Keywords
Acinetobacter baumannii, carbapenem resistance, nosocomial infection, mortality, ICU
Introduction
Carbapenem resistance in Acinetobacter spp has emerged as a significant health problem over the last decade, leaving limited option for antimicrobial therapy. [1] Surveillance studies indicate that the percentage of carbapenem-resistant isolates gradually increased over the last years in Europe, North America and Latin America. [1] The proportion of imipenem-resistant A. baumannii isolates at tertiary-care hospitals in Greece was 84,5% for intensive care units (ICU), 84,1% for surgical wards and 75,1% for medical wards between January and June 2010 (Greek system for Surveillance of Antimicrobial Resistance, GSSAR. CR-AB infections can extend ICU stay and hospital stay 15 and 30 days, respectively and have an attributable hospital mortality of 20% and ICU mortality of 4%. [2]
Hospital CR-AB outbreaks have been described previously. [3,4] Few case-control studies have been performed to determine risk factors of CR-AB infection. [2,3,5,6] Identified risk factors include blood transfusion, colonization density, [2] severity of disease, high TISS-28 score, [3] prolonged hospital stay, previous ICU stay, prior antibiotic exposure including exposure to aminoglycosides, carbapenems [5] and third - generation cephalosporins. [6]
In addition, controversy exists regarding the mortality attributable to CR-AB. [2,5] More data regarding the risk factors for CR-AB infection are needed in order to prevent CR-AB infection and to optimize therapy.
The aim of our study was to determine the risk factors for CR-AB infection and to investigate whether CR-AB infection significantly increases the 28-day ICU mortality rate.
Methods
Study Population
This study was conducted from January 2009 to March 2010 at the eight-bed Medical-Surgical ICU of “SOTIRIA” general hospital in Athens, Greece. During this period, all adults patients admitted to ICU and ventilated for at least 48 hours were included in the study. Comparisons were made between patients with CR-AB infections and patients without A. baumannii (AB) infections. All patients who acquired microbiologically documented CR-AB infection ≥48 hours after ICU admission were defined as case patients. Patients without microbiologically documented AB infection were defined as control patients. The controls were randomly chosen from the list of all ICU patients without AB infections during the study period. One control was individually matched to each case based on APACHE (Acute Physiology And Chronic Health Evaluation) II score (±5 points), age (±5years), and length of ICU stay (±2days) before onset of CR-AB infection (for cases) or before ICU discharge (for controls). During the study period, 53 of 156 patients met the case definition. Three of the cases were excluded from the study because it was not possible to find appropriate controls for them. Another five patients with carbapenem sensitive Acinetobacter baumannii (CS-AB) infection were excluded from the study. A total of 50 case-control pairs were the analysis population.
Data Collection
Surveillance of CR-AB infections included daily clinical examination of the patients, daily reviewing of the patient’s medical record, Kardex data, and all microbiological data of a positive blood culture result. Data were recorded on individual forms for each patient. The form included age, gender, cigarette smoking, alcohol intake, obesity, disease severity determined by the Acute Physiology and Chronic Health Evaluation (APACHE) II score at admission, co-morbidity determined by the weighted Charlson co-morbidity index at the time of admission diagnosis, transfer from another hospital or ward to the ICU, indwelling devices, culture and antimicrobial susceptibility test results, length of ICU stay and time between admission and isolation of the first positive A. baumannii culture. Prior exposures to antibiotics and other drugs were also recorded. All data were recorded by a well trained research nurse and reviewed by an infectious disease specialist. The study protocol was approved by the ethical committee of the hospital, in which the study was conducted.
Definitions
Health care-associated infections were defined according to the criteria of the Centers for Disease Control and Prevention. [7] Infection was confirmed in each patient by the physician in charge of his or her care. Until the microbiological documentation of micro-organisms, all patients received empirical antibiotic treatment based on the most common isolated pathogens in our ICU (A. baumannii and Pseudomonas aeruginosa). Attributable 28-day ICU mortality was calculated by subtracting the mortality of the controls from that of the cases. The 28-day ICU mortality ratio was defined as the ratio of the mortality of cases to that of the controls. Prior exposure to antibiotics was defined as the receipt of a systemic antimicrobial agent for at least 48-hour during the 14-day period prior to isolation of A. baumannii. CR-AB was defined if an A. baumannii isolate was resistant to both imipenem and meropenem.
