Review - (2022) Volume 13, Issue 6
Role of neurological and neuropsychiatric comorbidities in the long-term outcome of severe paediatric feeding and eating disorders - A caregiver perspective
Lydia Blank1*,
Georg Friedrich Hoffmann1 and
Rainer Blank2,3
1Department of General Paediatrics, Centre for Paediatric and Adolescent Medicine, University Hospital Heidelberg, Heidelberg, Germany
2Clinic for Child Neurology and Social Paediatrics, Child Centre Maulbronn, Maulbronn, Germany
3Heidelberg University, Heidelberg, Germany
*Correspondence:
Lydia Blank, Department of General Paediatrics, Centre for Paediatric and Adolescent Medicine, University Hospital Heidelberg,
Im Neuenheimer Feld 430, 69120 Heidelberg,
Germany,
Email:
Received: 10-Jun-2022, Manuscript No. ipjnn-22-12859;
Editor assigned: 12-Jun-2022, Pre QC No. P-12859;
Reviewed: 18-Jun-2022, QC No. Q-12859;
Revised: 24-Jun-2022, Manuscript No. R-12859;
Published:
30-Jun-2022
Abstract
Aim: To examine the long-term outcome of severe paediatric feeding
and eating disorders as perceived by their caregivers in relation to
predominant areas of comorbidities in the neurodevelopmental and
behavioural field.
Methods: A sample of 103 patients (ICD-10 F98.2, age below
7 years, all previously referred for a four-week inpatient eating
intervention between 2009 and 2016) was followed up by a parent
questionnaire with the target parameters being age-appropriate
eating and long-term improvement (Likert scale 1-10).
Four comorbidity subgroups were compared: 1) neurological
comorbidities (“Neuro-group”, n=28), 2) behavioural comorbidities
(“Psy-group”, n=24), 3) developmental delay without severe
neurological or behavioural disorders (“DD-group”, n=22) and 4)
without any neurodevelopmental or behavioural disorders (non-
DNP-group, n=29).
Results: After a mean follow-up period of 3.5 years (n=103),
the non-DNP-group (normal development and low rate of
comorbidities) achieved the best outcome. The Neuro-group had
the least age-appropriate eating behaviour on follow-up, while
their caregivers experienced good life satisfaction and surprisingly
low burden of disease; they were similarly satisfied with previous
treatment as caregivers of non-DNP-group. Caregivers of Psy-group
described a better outcome than those from the Neuro- and DDgroup,
however lowest satisfaction and felt highest burden of the
child eating problems.
Conclusion: Feeding and eating disorders are persisting for a long
time in children with disabilities. It seems that caregivers of children
with neurological disabilities adequately lowered their outcome
expectations and developed better coping strategies. This stays in
contrast to caregivers of children with behavioural disorders.
Keywords
Infantile eating disorders; Developmental disabilities;
Behavioural disorders; Cerebral palsy
Abbreviations
ARFID: Avoidant-Restrictive Food Intake
Disorder; ASD: Autism Spectrum Disorder; CP: Cerebral Palsy;
DD: Developmental Delay; GERD: Gastroesophageal Reflux
Disease; GMFCS: Gross Motor Function Classification System;
ICF: International Classification of Functioning, Disability and
Health; Non-DNP: Comorbidity Group without Developmental/
Neurological/Psychological Diagnoses
Introduction
Co-occurring medical or mental conditions are
common in children with severe paediatric feeding and
eating disorders of infancy or early childhood. The risk
of developing a feeding or eating disorder is increased in
children with intellectual or physical disabilities, [1,2]
autism spectrum disorders [3] and genetic syndromes,
further in premature born children [4]. Neurological
impairments manifesting as oral motor difficulties and
dysphagia often coexist in children with cerebral palsy
and other neurodevelopmental disorders [2,5-7] It is
often unclear whether the avoidant-restrictive food intake
problems can be explained by the comorbid disabilities
or are just associated. Therefore, the pathological foodintake
behaviour cannot exactly be classified as “Avoidant-
Restrictive Food Intake Disorder” (ARFID) because the
criteria D of ARFID according to DSM V cannot exactly
be determined in these children [1].
