Keywords
(eNOS), Mn(SOD), Polymorphism, Diabetic nephropathy
Introduction
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels [1].
Diabetic nephropathy is the leading cause of chronic renal failure. About 30–40% of all diabetic patients develop diabetic nephropathy putting them at high risk for end-stage renal disease (ESRD) and, in parallel, for severe micro and macro vascular complications [2]. Although type 1 and type 2 diabetes mellitus (insulin-dependent diabetes mellitus [IDDM] and non insulin dependent diabetes mellitus [NIDDM], respectively lead to ESRD, the great majority of patients are those with NIDDM [3]. Several studies have suggested that reactive oxygen species (ROS) are implicated in the etiology of type 1 diabetes [4]. Excess generation of ROS such as super oxide (O2), hydrogen peroxide (H2O2), hydroxyl radical (OH) and reactive nitrogen species such as nitric oxide oxidizes target cellular proteins, nucleic acids, or membrane lipids and damage their cellular structure and function [5]. Hyperglycemia stimulates the expression of nitric oxide synthase (NOS) and increases the production of nitric oxide, an intracellular second messenger. Increased nitric oxide generation favors the formation of peroxynitrite, a highly reactive oxidant. Antioxidant enzymes such as manganese superoxide dismutase (MnSOD) and endothelial nitric oxide synthase (eNOS) directly eliminate ROS [6]. The susceptibility of nitric oxide to nephropathy and cardiovascular disease depends to some extent on genetic factors, therefore, polymorphisms in the gene coding for endothelial nitric oxide synthase 3 (NOS3) can affect the risk of developing these diseases [7]. Hohenstein et al. [2] have reported the increased eNOS expression by the renal endothelium in type 2 diabetic nephropathy.
Mn(SOD) encoded by the superoxide dismutase (SOD2) gene, is translocated into the mitochondrial matrix where it scavenges superoxide radicals. A valine/alanine polymorphism has been identified in the targeting sequence of SOD2, and an in vitro study shows that valine (Val) instead of alanine (Ala) results in less efficient transport of MnSOD into the mitochondrial matrix and can compromise the ability to neutralize superoxide radicals in the cell [8]. In a Danish cohort of type 1 diabetes patients, it was concluded that MnSOD V16A polymorphism is involved in the development of nephropathy caused by type1 diabetes and seems to predict cardiovascular disease during follow up [7].
Smoking and homozygosity for the Mn(SOD) Val allele was found to be associated with an increased risk of diabetic nephropathy, which supports the hypothesis that oxidative stress contributes to the development of diabetic nephropathy. Mn(SOD) protects the cells from oxidative damage by scavenging free radicals. The demand for antioxidants is increased by smoking, which could disturb the balance between antioxidants and reactive radicals [9].
The overall aim of this study was to determine the relationship of eNOS and manganese superoxide dismutase Mn(SOD) gene polymorphisms in type 1 diabetic patients with nephropathy and end-stage renal disease in Pakistani population.
Material and Methods
Study subjects
The diagnosis of type 1 diabetes in patients was established by the medical experts of Shaikh Zayed Hospital, Lahore. The subjects had diabetic nephropathy history of 4-6 years. Patients having albumin excretion of >300 mg/24 h in two out of three consecutive urine samples, presence of retinopathy and absence of other kidney or urinary tract diseases were defined as diabetic nephropathy cases [10]. The study included ESRD patients who were maintained on hemodialysis at dialysis centers in Shaikh Zayed Hospital, Lahore for 1.5 to 2 years. The information about occupation and diabetic nephropathy family history was obtained from each patient. The polymorphism for manganese superoxide dismutase Mn(SOD) and endothelial nitric oxide synthase (eNOS) was compared with sex and age-matched healthy volunteers (control) who visited the hospital along with their patients for their treatment during this study period. The average time from the commencement of ESRD to the time of this study was 3.8-5.5 years. The creatinine clearance rates of ESRD patients were 20.5±13.6 mL/min and were hemodialysed 1-3 times in a week. The patients had a mean age of 50.3 ± 10.5 years. Informed consent was obtained from all patients and healthy volunteers participating in this study.
