancer. It is reported to kill leukemic stem and progenitor cells selectively [104]. Tigecycline showed anticancer potential foraccaptable various solid tumors, like gastric cancer, oral squamous cell, melanoma, neuroblastoma, and glioma tumors. Screening of tigecycline against gynecological cancer also provided significant results. Synergistically with cisplatin, the drug can inhibit hepatocellular carcinoma by inducing mitochondrial dysfunction. The completion of the phase 1 clinical trial (NCT01332786) of tigecycline to cure acute myeloid leukemia can be found on the NCI website. Tigecycline is metabolized via glucouronidation and amide hydrolysis followed by N-acetylyation to form N-acetyl-9-aminominocycline [105].
Doxycycline (DOXY): Another broad-spectrum tetracycline antibiotic, doxycycline, is reported to have anticancer proper-ties [106]. It is effective against breast, lung, cervical, and prostate cancer when considered a repur-posed drug [107]. A further study mentioned that doxycycline could be effective in glioblastoma by inducing oxidative stress and interfering in mitochondrial oxidative phosphorylation [108]. ROS-induced apoptosis via the signal-regulating kinase (ASK-1) Jun N-terminal kinase (JNK) pathway was found in doxycycline treatment to inhibit melanoma [109]. Through laser irradiation therapy, doxycycline produced ROS in situ, which helped to treat osteosarcoma. Doxy has been tested for treating renal cell carcinoma. Doxy-loaded nanoparticle formulation is tested on mice models to en-hance their efficacy and targeted delivery [110]. Furthermore, clinical trials (NCT02874430) of doxy with metformin are underway for controlling breast and uterine cancer. Another clinical trial (NCT02341209) for treating cutaneous T-cell lymphoma is continuing.
Anthelmintics
These drugs are used to treat infections caused by parasites in the gastrointestinal system. They expel parasitic worms and other intestinal parasites from the body. Some medications, like mebendazole, albendazole, and niclosamide, are repurposed for cancer treatment due to their ability to alter crucial cell signaling pathways via ROS induction and their role in mitochondrial dysfunction [111].
Niclosamide: Niclosamide is an FDA-approved antiparasitic drug with a history of 50 years of treating parasitic in-testinal infections [112]. It has been directed for cancer treatment to interfere in oncogenic signaling pathways like the NF-κB pathway and elevate ROS levels to induce apoptosis [113]. Recently, it has been documented that it can enhance the sensitivity of tumor cells for chemo and radiotherapy by controlling redox homeostasis. The drug also reduces renal carcinoma proliferation by inhibiting the Wnt/β-catenin signaling pathway and causing mitochondrial malfunction [114]. Niclosamide was re-ported to enhance the sensitivity of paclitaxel for cervical cancer cells through the ROSinduced mTOR inhibition mechanism [115]. Moreover, it was also reported to have a radiosensitizing effect on H1299 human lung cancer cells. An additional study demonstrated that niclosamide with gam-ma-ionizing radiation promotes c-Jun pathways and phosphorylation [116]. Further investigation in triple-negative breast cancer cells supported the ROS-based radiosensitizing nature of the drug in the signal transducer and activator of transcription 3 (STAT3) and B-cell lymphoma-2 (Bcl-2) inhibition [117]. The combination therapy of niclosamide with valproic acid showed a reduction in the cell via-bility of human lung cancer cells. The mechanistic pathway involved extrinsic apoptotic induction, endoplasmic reticulum stress activation, and loss of mitochondrial membrane potential [118]. The clinical trial (NCT02532114) of niclosamide with enzalutamide on prostate cancer patients was com-pleted.
Lu et al. demonstrated niclosamide drug metabolism by enzyme cytochrome 450 and UDP-glucuronosyltransferase using human liver microsomes (HLM) and found that NADPH-supplemented HLM generated 3-hydroxy niclosamide. UDPA-supplemented HLM mono-O-glucuronide [119].
Albendazole and Mebandazole: Both antihelminthic drugs have been thoroughly screened for their anti-tumor activity [120]. But their clinical trials could not be completed due to a lack of effect (NCT03628079). Hence, we are not discussing their anticancer potential.
Antimalarial: Artimisnin is a natural antimalarial drug. Artemisinin contains an endoperoxide bridge in its pharma-cophore [121]. This peroxide’s cleavage releases ROS, which can cause apoptosis and DNA damage and inhibit cell proliferation [122]. Extensive studies have been conducted to establish its ROS-induced anticancer potential on different kinds of cancer.