With regard to this heterogeneous group, it is not
surprising that a more precise classification remains
challenging. Different approaches to classify children with
feeding or eating disorders or to define subgroups have
been undertaken. Subgroups based on symptoms as in the
revised classification, DC:0-3R [8], and in a later approach
from Kerzner B, et al. [9] were defined. Both include
leading symptoms like food selectivity, restricted appetite/
infantile anorexia and fear of eating/posttraumatic feeding
disorder. Although the important role of of comorbidities
is evident in children with eating problems, only two
studies formed subgroups with respect to comorbidities
where cases were not assigned to one group exclusively but
in the majority to a combination of disorders. In the study
by Burklow KA, et al. [10] the cases were most frequently
assigned to the comorbidities “structural-neurologicalbehavioural”
(30/103) and second to the “neurologicalbehavioural”
group (28/103). Neurological diagnoses
included developmental delays, which were present in 75%
of the study population. The study sample of Rommel N,
et al. [11], on the contrary, contained only 11% (69/603)
neurodevelopmental disorders, whereas gastroesophageal
reflux disease was predominant with 60% of the cases
(228/380). Interestingly, oral motor problems were
present in 61% (427/700) while only few children showed
behavioural comorbidities (18.1%; 127/700).
On the other hand, we know from multimorbid
children with disabilities that treatment nowadays are
usually not built on ICD diagnoses but on goals based
on the International Classification of Functioning (ICF),
expecially on functional aspects during daily living and
participation including the environment and using a clientcentred
approach based on the expectances and goals of the
caregivers and if possible the patients themselves.
In consideration of the heterogeneous presentations
of study samples in the literature, comparing outcomes
of children with eating disorders is impeded. Moreover,
the long-term outcome has scarcely been examined with
a follow-up period of more than a year [5,12,13]. Existing
literature often focuses on specific patient characteristics
or differing outcome measurements, predominantly tube
weaning, weight gain or nutrition input as described
in a recent meta-analysis of Sharp WG, et al. [13]. They
describe outcomes of multidisciplinary interventions of
593 patients (age range, 15.7-48 months) in eleven studies,
also including two randomised-controlled trials of 454 tube
dependent patients 71% (95% CI, 54%-83%) were weaned
during intervention and finally, 80% of 414 patients by
follow-up (95% CI, 66%-89%). A decrease in disruptive
mealtime behaviour, parental stress and an increase in food
intake have been seen, whereas weight gain was low, mainly
due to the frequently performed "aggressive", rapid tube
weaning until discharge [13].
Most studies so far focussed on the above stated
“objective” outcome whereas parental perception of the
outcome has not been looked at. It is however crucial to
understand caregivers as they play crucial roles in decision
making, defining therapy goals and treatment; their
appraisal has of high importance for children with complex
chronic disorders.
This study aims to describe the different long-term
outcomes of severe paediatric feeding and eating disorders
from the parents’ perspective with regard to neurological,
neurodevelopmental and psychological comorbidities.
Methods
From a consecutive sample of 253 patients with the
diagnosis of severe feeding or eating disorder in early
childhood (ICD-10 F98.2 “Other feeding disorders of
infancy”) who had undergone an intensive multidisciplinary
inpatient treatment at the age of under 6 years between
2009 and 2016 [14].
The patients had previously received four weeks
of inpatient treatment in a parent-child setting by an
interdisciplinary team of paediatricians, child psychiatrist,
psychologists, speech therapists, physiotherapists,
occupational therapists, social worker, curative teachers and
nurses at a specialized centre for social and developmental
paediatrics.
Data sources/Measurements
Patient characteristics during inpatient stay - retrospective patient chart review (Time T1): Following
data were extracted from medical records: Gender, age at
hospital admission, number and type of comorbidities
(according to ICD-10), perinatal period (gestational age,
birth weight, APGAR scores), level of developmental
delay according to developmental age vs. chronological
age (severe, medium, slight or no delay), level according to
Gross Motor Function Classification System (GMFCS 1
to 5) in children with cerebral palsy, z-score of body-massindex,
successful tube weaning (yes/no) [15,16].