Extraction of DNA from Peripheral Blood
Peripheral blood from each patient was collected in EDTA vacutainer tubes and stored at -20°C. Genomic DNA was isolated from peripheral blood using conventional phenol-chloroform method. In an eppendorf tube, 0.75 mL blood and 0.75 mL of solution A (0.32 M sucrose, 10 mM Tris HCl (pH7.5), 5 mM MgCl2, Triton X100 1% v/v) were mixed by inverting the tubes 4 to 6 times and left at room temperature for 5 to 10 min. The supernatant obtained after centrifugation was discarded and resuspended the pellet in 400 μL of solution A. The nuclear pellet obtained after centrifugation was resuspended in 400 μL of solution B (10mM Tris HCl (pH 7.5), 400mM NaCl, 2Mm EDTA, 12 μL of 20 % SDS and 5 μL of protinase K). The mixture was incubated at 37°C for overnight.
Five hundred μL of solution C (phenol pH 8.0, 0.1% hydroxyquinoline, 0.1MTris HCl (pH 8) and solution D (chloroform, isoamyl alcohol; 24:1) was added, inverted and centrifuged at 13,000rpm for 10 min. The upper phase was collected in a new tube and added equal quantity of solution D. The aqueous phase (upper layer) obtained after centrifugation was transferred in a new tube and DNA was precipitated by adding 55 μL of sodium acetate and equal volume of isopropanol. Tube was inverted gently to precipitate DNA. The DNA pellet was washed, dried, dissolved in 150 μL of autoclaved distilled water and stored at 4°C.
PCR-RFLP analysis of Mn (SOD) gene
Manganese superoxide dismutase Mn( SOD) gene, containing Age I restriction site, was amplified from genomic DNA by polymerase chain reaction (PCR). The forward and reverse primer sequences were as follows: 5′-CCAGCAGGCAGCTGGCACCG-3′ and 5′-TCCAGGGCGCCGTAGTCGTAGG-3′, respectively. Amplifications were carried out in a total reaction volume of 25 μL containing 20 ng genomic DNA, 50 pM of each primer, 2 mM dNTPs, 2 mM MgCl2 and 5 U Taq polymerase (Fermentas). PCR was performed in a thermalcycler (Bio-Rad, Inc., USA) under the following conditions: initial step of denaturation at 95°C for 5 min, followed by 35 cycles at 94°C for 60 seconds, 60°C for 45 seconds (annealing) and at 72°C for 45 seconds (extension). PCR was completed by a final extension cycle at 72°C for 10 min. For RFLP analysis, 10 μL of PCR product, 19 μL of PCR water, 2 μL of buffer 0 and 2 μL of Age I restriction endonuclease (1U/μL) were mixed and incubated at 37°C for 16h. Ten μL of digested DNA fragments were electrophoresed on a 3% agarose gel and visualized by ethidium bromide staining.
PCR-RFLP analysis of eNOS gene
Intron of eNOS gene fragment was amplified by polymerase chain reaction with the forward and reverse primers 5'-AGGCCCTATGGTAGTGCCTTT-3' and 5'-TCTCTTAGTGCTGTGGTCAC-3', respectively (Fermantas). The PCR amplification was carried out with 20 ng DNA, 2 mM MgCl2, 2 mM dNTPs, 50 pM of each primer and 5 U of Taq polymerase in a total volume of 25 μL. Amplification was performed with an initial denaturation step at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 2 min. Predenaturation was done at 94°C for 4 min, and final extension for 7 min. Exon of the eNOS gene, which contains Ban II restriction site, was amplified with the following primers: forward 5'-TCCCTGAGGAGGGCATGAGGCT-3' and reverse: 5'- TGAGGGTCACACAGGTTCCT-3'. Amplification was carried out in a total reaction volume of 20 μL containing 20 ng genomic DNA, 50 pM of each primer, 2 mM dNTP, 2 mM MgCl2 and 5 U of Taq polymerase. Cycling conditions were denaturation at 94°C for 1 min, annealing at 71°C for 45 sec, and extension at 72°C for 45 sec. Final extension cycle was performed at 72°C for 10 min. For RFLP detection, 33 μL of reaction mixture was prepared by mixing 10 μL of amplification product, 2 μL of restriction endonuclease (1U/μL), 2 μL of buffer O and 19 μL PCR water. The mixture was incubated at 37°C for 18 h and an aliquot of 5 μL of digested mixture was analyzed by electrophoresis on a 3% agarose gel containing 0.5 μg/mL ethidium bromide as fluorescent tag and photographed.