Microbiological Analysis
Antimicrobial susceptibilities were determined by disk diffusion method and an automated method (bioMerieux, Vitek II, France). Isolates resistant to imipenem and meropenem (minimum inhibitory concentration>8mg/L) were considered carbapenem-resistant regardless of their susceptibility to other antibiotics. Intermediately susceptible strains were accepted as resistant.
Statistical analysis
Potential risk factors for CR-AB infections were tested using univariate and multivariate conditional logistic regression analysis in 50 cases with CR-AB infection and 50 controls without AB infection. Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as a percentage of the total number of patients analyzed. Variables with a P-value less than 0.2 in the univariate analysis were entered into the multivariate analysis. The association between CRAB infection and death at the ICU was tested using univariate and multivariate logistic regression models after controlling for several confounders and checking for co linearity. All tests were two-tailed and a p-value below 0.05 was considered significant. Odds ratios (OR) and 95% confidence intervals (95% CI) were computed. All statistical analyses were performed using SPSS version 16.
Results
During the study period, a total of 156 patients with 3.151 patient days were hospitalized in the ICU. The incidence of CR-AB infection was 33.9% and the incidence density was 16.8 cases/1000 ICU days. The most frequent types of infections were ventilator-associated pneumonia (70%), blood stream infections (24%), and catheter associated urinary tract infections (6%).
All isolates were susceptible to colistin. The resistance rates to tigecycline, amikacin/gentamycin and ampicillin-sulbactam were 62%, 72%, and 64% respectively.