Long-term outcome from parent perspective (Time
T2): Parent Questionnaire on recent status and outcome
of feeding/eating disorder, Burden of Disease and Life
Satisfaction.
A sixty-eight item questionnaire for caregivers was
applied. The questionnaire consisted of the diagnostic
questionnaire for feeding and eating problems developed
including tube feeding in German by Wilken and Jotzo16.
Further, we included questions on anthropometrics
(weight, height), a standardised questionnaire on the
parents’ life satisfaction (LiSat-11), two questions on the
burden of disease experienced by the primary caregiver and
the family and finally, feedback questions on the long-term
outcome in general and in specific areas with respect to
previous intensive inpatient treatment. We focus on the
analysis of following items in the parent questionnaire:
Target parameters:
1. Score of long-term outcome after the inpatient
treatment: "Eating improved in the long term
after this inpatient stay." (10-point Likert scale: 1
"strongly disagree" to 10 "strongly agree.")
2. Score of age-appropriate "normal" mealtime
behaviour: "From my point of view, my Treatment
satisfaction in retrospect: “I am satisfied with the
result of the inpatient eating therapy.” (10-point
Likert scale)
Further questions were:
“Is your child fed by tube/PEG?” (Yes/No, not
anymore/No, never.)
“My child's eating problems are a burden for the
family.” (5-point Likert scale: "Does not apply", "Applies
little", "Applies moderately", "Applies fairly" and "Applies
very much")
“The eating problems burden me as a mother/caregiver.”
(5-point Likert scale)
LiSat-11 item 1: “I feel my life in general is …”
(6-point Likert scale: "very unsatisfied", "unsatisfied",
"rather unsatisfied", "rather satisfied", "satisfied", and
"very satisfied")
Procedure
The first version of the questionnaire was adapted with
support of the multidisciplinary team and checked for comprehensibility in a pilot study of 8 participants and
then approved by a further independent researcher. The
questionnaire was sent by mail including an introductory
letter and a consent form to the 253 eligible earlier
patients. For the planned analysis of four subgroups, a
minimal sample size of 100 participants was calculated. To
reach the calculated return rate the families were reminded
with a phone call and if necessary the questionnaires were
sent again. If needed the questionnaire was completed in a
telephone interview. For inclusion in the study a declaration
of consent signed by both parents and a fully completed
questionnaire were necessary. Then, baseline data were
extracted and previous therapy reports were reviewed for
this sample.
Statistical Analysis
The analysis was carried out with SPSS for Windows
version 27.0 (IBM Corp., Armonk, NY). Data distribution
and descriptive statistics were calculated for all variables
of interest. Drop out analysis was conducted with nonparametric
tests (median two-sample test and Fisher’s exact).
Depending on the level of measurement, the subgroups were compared either with a chi-square independence test
and effect size of Cramer’s V for all nominal variables, with
a Kruskal-Wallis-test for all ordinal scaled variables (Dunn-
Bonferroni post-hoc test and Cohen’s d effect size), or with
a one-sided ANOVA for all metric variables.
Results
From a consecutive sample of 253 treated patients, 107
caregivers were willing to fill in the questionnaires. Four of
them were excluded because of incompleteness or lack of
signatures of both parents. Finally, a sample of 103 patients
(46 girls and 57 boys; mean age 3;3 yrs;months at T1; mean
age 6;9 yrs;months at T2) was included reaching the goal of
areturn rate of at least 40% (103 of 253). The mean time
interval after inpatient treatment was 3 years and 6 months
(range 6 months-7 years) after inpatient treatment. A dropout
analysis revealed no systematic differences between
responders and non-responders with respect to gender,
age distribution during inpatient stay and at follow-up,
year of treatment, and number of comorbidities. Further
information is gathered in Tab. 1.