Statistical Analysis
SPSS version 19 (Statistical Software) was used for the statistical analysis. X2 test was performed to determine the association and significance of different genotypes. X2 test was applied on allele and genotype frequencies between patient’s group and controls for the association study. The Probability value of P<0.05 was considered as statistically significant.
Results
RFLP for Screening of Mn(SOD) mutation
Genotypes, age group and gender of patient groups and control were examined using X2 test. X2 test was used to find out the association between two variables. Table 1 presents genotype and allele frequencies for Mn-SOD. No association was observed between ESRD patients group and controls in terms of gender as shown by statistical analysis result (X2 = 7.424; d.f. = 1; (p = 0.064) but significant difference was found in the age distribution between patients group and control as (X2 = 7.111; d.f. = 1; (p = 0.01)). It was obvious from the data that patients falling between the ranges of 45-70 year faced increased risk of disease.
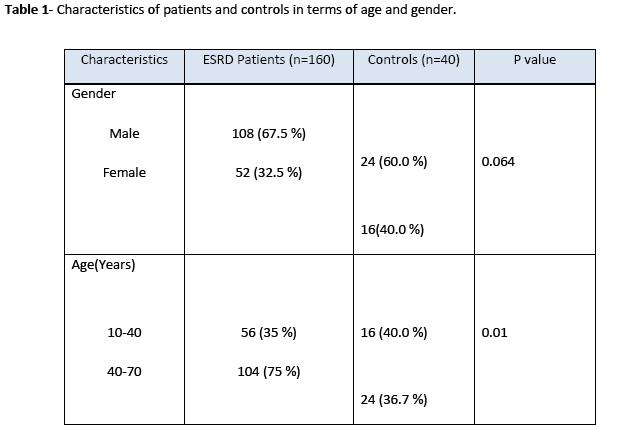
The amplified product of Mn(SOD) (91bp) on treatment with Age I restriction enzyme produced three bands of 91 bp, 74 bp, and 17 bp size indicating that samples were heterozygous for Val and Ala as shown in Fig. 1. However, lane 3, 4 and 6 showed a single band of 91 bp demonstrating that subjects were homozygote for Ala. Lane 7 revealed two bands at 74 bp and 17 bp positions and found to be homogenous for Val. Genotype distribution of Mn(SOD) gene was not significantly different from controls but the restriction pattern was dissimilar in diabetic nephropathy patients and healthy subjects (Table 2). Even though, T allele frequency was greater in diabetic nephropathy patients than controls, however, this difference was statistically non significant (p>0.05).