Out of the total of 156 hospitalized patients, 50 case-control pairs were studied. The mean age of patients with CR-AB and without AB infection was 67.5±13.8 and 68.7 ±14,5 years respectively. The mean APACHE II score at admission of patients with CR-AB and without AB infection was 16.6±5.2 and 16.3±4.5 respectively. The mean length of ICU stay for the cases before onset of CR-AB infection was 13.9±7.6 days and the mean length of ICU stay after onset of CR-AB infection was 17.6±16.0 days. The overall mean length of ICU stay of patients with CR-AB and without AB infection was 29.7±18.7 and 14.9±10.1 days, respectively.
Tables 1 and 2 show the univariate conditional logistic regression analysis of the potential risk factors for CR-AB infection related to clinical characteristics, invasive procedures and prior therapy. Tobacco smoking (p=0.19), Charlson co-morbidity index larger than two points (p=0.04), diabetes mellitus (p=0.14), intra-abdominal surgery (P=0.07) and cardiovascular disease as a reason for ICU admission (p=0.18), use of gastric ulcer prophylaxis medications (p=0.15) and previous administration of more than three antibiotic classes (p=0.01) were risk factors for CR-AB infection at the 0.20 p-value level. Receipt of carbapenem was not a risk factor for CR-AB infection (p=0.53).
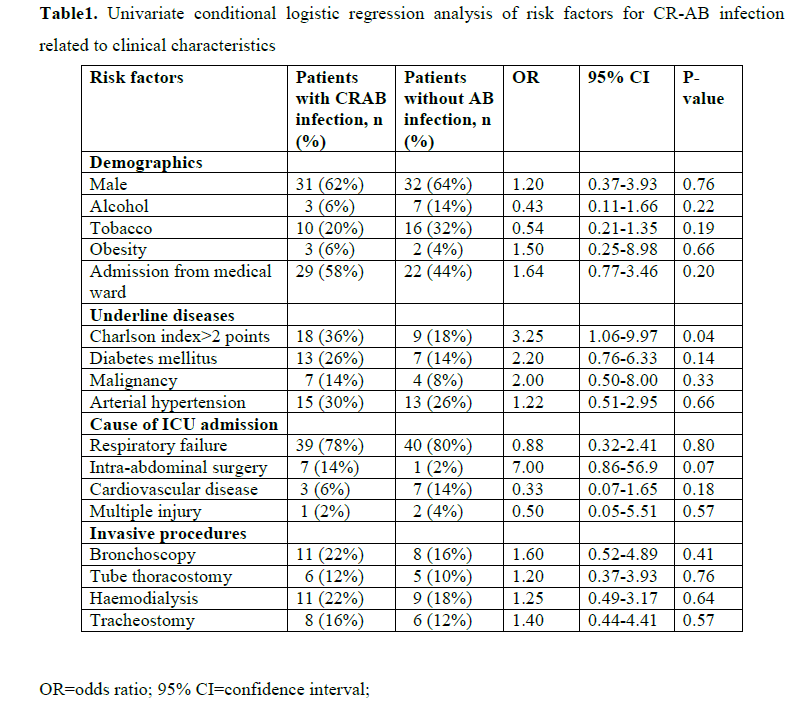
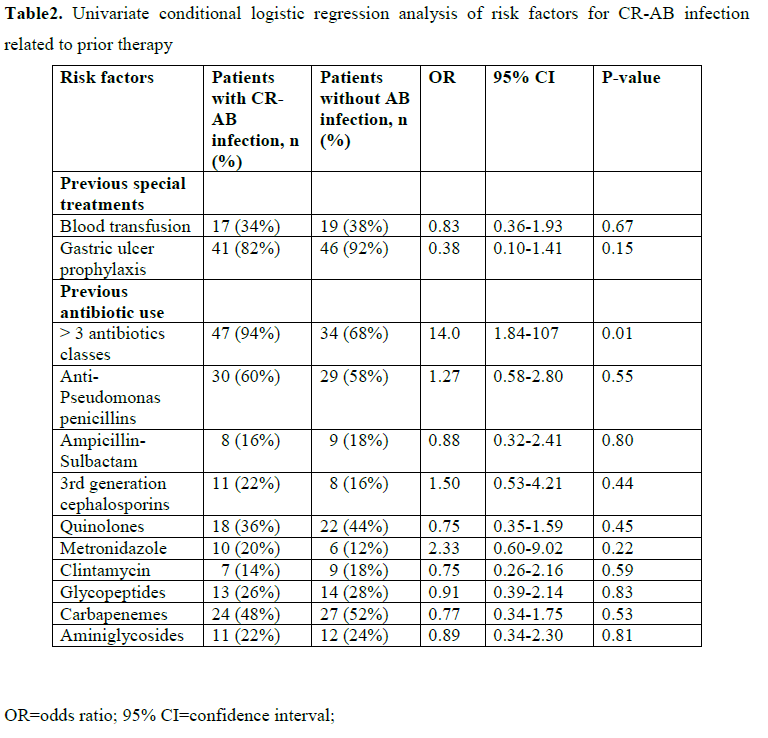
When all these variables were inserted into a multivariate conditional logistic regression model, only previous administration of more than three antibiotic classes (OR=34.0, 95% CI 2.22-522, P=0.01) was an independent risk factor for CR-AB infection (data not shown).
The overall mortality was 42% on day 14, and 54% on day 28. A total of 27 of 50 patients (54%) with CR-AB infection died within 28-ICU days, and 26 of 50 (52%) without CR-AB died. The crude 28-day ICU attributable mortality was 0.02, (95% CI=-0.18, 0.22, P=0.84).
Tables 3 and 4 show the univariate logistic regression analysis of risk factors for 28-day ICU mortality related to clinical characteristics, invasive procedures and prior therapy. Only APACHE II score at admission (p=0.002), haemodialysis (p=0.01), tracheostomy (P=0.005), and use of gastric ulcer prophylaxis medications (p=0.03) were significantly associated with 28-day ICU mortality.