| Characteristics |
Value |
| Gender, n (%) |
Female |
46 (44.7) |
| Male |
57 (55.3) |
| Age, mean in y; mo. (SD) (range) |
Inpatient stay (T1) |
3;3 (1;11) (0;4-8;10) |
| Follow-Up (T2) |
6;9 (2;10) (1;11-13;6) |
| Time Point of Follow-Up, mean in years; months (SD) (range) |
3;6 (1;11) (0;6-8;11) |
| Comorbidities, mean (SD) |
5.36 (3.1) (0-17) |
| Medical Concerns, n (%) |
Neurological disorders |
35 (34.0) |
| Cerebral palsy (GMFCS mean 4.5) |
20 (19.4) |
| Motor, language or cognitive developmental disorders |
77 (74.8) |
| Level of Developmental Delay |
Slight or None |
46 (44.7) |
| Medium |
33 (32.0) |
| Severe |
24 (23.3) |
| Autism spectrum disorder |
9 (8.7) |
| Psychological-behavioural disorders |
27 (26.2) |
| Other |
Congenital malformations, deformations and chromosomal abnormalities (Q00-99) |
58 (56.3) |
| Chromosomal abnormalities (Q90-99) |
21(20.9) |
| Down syndrome |
9 (8.7) |
| Congenital malformations of the circulatory system |
7 (6.8) |
| Congenital malformations of oesophagus |
3 (2.9) |
| Cleft palate |
3 (2.9) |
| Endocrine, nutritional and metabolic diseases (E00-89) |
17 (16.5) |
| Diseases of the digestive system |
12 (11.7) |
| Gastroesophageal reflux disease |
3 (2.9) |
| Perinatal Period |
Prematurity (< 37 wks.) (n=92) |
49 (53.3) |
| Low Birth Weight (<2500 g) (n=88) |
45 (51.1) |
| Z-Score of BMI, mean (SD) (range) |
Inpatient Stay (T1) (n=97) |
-1.38 (1.64) (-7.00-3.00) |
| Follow-Up (T2) (n=90) |
-1.15 (1.76) (-4.70-3.60) |
| Tube feeding at one point of life, n (%) |
61 (59.2) |
| Tube feeding during inpatient stay (T1), n (%) |
45 (43.7) |
| Tube weaned during inpatient stay (T1), n (%) |
5 out of 45 (11.1) |
| Tube weaned at Follow-Up (T2), n (%) |
26 out of 45 (57.8) |
Tab. 1. Patient characteristics (N=103).
Subgroup analysis
Definition of subgroups: The subgroups are based
on three predominant areas of comorbidities according to
ICD-10 in order to compare their role on the outcome of
ARFID diagnoses as shown in Tab. 1.
The first group contains 28 children (27.2%) with
neurological diagnoses (“Neuro-group”), mainly cerebral
palsy (G80.x), epilepsy (G40.x) and also spinal muscular
atrophy (G12). Twenty-four children (23.3%) with
prevailing psychological or behavioural diagnoses (F00.x
to F98.x) additionally to the eating or feeding disorder
were assigned to the second group (“Psy-group”). The third
subgroup comprises 22 children (22.7%) with notable
developmental delays including genetic syndromes and
metabolic disorders without additional neurological or
psychiatric diagnoses (“DD-group” i.e. neither "Neuro"
nor "Psy"). Finally, a group of 29 patients (28.2%) with
normally developing children without neurological and
psychiatric/behavioural disorders (“Non-DNP-group” i.e.
no developmental/neurological/psychiatric comorbidity)
was assembled.
Characteristics
Age at inpatient stay differs significantly between groups
(F(3.99) = 4.11; p = 0.009). Pairwise comparison shows a
significant difference between the Non-DNP-group with a
mean age of 2.46 (SD 1.63) and the Psy-group with a mean
age of 4.1 years (SD 1.36; p = 0.005). Mean age of Neurogroup
was 3.0 yrs. (SD 1.8 yrs. and of the DD-group 3.4
yrs. (SD 2.0).
On average, 6.4 (SD 3.1, range 1-18) diagnoses
according to ICD-10 were given per patient. A significantly
lower number of diagnoses was assigned to the Non-DNPgroup
with a mean of 4.1 (SD 2.14) in contrast to the other
groups (F(3,99) = 7.766; p < 0.001) (mean/SD: Psy-group
7.83/2.93, Neuro-group 7.54/3.27, DD-group 6.23/2.71).
Group differences in the degree of developmental delay
are highly significant (χ2(6) = 66.82, p < 0.001, V = 0.57)
as indicated in the subgroup characteristics.