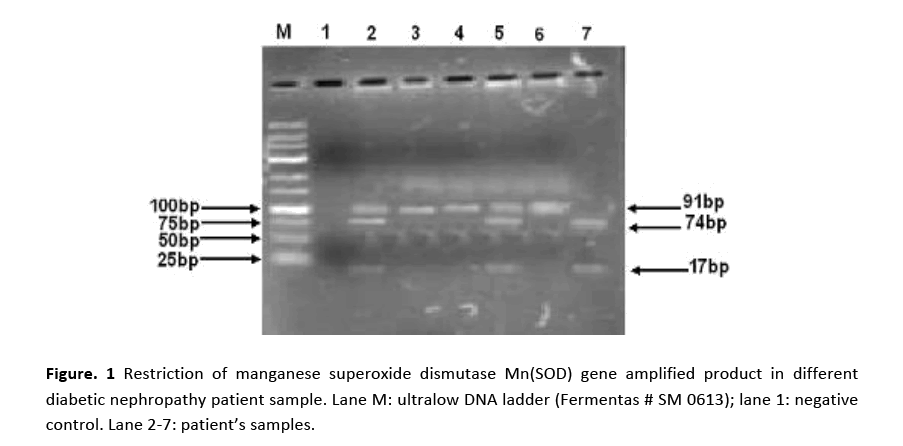
Figure 1: Restriction of manganese superoxide dismutase Mn(SOD) gene amplified product in different diabetic nephropathy patient sample. Lane M: ultralow DNA ladder (Fermentas # SM 0613); lane 1: negative control. Lane 2-7: patient’s samples.
Genotype analysis and allele frequencies for eNOS gene
PCR amplification for eNOS (intron) gene gave two bands of 420 bp and 393 bp molecular sizes are shown in Fig. 2. Amplified product of 420 bp indicates 4b allele (five tandem repeats) while 393 bp 4a allele (four tandem repeats). Samples showing a single band at 420 bp position indicated the presence of 4b allele. Amplification of eNOS (exon) produced a 457 bp fragment in all the patients and the controls as shown in Fig. 3. RFLP assay of amplified product with Ban II restriction enzyme revealed two bands of 320 bp and 137 bp indicating that patients were homozygous for (GG). The appearance of three bands of 457 bp (mutant), 320 bp and 137 bp on agarose gel demonstrated heteroztgosity as shown in Fig. 4.
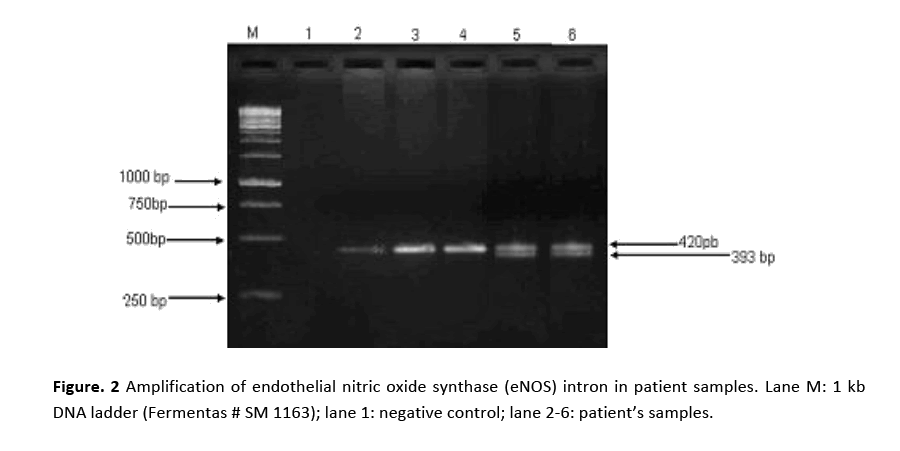
Figure 2: Amplification of endothelial nitric oxide synthase (eNOS) intron in patient samples. Lane M: 1 kb DNA ladder (Fermentas # SM 1163); lane 1: negative control; lane 2-6: patient’s samples.