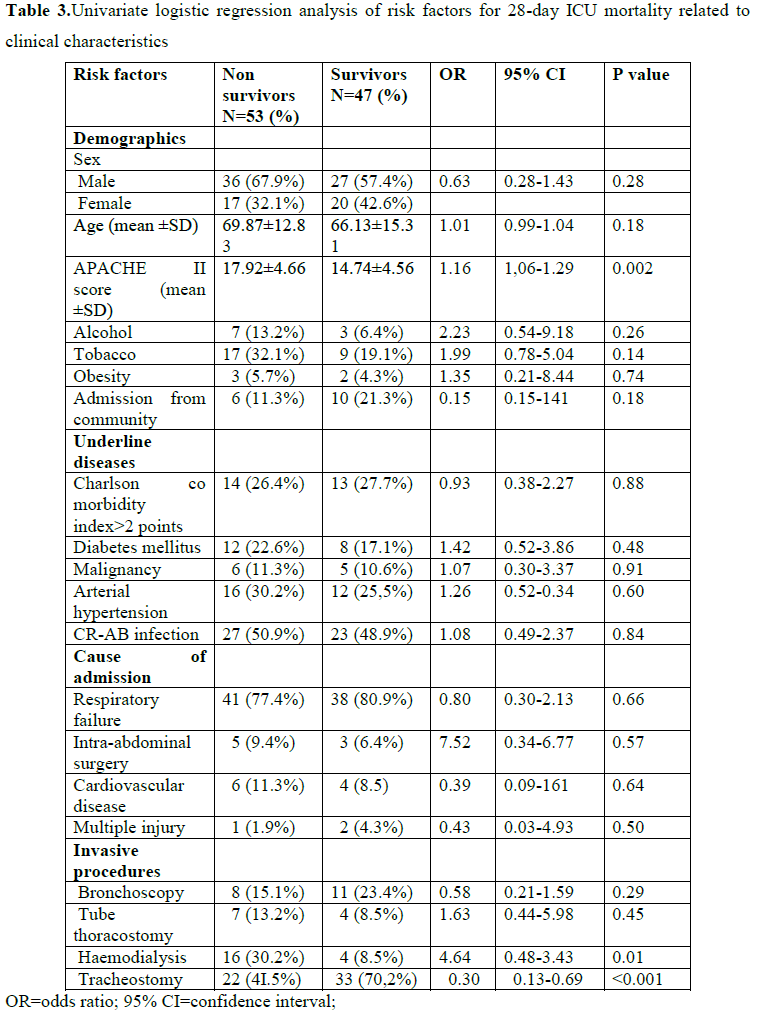
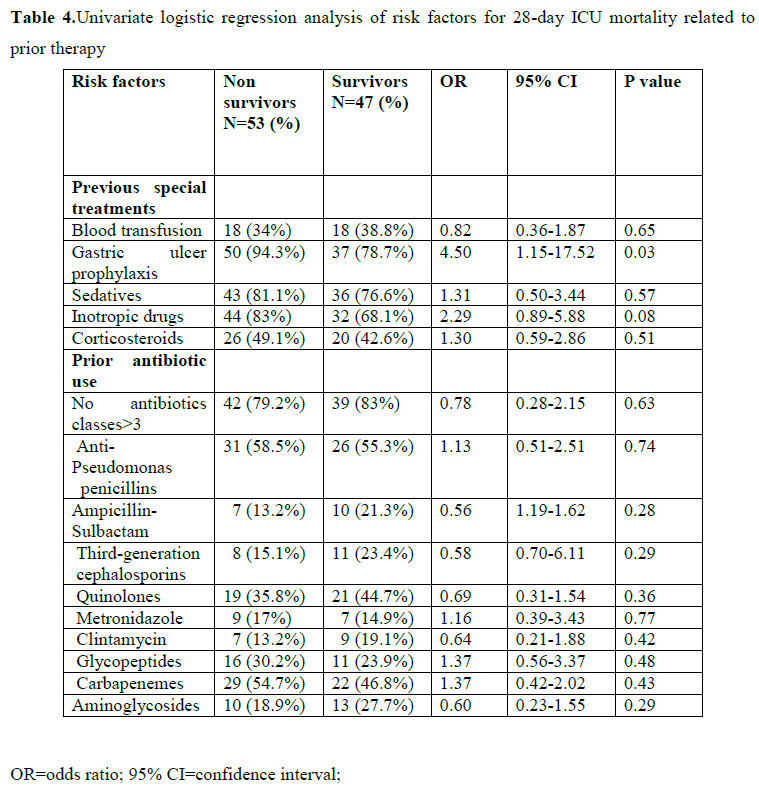
The crude 28-day ICU mortality ratio for CR-AB vs. no AB infection was 1.08 (95% CI 0.49-2.38, P=0.84). When we adjusted this association for all the variables that were associated at the p-value level of 0.20 either with CR-AB infection or with mortality, CR-AB infection was still not associated with death at the ICU (OR=1.40, 95% CI 0.46-4.22, P=0.55). However, in that same model, APACHE II score at admission (OR=1.13, 95% CI 1.00-1.28, P=0.05), presence of tracheostomy (OR=0.04, 95% CI 0.004-0.35 P=0.004), and use of gastric ulcer prophylaxis drugs (OR=6.65, 95% CI 1.10-40.0, P=0.04) were significantly associated with 28-day ICU mortality (data not shown).