Cardiovascular Drugs
Statin drugs, like atorvastatin and simvastatin, are used to inhibit the enzyme 3-hydroxy-3-methylglutarylcoenzyme a (Hmg-CoA), which plays a key role in the synthesis of choles-terol. A high level of cholesterol is risky for coronary disease and atherosclerosis. Hmg-CoA inhibitors are redirected for their anticancer activity [123]. Simvastatin is highly documented and widely used as a drug for cardiovascular disease. It is used to maintain cholesterol by inhibiting 3-hydroxy-3-methylglutarylcoenzyme a (Hmg-CoA) reductase in the mevalonate pathway. The drug is repurposed for anticancer treatment because of its sensitizer potential for chemotherapy and radio-therapy for treating different cancers. Simvastatin alone or synergistically with dox works as an ROS inducer and inhibits breast cancer cell lines MCF7 [124]. Synergistically, pentoxifylline effectively stimulated ERK/AKT, enhanced ROS levels, downregulated p-38, and inhibited the NF- κB signaling pathway in triple-negative breast cancer cell lines [125]. Additionally, simvastatin and atorvastatin enhanced ROS levels in the humanclogiocarcina KKU-100 cells [126]. Simvastatin has been considered for clinical trials for the treatment of various cancers, such as breast cancer (nct00334542) and metformin in bladder cancer (nct02360618). The oral administration of simvastatin in the form of inactive lactone gets hydrolyzed to the active simvastatin acid by carboxyesterase (CES1) and paraoxonase (PON1) [127]. The simvastatin acid acts as an inhibitor of HMG-CoA reductase. Simvastatin acid can be converted back to simvastatin through glucuronidation in the presence of UGT1A1 or UGT1A3. The extensive metabolization of simvastatin and simvastatin acid by CYP3A4/5 converts them to 3-hydroxysimvestatin and 6-hydroxymethyl-simvestatin and -simvastatin acid. Due to the creation of harmful reactive p-quinoneimine intermediates, gene profiling of various statin medications, such as fluvastatin and atorvastatin, revealed alterations in the expression of 857 and 1091 transcripts. However, in the case of simvastatin, only 102 transcripts were differentially expressed due to the lack of nitrogen and an arene ring. These differentially expressed specific genes are related to apoptosis, cell cycle regulation, metabolism, and cell defense [128].
Antipsychotics
The phenothiazine (PTZ) class of antipsychotic drugs is used for the treatment of schizophrenia and anxiety. It has tricyclic phenothiazine with a thiazine ring in the center. Functional groups present in PTZ at the second and tenth positions exert their pharmacological antipsychotic activities. The elec-tronwithdrawing group at the second position and piperazine and piperidine linkage at the tenth po-sition enhance the therapeutic efficiency of PTZ drugs [129]. PTZ alters mitochondrial membrane po-tential, which causes apoptosis.
Fluphenazine is an antipsychotic drug with an antagonistic nature with dopamine D1 and D2 recep-tors and has a strong affinity toward 5-HT receptors. It is used for the treatment of patients with schizophrenia and bipolar disorders [130]. Constructive evidence has shown a reduced rate of cancer in such patients, indicating the anticancer potential of antipsychotic drugs [131]. Screening of tri-ple-negative breast cancer cell lines showed enhanced ROS levels, which could work by impairing the mitochondria membrane potential and ROS-induced cancer cell death [132]. Hela cancer cells showed effective photodynamic mechanisms when treated with fluphenazine and UVA light [133]. A clinical trial (NCT00335647) was also conducted for treating patients with refractory advance multiple myeloma. The fluphenazine metabolism involves sulfoxidation, oxidation, and the elevation of serum aminotransferase in the liver, which produces toxic reactive intermediates, such as ROS [134]. Table 1 shows how drugs producing reactive oxygen species can be used in various antineoplastic therapies and their mechanism of action on tumor cells [135].