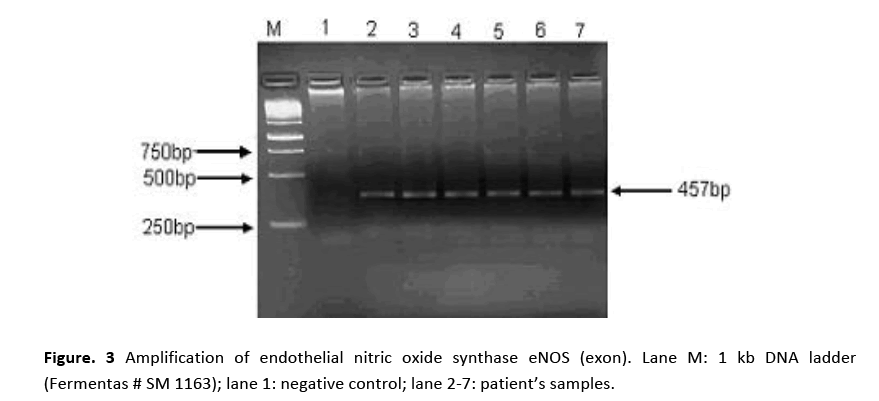
Figure 3: Amplification of endothelial nitric oxide synthase eNOS (exon). Lane M: 1 kb DNA ladder (Fermentas # SM 1163); lane 1: negative control; lane 2-7: patient’s samples.
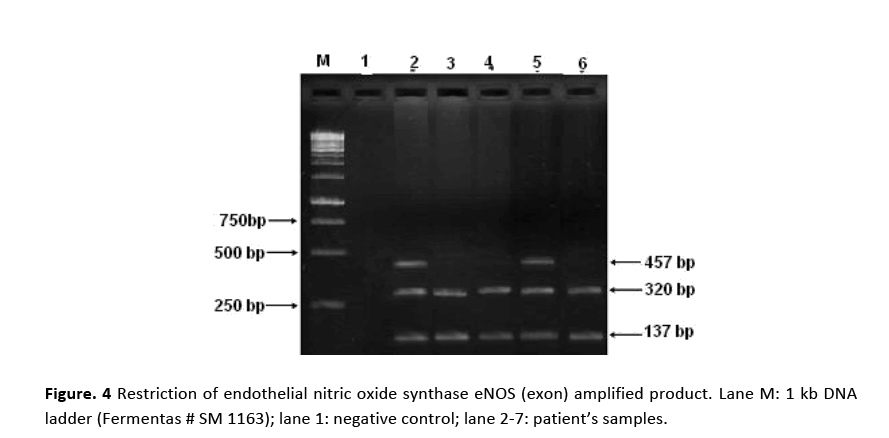
Figure 4: Restriction of endothelial nitric oxide synthase eNOS (exon) amplified product. Lane M: 1 kb DNA ladder (Fermentas # SM 1163); lane 1: negative control; lane 2-7: patient’s samples.
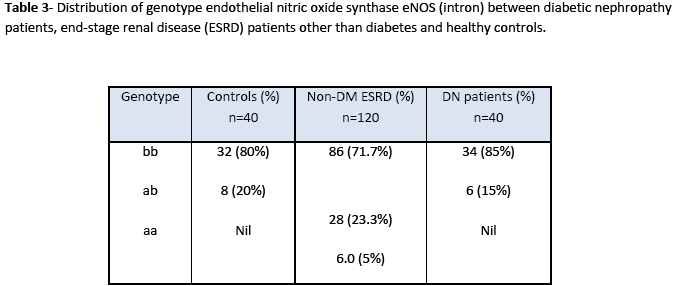
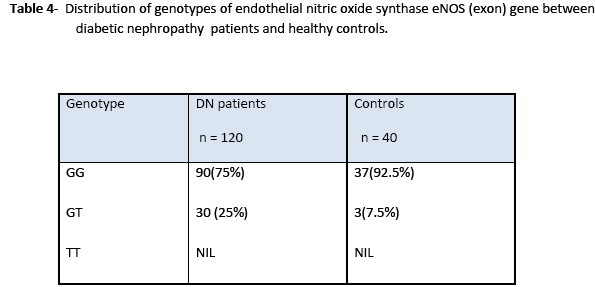
Statistical analysis of genotype distribution of eNOS (intron) gene indicates that there is a significant difference in genotypes (ab) between non-diabetic ESRD patients and control (X2 =13.3; 95%CI; p= 0.01). However, there is no significant difference in genotypes (ab) between diabetic nephropathy patients and control (X2 =0.049; 95%CI; p= 0.656). Heterozygous allele ab was more frequent in non-diabetic ESRD patients as compared to that in diabetic nephropathy patients and healthy controls (Table 3). Statistical analysis of genotype eNOS (exon) gene showed significant differences in genotypes between patients and control groups as heterozygous (df 39; 95%CI (0.055-0.395); p=0.011). Heterozygote G/T allele was present in patients more frequently as compared to that in control while homozygote T/T allele polymorphism was not found in the study samples as shown in Table 4.