The level of care rated by the official health insurance
system in Germany differs highly between subgroups
(F(3,99) = 25.40; p <0.001) in the following ascending
order: Non-DNP-group (mean 1.38), Psy-group (2.71),
DD-group (3.41) and finally Neuro-group (4.5). This is
consistent with the high mean GMFCS-level of 4.5 of
children with cerebral palsy in the Neuro-group.
The average gestational age was generally low (34.7
weeks; SD 5.5). For the non-DNP-group, a non-significant
trend towards lower birth weight and gestational age has
been observed. 67.9% of Non-DNPs (19 of 29, Missing
1) were premature born in contrast to a range of 40.0 to
57.1% in the other groups. Low birth weight (<2500 g)
was most found in Non-DNP-cases (18 of 29, 66.7%,
Missing 2) and only in 41.2 to 45.8% of the other groups.
No significant group difference was found for 5-minute Apgar score, gender, follow-up period, and age at followup.
Both time points evaluated indicated a mean BMI
z-score below average with -1.38 at T1 and -1.15 at T2
(T1 vs. T2 n.s.). No statistical intergroup difference in both
time points was found.
Target parameters
General long-term outcome: From the parents’ point
of view, the outcome results were heterogeneous (Fig. 1).
The long-term outcome at follow-up (T2) was described as
positive by 57.3% of the parents (scale scores 6-10 out of
10). The ratings on long-term outcome varied significantly
among groups (p = 0.01; H = 11.27; n = 102). In pairwise
comparison, the best group on average, the Non-DNPgroup
(Median 8), differed greatly from the group with
lowest ratings, the Psy-group (Median 5, p = 0.006; r =
-3.3).
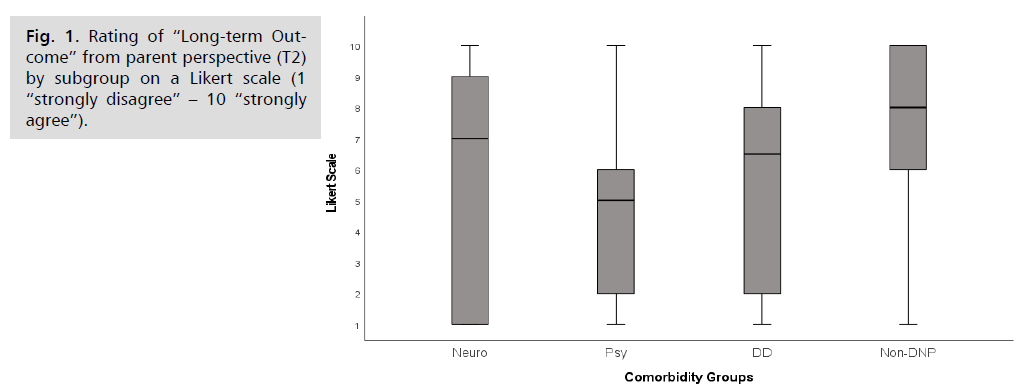
Fig 1: Rating of “Long-term Outcome” from parent perspective (T2) by subgroup on a Likert scale (1 “strongly disagree” – 10 “strongly agree”).
Age-appropriate eating: A large proportion of children
have by no means achieved age-appropriate eating by
follow-up ((mean 4.04 out of 10; s. (Fig. 2)). 37.6% of
the children were still rated with the lowest scale value
(score 1 out of 10). On the other hand, merely 22.8% of
the children were described as eating appropriately for their
age (scale scores 8-10 out of 10). General differences were
found between the subgroups (p = 0.001; H = 16.26; n =
101). The Neuro- (Median 1) and the DD-group (Median
2) showed the least age-appropriate mealtime compared
to the Non-DNP cases with significantly better results
(Median 6, p < 0.001 and p = 0.044).
