Hasan Khosravi1,2, Maryam Abdollahi2, Mehrnoosh Badakhsh2, Tahereh Soori2, Masoud Jafari2,Gordon Bae1,3 and Maryam Daneshpazhooh2*
1Harvard Medical School, Boston, USA
2Autoimmune Bullous Diseases Research Center, Department of Dermatology, Tehran University of Medical Sciences, Tehran, Iran
3Brigham and Women’s Hospital, Department of Medicine, Boston, USA
- *Corresponding Author:
- Maryam Daneshpazhooh
Autoimmune Bullous Diseases Research Center, Department of Dermatology
Tehran University of Medical Sciences, Tehran, Iran
Tel: 982155618989
E-mail: daneshpj@tums.ac.ir
Received date: February 15, 2017; Accepted date: March 25, 2017; Published date: March 31, 2017
Citation: Khosravi H, Abdollahi M, Badakhsh M, et al. Rituximab Induced Neutropenia in a Patient with Bullous Pemphigoid. Arch Med. 2017, 9:2. doi: 10.21767/1989-5216.1000208
Copyright: © 2017 Khosravi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Rituximab, a chimeric monoclonal antibody against the CD20 B-cell antigen, is used to treat B-cell malignancies, rheumatoid arthritis, and autoimmune blistering diseases. Adverse events seen with rituximab include infusion reactions, infections, and late or early-onset neutropenia. Specifically, neutropenia is classified as grade III, an absolute neutrophil count (ANC) of 0.5-1.5 x 109/L, or grade IV, an ANC less than 0.5 x 109/L. In this case, we present a bullous pemphigoid (BP) patient with grade IV rituximabinduced neutropenia and safe re-treatment after two years.
Keywords
Rituximab; Neutropenia; Bullous pemphigoid; Granulocyte colony stimulating factor
Abbreviations
BP: Bullous Pemphigoid; DIF: Direct Immunofluorescence; EON: Early-onset Neutropenia; GCSF: Granulocyte Colony Stimulating Factor; LON: Late-onset Neutropenia; WBC: White Blood Count
Case Report
A 66-year-old woman presented with 2 weeks of pruritus, oral erosions, urticarial plaques and tense bullae on the abdomen, scalp, and upper extremities. Biopsy and direct immunofluorescence (DIF) demonstrated linear IgG and C3 deposition at the basement membrane consistent with BP; a DIF on salt split skin ruled out epidermolysis bullosa acquisita. Of note, Anti-BP 180 ELISA was greater than 200 μ/ml and Anti-BP 230 was 2.3 μ/ml. The patient was subsequently treated with topical clobetasol with refractory bullae unresponsive to 80 mg daily prednisolone and such immunosuppressive medications as 150 mg daily azathioprine, 2 g daily mycophenolate mofetil, and 15 mg methotrexate weekly. Due to refractory disease, four years after the diagnosis, methotrexate was discontinued, and the patient was started on 500 mg weekly infusions of a rituximab biosimilar (RedituxTM) for 4 weeks along with 10 mg prednisolone daily. Eighteen days after the fourth infusion, the patient was afebrile with a screening CBC demonstrating a white blood count (WBC) of 1.9 x 109/L, ANC of 0.437 x 109/L, hemoglobin of 12.4 g/dl, and platelet count of 188 x 109/L. Two days later, the patient returned with a fever of 38.5°C, weakness, malaise, and WBC of 1.2 x 109/L. She was hospitalized and treated with vancomycin 4 g BID, meropenem 4 g TID, acyclovir 400 mg BID, fluconazole 100 mg BID, and 300 mcg filgrastim, a granulocyte colony stimulating factor (GCSF), for two days. No sources of infection were identified. The patient became asymptomatic on day 2 of her hospitalization, and blood counts recovered to a WBC of 4.7 x 109/L and ANC of 2.44 x 109/L. The overall trend in ANC is depicted in Figure 1. After 2 years, the patient had recurrence of bullae requiring an additional course of a different rituximab biosimilar agent (ZytuxTM), 500 mg weekly for four weeks, with no subsequent neutropenic episodes [1-3].