| Scope of ROS Treatment |
Application of ROS |
Action on Tumor Cells |
References |
| ROS-mediated processes in radiotherapy |
Anti-neoplastic treatment based on ROS. |
Sensitivity of tumor cells toward radiation. |
[64,65] |
| Ionizing radiation-induced damages |
Elevated ROS levels associated with radiation-induced genomic instability. |
Severely damage mitochondrial DNA, altering the expression of various genes that encode critical proteins. |
[67] |
| Cellular protection mechanism against ionizing radiation |
Normal cells get effected by ROS. |
Mn-SOD appears to play a protective role for normal cells. |
[73] |
| ROS-mediated processes in photodynamic therapy |
A non-toxic photosensitizer is used for ROS production in cancer cells. |
PDT causes a molecular interaction of cell death pathways, balancing apoptosis, necrosis, and autophagy. |
[79] |
| ROS in chemotherapy |
Drug-induced oxidative stress superimposes on the intrinsic one in cancer cells, resulting in potent ROS-mediated cytotoxic processes that preferentially kill tumor cells. |
Acridine-based anticancer drugs bind to DNA via intercalation and either donate or accept electrons from DNA to perform anti-neoplastic activity. |
[85] |
| Endogenous antioxidants as pro-oxidants in tumor treatment |
Endogenous antioxidants are supportive for the prevention of cancer but suffer contention due to ROS scavenging ability. |
Thioredoxin can confer resistance to ROS-generating anticancer drugs on cancer cells. |
[89] |
| ROS-generated repurposed drugs for cancer treatment |
ROS is the byproduct of the drug’s metabolism. |
Catalytic oxidation of the drug by CYP450 causes a loss of electrons from the drug and makes it inactive drug metabolite. |
[96] |
| Antibacterial drugs |
Some of the antibiotics are tested for cancer management as they target DNA non-specifically. |
Doxorubicin is widely directed to treat breast cancer. Phase I/II clinical trial dox has been completed, (NCT02888665) with Pembrolizumab. |
[97–99] |
| Completion of the phase 1 clinical trial (NCT01332786) of tigecycline to cure acute myeloid leukemia can be found on the NCI website. |
| Anthelmintics |
Some medications, like mebendazole, albendazole, and niclosamide, are repurposed for cancer treatment by altering crucial cell signaling pathways via ROS induction and their role in mitochondrial dysfunction. |
Niclosamide reduces renal carcinoma proliferation by inhibiting the Wnt/β-catenin signaling pathway and causing mitochondrial malfunction. |
[111] |
| The clinical trial (NCT02532114) of niclosamide with enzalutamide on prostate cancer patients was completed. |
| Antimalarial drugs |
Antimalarial drug. Artemisinin contains an endoperoxide bridge in its pharmacophore. This peroxide’s cleavage releases ROS. |
DNA damage, apoptosis, and inhibition of cell proliferation. |
[122] |
| Cardiovascular drugs |
Repurposed for anticancer treatment because of its sensitizer potential for chemotherapy and radiotherapy for treating different cancers. |
Simvastatin alone or synergistically with dox works as an ROS inducer and inhibits breast cancer cell lines MCF7. |
[127] |
| Simvastatin has been considered for clinical trials for the treatment of various cancers, such as breast cancer (NCT00334542) and metformin in bladder cancer (NCT02360618). |
| Antipsychotic drugs |
The electron-withdrawing group at the second position and piperazine and piperidine linkage at the tenth position enhance the therapeutic efficacy of PTZ drugs. |
A clinical trial (NCT00335647) was also conducted for treating patients with refractory advance multiple myeloma. |
[129] |
Table 1. Summary of the ROS in anti-neoplastic therapies.
Conclusion
Cancer is a chronic disease, and different modes of treatment are developed to combat the disease. A detailed transcriptome and proteome analysis provided several benefits. In recent research, the role of mitochondria in the generation of ROS has pioneered an effective route for cancer therapy. Understanding the mechanisms and consequences of tumor redox balance has accelerated the development of potentially effective ROS mediated therapies. Many ROS-generating drugs, such as doxorubicin, niclosinamide, fluphenazine, and simvastatin, have given promising results in cancer clinical trials. But patients have to compromise the toxicity of these drugs during drug metabolism. The double-edged sword behavior of ROS may initiate several unknown complicated mechanisms in our bodies. However, targeted delivery using the potential delivery system is one of the approaches but not the complete solution for this complicated uncontrolled mitosis. Therefore, a better understanding of ROS contributions and redox vulnerabilities should be considered for the development of ROS-mediated therapies to overcome treatment resistance and prevent disease recurrence and progression.