Amal O. Alotaibi1, Ghada A. Alsowailmi1*, Sara I. Alshahwan1, Afnan A. Alsahli1 andYaser M. Almalik1,2
1King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
2Division of Neurology, Department of Medicine, King Abdulaziz Medical City, Riyadh, Saudi Arabia
- *Corresponding Author:
- Ghada A
Al-Sowailmi, College of Medicine
King Saud Bin Abdulaziz University for Health Sciences
Riyadh, Saudi Arabia
Tel: +966548661011
E-mail: alsowailmi291@ksau-hs.edu.sa
Received Date: January 13, 2020; Accepted Date: May 04, 2020; Published Date: May 11, 2020
Citation: Amal OA, Ghada AA, Sara IA, Afnan AA, Yaser MA (2020) Renal Infarction Following Initiation of Fingolimod. J NeurolNeurosci Vol.11
No.3: 317.
DOI: 10.36648/2171-6625.11.1.317
Keywords
Multiple sclerosis; Fingolimod; Sphingosine-1- phosphate; Renal infarction; Vascular complication
Introduction
Fingolimod is an oral drug usedto treat relapsing remitting multiple sclerosis (RRMS). By interacting withsphingosine-1- phosphate (S1P) receptor subtype 1, fingolimod causes sequestration of lymphocytes and reducestheir infiltration into the central nervous system (CNS), lowering their counts by 73% [1]. Fingolimod delays the progression of neurologic disability in patients with RRMS, reducing the rate by 30%.It was also shown to reduce the frequency of clinical relapses by 54% [1].
In a randomized trial assessing the safety of fingolimod over 4 years, the investigators reported headaches, back pain, influenza, cough, and diarrhea as the most frequent adverse effects, with a reported incidence of 10% or more. Other adverse effects included macular edema (0.4%) and mild hypertension (6.5%). Moreover, bradycardia and atrioventricular conduction blockwereoccasionally observed on theinitiation of treatment [2].
Fingolimod is a relatively new drug, and its long-term side effects remain to be fully described. Herein, we report a potential association between fingolimod use and renal infarction in a 45-year-old Saudi male patient with RRMS, undergoing fingolimod therapy for 32 months.
Case Description
A 45-year-old male with multiple sclerosis diagnosed in July 2015 was treated with 0.5 mg of fingolimod once daily since September 2015. The patient was admitted in April 2018 for the management of right renal infarction diagnosed by an abdominal computed tomography (CT) scan with contrast dye. The CT scan exhibited a right renal cortical infarction, mainly involving themiddle and lower lobes (Figure 1). The patient’s past medical history was unremarkable.
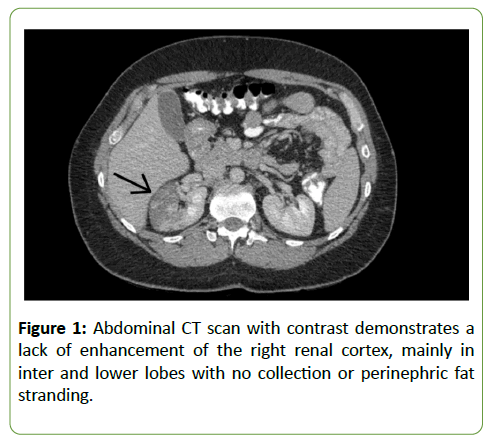
Figure 1: Abdominal CT scan with contrast demonstrates a lack of enhancement of the right renal cortex, mainly in inter and lower lobes with no collection or perinephric fat stranding.
On examination, the patient was found to be conscious, oriented, and hemodynamically stable. The systemic examination was unremarkable, except for mild tenderness in the right iliac fossa. Mild left finger dysmetria (established by finger-to-nose test) was noted and the Expanded Disability Status Scale (EDSS) score was 0.5.
Extensive lab tests were ordered, including hepatic, renal, and thyroid profiles, complete blood count, coagulation profile, and blood chemistry. All investigations were within normal limits. Lupus anti-coagulant test was negative. Protein S activity and activated protein C resistance ratio were normal. The patient was found to be negative for hepatitis B and C, as well as syphilis antibodies. Anti-DNA and anti-nuclear antibody tests were negative. Urinalysis showed clear urine with pH 6.5, 1 WBC, and 2 RBCs. Urine culture was negative.