Discussion
The association of proteinuria with diabetes was first recognized in the eighteenth century; however, Kimmelstiel and Wilson [11] defined the condition by describing the glomerulosclerosis and the association with proteinuria and hypertension in type 2 diabetes. These features represent a late stage in the progression of the condition. Subsequent work, mainly on type 1 diabetes, led to the definition of several distinct phases in the evolution of the disease [12]. The pathology of diabetic nephropathy is characterized by glomerular basement membrane thickening and mesangial expansion with increased extracellular matrix deposition. Patients with type 1 diabetes are at the highest individual risk of nephropathy, but those with type 2 diabetes are at significant risk [13]. There is evidence that the incidence of renal failure in diabetes type 1 may be decreasing, perhaps due to better preventive treatment [14].
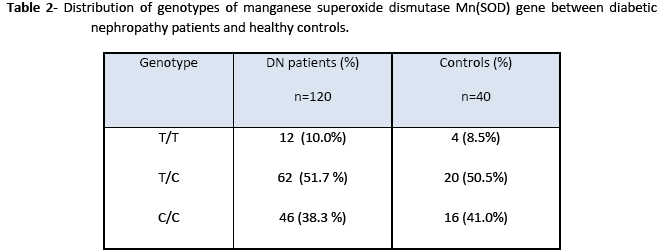
In Val-9Ala polymorphism of Mn(SOD), 91 bp amplified product with the T allele (Val) yielded two fragments of 74 and 17 bp molecular size after digestion with Age I. The PCR product with the C allele (Ala) produced a single fragment, since the amplified product was not cut with the restriction enzyme. For the heterozygosity of Val (GTT) at codon (-9), the restriction of amplified product produced three DNA fragments having molecular sizes of 91 bp, 17 bp and 74 bp. For homozygosity of Val alleles (Val/Val), the digestion resulted in two DNA products, with only one bright band of 72 bp. These observations are in accordance with El Masry et al [15]. Our findings showed that the frequency of Val/Val, Val/Ala, Ala/Ala were 8.5, 50.5 and 41.0% in healthy controls and 10, 51.7 and 38.3% in diabetic nephropathy patients, respectively. There was no significant difference in the frequency of the Ala/Ala and homozygous Val/ Val genotype. The results suggest that the Ala(-9) Val polymorphism in the Mn(SOD) gene is not associated with a risk of the development of diabetic nephropathy. El Masry et al [15] have also reported that in Egyptian diabetic children Ala(-9)Val substitution in the Mn-SOD2 gene is associated with diabetic neuropathy (DN) but not a significant factor in diabetic nephropathy patients. However, an association between Val(-9) Ala polymorphism and schizophrenia suggests that this polymorphism may contribute to neurodegenerative disorders. Mn(SOD) gene may not be involved in the pathogenesis of diabetic nephropathy, but this mutation is more likely to be involved in neurodegenerative disorders [16]. Möllsten et al. [7] have described that in a Danish cohort of type 1 diabetes patients, MnSOD V16A polymorphism is involved in the development of nephropathy caused by type 1 diabetes and seems to predict cardiovascular disease during follow up. On the other hand, Strokov et al. [17] showed that Ala(-9)-Val polymorphism in a Russian population is associated with a high risk of the development of neuropathy in type 1 diabetic patients.