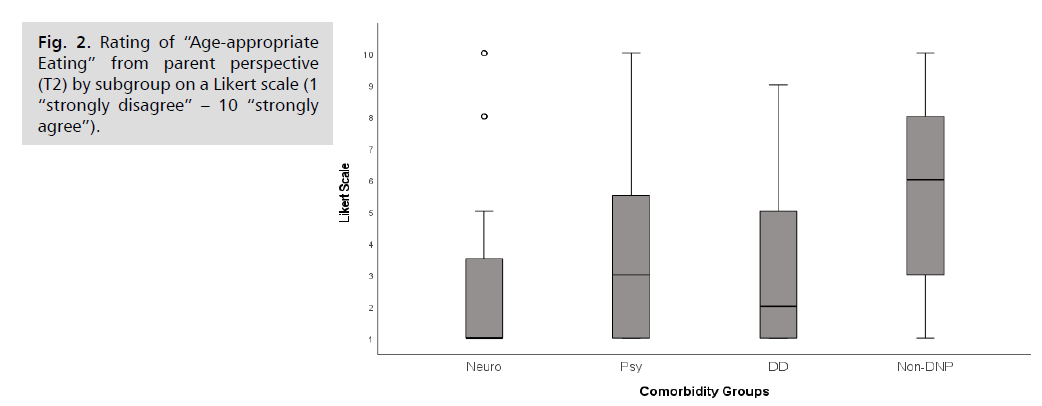
Fig 2: Rating of “Age-appropriate Eating” from parent perspective (T2) by subgroup on a Likert scale (1 “strongly disagree” – 10 “strongly agree”).
Other items of the parent questionnaire
At follow-up 61% of the parents have been rather
satisfied with the result of the former inpatient treatment
(Fig. 3). A general difference between groups was found
(p = 0.038; H = 8.44; N = 100) with pairwise differences
in between the most satisfied group, the Non-DNP-group
(Median 9), and the Psy-group (Median 5; p = 0.05) with
the lowest results. The ratings of the Neuro-group were
similar to the Non-DNP-group (Median 8, Interquartile
range 8; DD-group: Median 5.5).
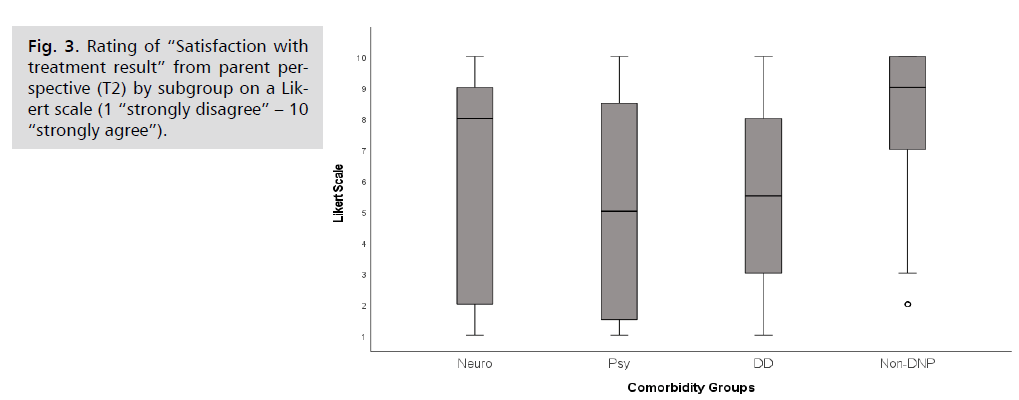
Fig 3: Rating of “Satisfaction with treatment result” from parent perspective (T2) by subgroup on a Likert scale (1 “strongly disagree” – 10 “strongly agree”).
During the inpatient stay, tube feeding was performed
in 45 out of 103 cases. At follow-up, 12 out of 13 patients
(92.3%) of the Non-DNP cases were successfully tube
weaned, compared to less than half of the initially tube-fed
children in all other groups (Neuro: 7 of 17; Psy: 2 of 6;
DD: 5 of 9). However, requirements for statistical testing
were not met.
On average, three years after the inpatient stay, the
burden of disease was still high for many of the families and
primary caregivers. 35.4% of the families and 39.2% of
the primary caregivers continued to be negatively affected
by the child's eating disorder (scale scores 4-5 out of 5; N
= 96) while only 40% of the parents or caregivers are not
or only slightly burdened (scale scores 1-2 of 5). Again, significant group differences were found (H = 8.1; p =
0.044; mean ranks: Psy 60.9, n = 19; DD 53.1, n = 22;
Neuro 45.4, n = 28; Non-DNP 39.2, n = 27). The most
stressed families were among the children in the Psy-group
with the highest mean rank and differing significantly from
the least stressed group, the Non-DNP group in post-hoc
analysis (z = 2,66; p = 0.046).