Discussion
In 2001, the International Network for the Study and Prevention of Emerging Antimicrobial Resistance (INSPEAR) defined the emergence of carbapenem resistance in Acinetobacter species as a sentinel event, warranting prompt epidemiological and microbiological interventions. [8] Carbapenem resistance has also become a great concern for the treatment of serious Acinetobacter infections due to concomitant resistance against multiple antimicrobial agents.
Few data exist on the incidence and incidence density of CR-AB infection. In the present study, the incidence (33.9%) and incidence density of CR-AB infection (16.8 /1000 patient-days) were quite high. In contrast, low incidence rates of CR-AB infection (4.6%) were found in other studies. [2] This difference likely reflects variation in the characteristics of the cases, diagnostic strategies or infection definitions, and highlights the development and implementation of a multidisciplinary approach and a comprehensive infection control program (‘bundle’), the importance of which has been demonstrated in many studies. [4]
Few case-control studies have been performed to determine the risk factors specific for CR-AB infections. Risk factors of CR-AB infection include not only prior exposure to antibiotics, but also variable related to host defenses. [2,3,5,6] In our study, the previous exposure to more than three different antibiotic classes was the only independent risk factor for CR-AB infection. The risk for CR-AB infections was 34 times higher for patients who received more than three different antibiotic classes. The identification of previous antibiotic selection pressure: exposure to more than three different antibiotic classes as a risk factor is not unexpected. It has also been observed by Playford and colleagues for the acquisition of CR-AB [2] and by Depuydt and colleagues in patients with multidrug resistance ventilator-associated pneumonia. [9] These findings emphasize the importance of continued surveillance of microorganisms and antimicrobial agents to provide clinicians with information for choosing empirical therapy and revision of antimicrobial prescribing policies.
Furthermore, the lack of association between CR-AB infection and specific antibiotic classes, including carbapenemes is consistent with previous studies [2,10,11] and may reflect the high prevalence of the carbapenem resistant strains in our ICU, or the large diversity of antibiotic usage. Treatment with a large number of broad-spectrum antibiotics is a surrogate marker of disease severity [12-14] and could ablate a patient’s pre-existing microflora. [2]
The influence of carbapenem resistance on the mortality of patients infected with A. baumannii is still subject to debate because of methodological heterogeneity among studies. Some studies have reported that CR-AB infection is associated with significantly increased mortality rate. [5,15-17] However, other studies have found no significant difference in mortality between patients with ventilator-associated pneumonia (VAP) and bacteremia caused by CR-AB compared to patients with VAP and bacteremia due to CS-AB isolates. [18-20] Similarly, Daniels and colleagues in a retrospective, propensity score matched study have reported that the 28-day mortality of patients with MDR A. baumannii HCAI was not significantly different from that of patients with non–MDR A. baumannii HCAI (4.8%). [21] Sunenshine and colleagues [22] conducted also a retrospective cohort study to examine outcome of patients with MDR Acinetobacter infection compared with patients with susceptible Acinetobacter infections and patients without Acinetobacter infections. They demonstrated that patients infected with MDR strains of Acinetocter have longer lengths of stay in both the hospital and ICU than patients infected with drug-susceptible Acinetobacter and patients without Acinetobacter infection when they controlled for severity of disease. They also found a trend toward increased mortality rates among patients with MDR Acinetobacter infection. However, the difference was not statistically significant when they controlled for severity of disease. Our matched case-control study examined the 28-day ICU mortality rate of patients with CR-AB infections compared with patients without AB infections and demonstrated that CR-AB infections are not independently associated with increased 28-day ICU mortality. These results should be interpreted with caution because of the small patient number and highlight the need for multicentre studies involving a larger number of patients to advance our understanding of the mortality associated with CR-AB infection. Additionally, the non significant attributable mortality for CR-AB infection could reflect the administration of appropriate antibiotic therapy, the importance of which has been demonstrated in many studies. [23-25]
An important finding in the present study was that the high crude 28-day ICU mortality rate (54%) of patients with CR-AB infection, which could be attributed to the underlying disease severity, as the 28-day ICU mortality in the control group was also high (52%). [26] These findings justify the effort to reduce the nosocomial transmission of CR-AB. In addition to the decreased mortality and the economic benefits that would result from reducing CR-AB infections, such efforts may also provide benefits in reducing the risk of transmission of other multiresistant organisms and the use of broad-spectrum antibiotics.