Nitric oxide, an extensively studied endothelium derived relaxing factor is reported to be very potent regulator of intra renal haemodynamics [18]. Nitric oxide is produced from L-arginine by nitric oxide synthase. Two alleles have been identified in intron 4; the one has five tandem 27 bp repeats. The first three repeats have A and the last two have G at the 19 base of the 27 bp repeats, respectively. However, the second allele has only four repeats, in which first two repeats have A and the last two have G at the 19 base of the repeat, respectively. These two alleles were denoted as eNOS4a for four repeats and eNOS4b for five repeats [19]. PCR of eNOS (intron) amplified 420 bp and 39 3bp fragments. Amplified product of 420 bp indicates 4b allele and 393 bp 4a allele. The frequencies of eNOS4b/b, eNOS4b/a, eNOS4a/a were 80, 20 and 0.0% in control group, 71.7, 23.3 and 5.0% in non diabetic ESRD and 85, 15 and 0.0% in diabetic nephropathy patients, respectively. Our study suggests that the frequency of a allele in eNOS intron 4 was significantly higher in non diabetic ESRD patients as compared to that in healthy subjects. These results agree with those of Yokoyama et al. [20] demonstrating that eNOS intron 4 polymorphism is a risk factor for ESRD in non diabetic renal disease. However, Wang et al. [21] have reported that a smoking dependent risk of ischemic heart disease is associated with a polymorphism ecNOS4a/b in intron 4 of the eNOS gene.
In this study, genotype distribution and the allele frequencies of eNOS (exon) showed the percentage of heterozygotes (GT) in diabetic nephropathy patients was 25% as compared to 7.5% in control subjects. The results demonstrate that patients with renal diseases, including diabetes mellitus, with Glu298Asp mutation, are at higher risk. The eNOS (GT) genotype was found to be associated with the rapid deterioration of renal function and a risk factor for ESRD.
These findings reinforce the observation that nitric oxide synthase activity is stimulated during diabetic nephropathy, however, some reports reveal either no or reduced eNOS expression [22]. Our results are supported by several research reports suggesting an association between eNOS polymorphism and advanced diabetic nephropathy [23]. Shin et al. [24] reported that eNOS genotype increases susceptibility to nephropathy and influences the rate of progression in type 2 diabetic nephropathy in Korean patients.
The present study shows an important association of Glu298Asp mutation with diabetes mellitus as a cause of end stage renal diseases and recommends that Glu298Asp variant of the eNOS gene is a susceptible genetic risk factor for the accelerated nephropathy in type 1 diabetes mellitus. Large numbers of studies are available which present data for worldwide association between different genetic variants and diabetic nephropathy. After proper identification of these variants, susceptible diabetic nephropathy gene can be replaced by means of advanced molecular biology techniques. The information regarding these disease vulnerable genes will help to increase the understanding of disease pathophysiology, prognosis and consequently develop potential strategies for their treatment.
Conclusions
Our study suggests that the frequency of a allele in eNOS intron 4 was significantly higher in the patients from non diabetic ESRD as compared to healthy subjects. The frequency of the eNOS 4a allele was also significantly higher in dialyzed patients than in healthy control subjects. Analysis of genotype distribution of eNOS (intron) gene indicates that there is a significant difference in genotypes (ab) between non diabetic ESRD patients and control but there is no significant difference in genotypes (ab) between diabetic nephropathy patients and control. Genotype eNOS (exon) gene showed significant differences in genotypes between patients and control groups.
The study of Ala(-9)Val polymorphism showed that the Ala(-9)Val polymorphism in the Mn-SOD gene is not associated with a risk in the development of diabetic nephropathy.
5310
References
- O'Sullivan JB, Mahan CM. Diagnosis and classification of diabetes mellitus. Am Diabetes Assoc 2009; 32 (Supp. 1):S62-S67.
- Hohenstein B, Hugo CPM, Hausknecht B, Boehmer KP, Riess RH, Schmieder RE. Analysis of NO-synthase expression and clinical risk factors in human diabetic nephropathy. Nephrol Dial Transplant 2008;23(4):1346–54.
- Soman SS, Soman AS. Diabetic nephropathy [Website].- [received on 19 November 2009]- Retrieved March 3, 2011-Available on the internet: < https://emedicine.medscape.com/article/238946-overview >
- Chen J, Gusdon AM, Terri CT, Clayton EM. Role of increased ROS dissipation in prevention of T1D. Ann NY AcadSci 2008;1150:157–66.