On admission, the patient was consulted by hematology and rheumatology specialists for the investigation of the hypercoagulable state and vasculitis. The hematology specialist advised starting the patient on apixaban for 6 months and subsequently switching to aspirin, while rheumatology evaluation reported no evidence of specific vasculitis. CT runoff angiogram showed a dissection of the upper branch of the right renal artery with right renal infarction involving the middle and lower poles of the kidney.
Discussion
In this report, we describe a patient with RRMS on fingolimod presenting with renal infarction. To our knowledge, there is no reported association between renal infarction and fingolimod treatment. However, a growing number of studies point to additional adverse effects of fingolimod that extend beyond its effect on lymphocytes. The ubiquitous expression of S1P receptors explains the unpredictable side effects of fingolimod. Additionally, active metabolites of fingolimod may affect several biological processes, including angiogenesis, endothelial cell-cell adhesion, and cardiovascular function alteration.
Out of the five subtypes of S1P receptor, fingolimod exhibits high affinity for S1P1, S1P3, and S1P4. Both S1P1 and S1P3 are found on vascular endothelium and smooth muscle cells, where they contribute to the maintenance of the endothelial barrier function and peripheral vascular tone [3]. Based on animal studies, S1P1 and S1P3 can cause vasodilation by activating endothelial nitric oxide synthase and thereby inducing a release of nitric oxide. Therefore, the functional antagonism of S1P1 and S1P3 can result in vasoconstriction. The initial dose of fingolimod was shown to act as an agonist at the S1P1 receptors, resulting in bradycardia, hypotension, and decreased vascular permeability. With repeated doses, fingolimod acts as a functional antagonist that binds, internalizes, and degrades the S1P1 receptors. This downregulation of the receptors results in vasoconstriction, mild hypertension, and macular edema [4].
Notably, a number of studies have reported unexplained vascular effects of fingolimod in patients with multiple sclerosis. One such report describedthe appearance of erythematous purpuric macules on the distal portion of the hand digits, which completely resolved after cessation of fingolimod treatment. After 2 months, fingolimod was reintroduced and the same reaction recurred [5]. Another study reported a case with necrosis of one hand due to severe vasospasm that started 7 days after the initiation of treatment with a high dose of fingolimod [6]. Additionally, one study reported painless ecchymotic angioedema-like cutaneous manifestation over the knees of a patient after 2 days of fingolimod therapy [7].
In regard to our case, the patient had an unremarkable medical history with no apparent factors that would predispose him to vascular pathologies. The evidence from the literature regarding the unexpected vascular complications of fingolimod suggests that the drug may have played a role in the patient’s adverse event.
Conclusion
Although the exact mechanism underlying the effects of fingolimod on vasculature is not completely understood, there is growing evidence in the published literature of possible unexpected vascular effects. Further research is warranted, with additional studies evaluating the long-term effects of the drug. Physicians need to be updated of any new findings, and should report any new or existing adverse effects.
28259
References
- Brust J (2011) Current diagnosis & treatment neurology. McGraw Hill Professional, USA.
- Kappos L, O'Connor P, Radue EW, Polman C, Hohlfeld R, et al. (2015) Long-term effects of fingolimod in multiple sclerosis: the randomized Freedoms extension trial. Neurology 84: 1582-1591.
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, et al. (2010) Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9: 883-897.
- Camm J, Hla T, Bakshi R, Brinkmann V (2014) Cardiac and vascular effects of fingolimod: Mechanistic basis and clinical implications. Am Heart J 168: 632-644.
- Russo M, Guarneri C, Mazzon E, Sessa E, Bramanti P, et al. (2015) Fingolimod-associated peripheral vascular adverse effects. Mayo Clin Proc 90: 1424-1427.
- Schwarz A, Korporal M, Hosch W, Max R, Wildemann B (2010) Critical vasospasm during fingolimod (FTY720) treatment in a patient with multiple sclerosis. Neurology 74: 2022-2024.
- Masera S, Chiavazza C, Mattioda A, Superti G, Beggiato E, et al. (2014) Occurrence of ecchymotic angioedema-like cutaneous lesions as a possible side effect of fingolimod. Mult Scler J 20: 1666-1667.