Finally, in consistence with other studies, [27,28] it was found that CR-AB infection was not associated with 28-day ICU mortality. However, APACHE II score at admission, use of gastric ulcer prophylaxis drugs were significantly associated with 28-day ICU mortality in a multivariate model. These factors related to disease severity and are largely unmodifiable. The protective relationship between tracheotomy and ICU mortality highlights the benefits of tracheotomy and such invasive procedures should be encouraged in order to decrease the high 28-day ICU mortality of studied population.
The strengths of our study were the sound selection of controls matched to the cases on APACHE score, age and length of ICU stay that limit the possibility of selection bias, and the study of multiple risk factors for CR-AB infection. We had also several potential limitations. The sample was relatively small and this led to several large confidence intervals, although this is a common problem in studies assessing risk factors for infections because of multidrug-resistant microorganisms. Moreover, the study was performed at one ICU, and the results may not be generalized to other settings. Our findings highlight the need for a multicentre study involving a larger number of patients.
Conclusion
In conclusion, this matched case-control study verified a large incidence, incidence density and 28-day ICU mortality due to CR-AB infection, which justify the continued study of this common infectious disease problem. Previous exposure to more than three different antibiotic classes was the only independent risk factor for the development of CR-AB infection. CR-AB infection was not associated with 28-day ICU mortality before or after adjustment for several other risk factors. Special attention should be paid to infection control practices and prudent antibiotic use in ICUs, where CR-AB infections are endemic. Large multicentre studies of CR-AB infection are further warranted.
2761
References
- Peleg A, Seifert H, Paterson D. Acinetobacterbaumannii: emergence of a successful pathogen. ClinMicrobiol Rev 2008; 21(3):538-82.
- Playford FG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacterbaumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 2007;65(3): 204-11.
- Kohlenberg A, Brümmer S, Higgins P, Sohr D, Piening B, De Grahl C, et al. Outbreak of carbapanem-resistant AcinetobacterBaumannii carrying the carbapenemase OXA-23 in German university medical centre. JMM 2009;58(11):1499-507.
- Enoch D, Summers C, Brown N, Moore L, Gillham M, Burnstein R, et al. Investigation and management of an outbreak of multidrug-carbapenem-resistant Acinetobacterbaumannii in Cambridge, UK. J Hosp Infection 2008;70(2) :109-18.
- Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, Liu JW, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacterbaumannii. IJID 2010;14(9):764-9.
- Baran G, Erbay A, Bodur H, Onguru P, Akinci E, Balaban G, et al. Risk factors for nosocomial imipenem-resistant Acinetobacterbaumannii infections. IJID 2008;12(1):16-21.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36(5):309-32.
- Richet H, Mohammed J, McDonad L, Jarvis W. Building Communication Networks: International Network for the Study and Prevention of Emerging Antimicrobial Resistance. Emerg Infect Dis 2001;7(2): 319-22.
- Depuydt P, Vandijck D, Bekaert M, Decruyenaere J. Blot S, Vogalaers D, et al. Determinants and impact of multidrug antibiotic resistance in pathogens causing ventilator-associated-pneumonia. Critical Care 2008; 12(6):R142.
- Young LS, Sabel AL, Prise CS. Epidemiology, clinical and Economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacterbaumanni infection in surgical intensive care unit. Infect Control HospEpidemiol 2007;28(11):1247-1254.
- De Castro Romanelli RM, de Jesus LA, Clemente WT, Lima SS, Rezente ED, CoutinoRL,et al. Outbreak of Resistant AcinetobacterBaumannii-measures and Proposal for Prevention and control BJID 2009;13(5):341-347.