- Hovink T, Dolzan V, Bratina NU, Podkrajsek KT, Battelino T. Genetic polymorphisms in genes encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care 2009;32(12):2258–62.
- Ceriello A. New insights on oxidative stress and diabetic complications may lead to a causal antioxidant therapy. Diabetes Care 2003; 26:1589 –96.
- Möllsten A, Lajer M, Jorsal A, Tarnow L. The endothelial nitric oxide synthase gene and risk of diabetic nephropathy and development of cardiovascular disease in type 1 diabetes. Mol Genetics Metabolism. 2009;97(1):80-4.
- Freedman BI, Bostrom M, Daeihagh P, Bwden DW. Genetic factors in diabetic nephropathy. Clin J Am SocNephrol 2007;2:1306-16.
- 9. Mollsten A, Marklund SL, Wessman M, Svensson M, Forsblom C, Parkkonen M, et al. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes 2007;56(1):265-69.
- Parving H-H, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner BM (ed) Brenner and Rector's The Kidney, 7th edn. WB Saunders, Boston MA. 2004: 1777-818.
- Kimmelstiel P, Wilson C. Intracapillary lesions in the glomeruli in the kidney. Am J Pathol 1936;12:83-7.
- Mogensen CE. Microalbuminuria, blood pressure and diabetic renal disease: origin and development of ideas. Diabetologia 1999;42(3):263-85.
- Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int 1999;55(1):1-28.
- Bojestig, M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med 1994;330(1):15-8.
- El Masry TM, Zahra MAMA, El Tawil MM, Khalifa RA. Manganese superoxide dismutase alanine to valine polymorphism and risk of neuropathy and nephropathy in Egyptian type 1 diabetic patients. Rev Diabetic studies 2005;2(2):70-4.
- Akyol O, Yanik M, Elyas H, Namli M, Canatan H, Akin H, et al. Association between Ala (-9) Val polymorphism of Mn(SOD) gene and shezophrenia. ProgNeuropsychopharmacolBiol Psychiatry 2005;29(1):123-31.
- Strokov IA, Bursa TR, Drepa OI, Zotova EV, Nosikov VV, Ametov AS. Predisposing genetic factors for diabetic polyneuropathy in patients with type 1 diabetes: a populationbased case-control study. ActaDiabetol 2003;40(Supp 2):S375-79.
- Zate R, De Nucci G. Effects of acute nitric oxide inhibition on rat glomerular microcirculation. Am J Phystol 1991;261(2 Pt 2):F360-63.
- Wang Y, Kikuchi S, Suzuki H, Nagase S, Koyama A. Endothelial nitric oxide synthase gene polymorphism in intron 4 affects the progression of renal failure in non-diabetic renal diseases. Nephrol Dial Transplant 1999;14(12):2989-902.
- Yokoyama K, Tsukada T, Matsuoka H, Hara S, Yamada A, Kawaguchi Y. High accumulation of endothelial nitric oxide synthase ; a gene polymorphism in the patients with end stage renal disease. Nephron. 1998;79(3):360-1.
- Wang XL, Sim AS, Badenhop RF, McCredie RM, Wilcken DEL. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nature Med. 1996; 2:41–5.
- Schwartz D, Schwartz IF, Blantz RC. An analysis of renal nitric oxide contribution to hyperfiltration in diabetic rats. J Lab Clin Med 2001;137(2):107–14.
- Noiri E, Satoh H, Taguchi J, Brodsky SV, Nakao A, Ogawa Y, et al. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension 2002;40(4):535-40.
- Shin YS, Baek SH, Chang KY, Park CW., Yang CW, Jin DC, et al. Relations between eNOS Glu298Asp polymorphism and progression of diabetic nephropathy. Diabetes Res Clin Practice 2004;65:257–65.