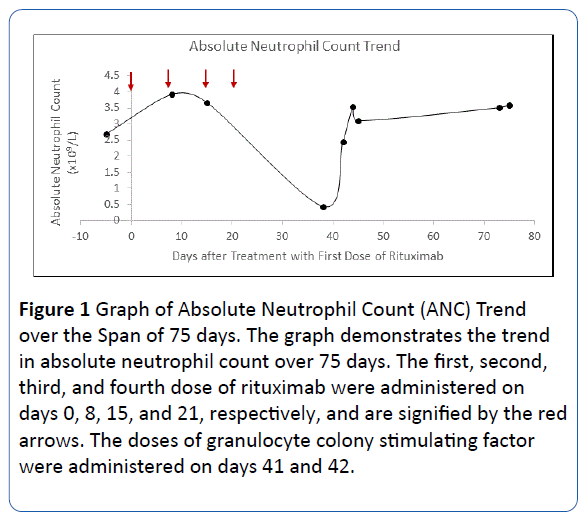
Figure 1: Graph of Absolute Neutrophil Count (ANC) Trend over the Span of 75 days. The graph demonstrates the trend in absolute neutrophil count over 75 days. The first, second, third, and fourth dose of rituximab were administered on days 0, 8, 15, and 21, respectively, and are signified by the red arrows. The doses of granulocyte colony stimulating factor were administered on days 41 and 42.
Discussion and Conclusion
In conclusion, we present a BP patient that developed grade IV neutropenia eighteen days after completing 4 weeks of treatment with rituximab without subsequent neutropenia on re-treatment. The differential for this neutropenia included infection and other neutropenia-inducing drugs, but these were unlikely given no signs of infection and the lack of additional neutropenic-inducing drugs [4]. Prior cases of late-onset neutropenia (LON), occurring 4 weeks after the last rituximab infusion, have been reported in chronic lymphocytic leukemia and autoimmune rheumatologic treatment; however, we only found 6 cases of rituximab-induced early-onset neutropenia (EON), occurring 4 weeks after the first infusion of rituximab [3,5-8]. Interestingly, this case of rituximab-induced neutropenia, occurring 18 days after the last rituximab infusion, is neither EON nor LON. Suggested mechanisms for rituximabinduced LON include anti-neutrophil IgG antibodies, large granular lymphocytes inducing neutrophil apoptosis, aberrant Bcell reconstitution, and disrupted granulocyte homeostasis [3,8,9]. However, these mechanisms have not been proposed for early-onset neutropenia. Lastly, studies have proposed rituximab-retreatment after LON, suggesting a possibility of retreatment without future neutropenic episodes [10]. In this case report, we not only document the first case of rituximabinduced neutropenia in a dermatologic patient that is neither LON nor EON, but we also demonstrate the safe readministration of rituximab, using a different biosimilar drug, allowing continued, effective treatment without concern for future neutropenic episodes.
18983
References
- Breuer GS, Ehrenfeld M, Rosner I (2014) Late-onset neutropenia following rituximab treatment for rheumatologic conditions. Clin Rheumatol 33: 1337-1340.
- Shetty S, Ahmed AR (2013) Treatment of bullous pemphigoid with rituximab: critical analysis of the current literature. J Drugs Dermatol 12: 672-677.
- Arroyo-Ávila M, Fred-jiménez RM, Vilá LM (2015) Early-onset neutropenia induced by rituximab in a patient with lupus nephritis and hemolytic anemia.
- Andersohn F, Konzen C, Garbe E (2007) Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 146: 657-665.
- Enríquez R, Borrás-blasco J, Sirvent AE, Masía M, Amorós F (2007) Early onset neutropenia after rituximab in lupus nephritis. Clin Exp Rheumatol 25: 345.
- Gottenberg JE, Guillevin L, Lambotte O (2005) Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis 64: 913-920.
- Mealy MA, Levy M (2015) Favorable outcome of granulocyte colony-stimulating factor use in neuromyelitis optica patients presenting with agranulocytosis in the setting of rituximab. J Neuroimmunol 287: 29-30.
- Voog E, Morschhauser F, Solal-céligny P (2003) Neutropenia in patients treated with rituximab. N Engl J Med 348: 2691-2694.
- Grant C, Wilson WH, Dunleavy K (2011) Neutropenia associated with rituximab therapy. Curr Opin Hematol 18: 49-54.
- Reitblat T, Wechsler A, Reitblat O (2015) Rituximab-related late-onset neutropenia in patients with rheumatic diseases: successful re-challenge of the treatment. Am J Case Rep 16: 211-214.