- Wong TH, Tan BH,Ling ML, Song C. Multi-resistant Acinetobacterbaumannii on a burns unit-critical risk factors and prognosis. Burns 2002;28(4):349-357.
- Smolyakov R ,Borer A , Riesenberg K, Schiaeffer F, Alcan M, Porath A, et al. Nosocomial multi-drug resistant AcinetobacterBaumanni bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hospital Infect 2003;54(1):32-58.
- Akinci E, Colpan A, Bodur H, Balaban N, Erbay A . Risk factors for ICU-acquired imipenem-resistant Gram-negative bacterial infections. J Hospital Infect 2005;59(4):317-32.
- Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, et al. Impact of imipenem resistance on mortality in patients with Acinetobacterbacteraemia. J Antimicrob Chemotherapy 2007;59(3):525-30.
- Hernandez-Tores A, Garcia-Vanquaez E, Gomez J, Canteras M, Ruiz J, YaqueG.Multidrag and carbapenem-resistant Acinetobacterbaumanni infections: Factors associated with mortality. Med Clin 2012;138(15):650-655.
- Zheng YL, Van YF, Zhou LH, Ye ML, Liu S, Xu CQ, He YQ, et al. Risk factor and mortality of patients with nosocomial carbapenem-resistant Acinetobacterbaumanni pneumonia. Am J Infect Control 2013:e1-e (Epud ahead of print).
- Garnacho-Montero J, Ortiz-Leyba C, Fernandez-Hinojosa E, Aldabo-Pallas T, Cayuela A, Marquez-Vacaro JA, et al. Acinetobacterbaumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 2005;31(5): 649-55.
- Lautembach E, Synnestvedt M, Weiner M, Bilker W, Vo L, Schein J, et al. Epidemiology and impact of imipenem resistance in Acinetobacterbaumannii. Infect Control HospEpidemiol 2009;30(12):1186-92.
- Chen SJ, Chao TF, Chiang MC, Kuo SC, Chen LY, Chiang DH, et al. Predictors of mortality in surgical patients with Acinetobacterbaumannii bacteremia. J MicrobiolImmunol Infect 2005;38(2):127-36.
- Daniel T, Deppen S, Arbogast P, Griffin M, Schaffner W, Talbot T. Mortality rates associated with Multidrug-resistant Acinetobacterbaumannii infection in surgical intensive care units. Infect Control HospEpidemopl 2002;23(2):106-8.
- Sunenshine R, Wright M, Maragakis L, Ηarris A, Song X, Hebden J. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007;13(1):97-103.
- Moine P, Timisit J, DeLassence A, Troche G, Fosse J, Alberti C, et al. Mortality associated with late-onset pneumonia in the intensive care unit: results of a multicenter cohort study. Intensive Care Med 2002;28(1):154-63.
- Kim YJ, Kim SI, Hong KW, Kim YR, Park YJ, Kang MW. . Risk factors for mortality in patients with carbapenem-resistant Acinetobacterbaumannii bacteremia: impact of appropriate antimicrobial therapy. J Korean Med Sci 2012; 27(5):471-475.
- Huang ST, Chiang MC, kuoSc, Lee YT, Chiang TH, Yang SP. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacterbaumannii bacteremia. J MicribiolImmunol Infect 2012; 45(5):356-362.
- Aydemir H, Celebi G, Piskin N, Oztoprak N, Keskin AS, Actas E, et al. Mortality attributable to carbapenem-resistant nosocomial Acinetobacterbaumannii infections in a Turkish university hospital. Jpn J Infect Dis 2012; 65(1):66-71.
- Kim SY, Jung JY, Kang YA, Lim JE, Kim EY, Lee SK, et al. Risk factors for Occurrence and 30-day mortality for carbapenem-resistant Acinetobacterbaumannii bacteremia in an Intensive Care Unit. J Korean Med Sch 2012; 27:939-947.
- Lemos EV, de la Hoz FP, Alvis N, Einarson TR, Quevedo E, Castaneda C, et al. Impact of carbapenem resistant on clinical and economic outcomes among patients with Acinetobacterbaumannii infection in Colombia. Clinical Microbiol Infect 2013 (Epud ahead of print.).