The caregivers’ general life satisfaction (item 1 of
LiSat-11) was good with a mean of 4.79 out of 6 (SD 1.10;
Median 5; variance 1.22; n = 100). A clear trend towards
a lower life satisfaction was observed among caregivers
of children in the Psy-group: 27.2% (6/22) rated their
"life overall" as "very unsatisfied" to "rather unsatisfied" compared to only 9% (2/22) of the DD-group, 7% (2/28)
of the Neuro-group, and 7% (2/28) of the Non-DNP.
Discussion
Paediatric feeding and eating disorders have already
been analysed with respect to comorbidities about 20
years ago [10,11]. This is the first study to examine the
differences of long-term outcomes of severe paediatric
feeding and eating disorders with regard to subgroups of
predominant neurological, developmental and behavioural
comorbidities. The caregivers’ perspective with respect
to these subgroups has been examined and related to the
outcome.
Intellectual and neurological disabilities as well as
neurobehavioral problems were found to be relevant
for long-term outcome with regard to aspects like ageappropriate
eating or burden of disease perceived by the
parents. Positive predictors seem to be age-appropriate
development, absence of severe neurological and behavioural
conditions and early age at treatment. Prematurity and low
birth weight may contribute to the development of early
eating disorder, [17] but seem to have no negative impact
on the long-term outcome when complex neurological or
behavioural conditions are absent.
Comparison of outcomes with the existing literature is
limited because insufficient details on patient characteristics
and symptom severity (e.g. developmental delay) are
provided as noted in a recent meta-analysis [13]. The
heterogeneity of outcome measurements poses additional
challenges [13].
Schadler G, et al. [5] analysed a population of preterm
born children presenting similar comorbidities as in the
present study (neurological impairment, developmental
delays and interaction problems) who achieved in 61.6%
(52 of 83 patients) an overall long-term obtainment of
the initial treatment success after a median follow-up of 3
years. However, subgroups have not been analysed.
With respect to the complex and severe comorbidities
in this study, it seems reasonable that the frequency of
successful tube weaning is relatively low (57.8%) compared
to the literature, e.g. with 80% of 414 patients by followup
(95% CI, 66%-89%) [13]. This stays in line with
literature describing a worse outcome in children with
neurodevelopmental issues or other comorbidities like
metabolic diseases [18,12] which is, however, not stated in
all studies [4]. Marinschek S, at al. [4] described a tube
weaning rate of 92.3%, independent of comorbidities like
genetic syndromes (26%), prematurity (23%), CP (7%)
and ASD (6%), after a follow-up period of 1 to 6 years
in a sample of 266 participants. At the same time, 68%
ate an age-appropriate diet at follow-up and only few ate
selectively (12.5%). However, the numbers of children
with neurodevelopmental and neurobehavioral issues were
possibly too small to detect significant differences. Further,
no information was provided on severity.
There are two studies consisting of populations with
prevailing gastrointestinal comorbidities using a similar
outcome measurement [19,20]. A trial with a follow-up
period of approximately one year examined the outcome
of 67 patients with a similar 5-point Likert scale reaching a
good improvement in 97% (Likert scale 4-5 of 5) hereafter
[20]. A randomized-controlled trial conducted in the same
clinic regarding a five day long intervention, reported after
a short follow-up (mean 36 days, 3 Missing Data) a mean
result of 3.6 on a 5 point Likert scale for age-appropriate
“normal” eating in the intervention group (n = 10) [19].
The mean of 4.04 on a 10 point Likert scale and a much
longer follow-up period in the present study, raises the
question whether the study samples are comparable. This
supports the hypothesis that eating disorders in children
with medical problems like congenital heart diseases, GERD and other gastrointestinal comorbidities have
better outcomes than those with neurodevelopmental and
behavioural comorbidities.
There are indications that general life satisfaction is
lower among caregivers of children with major behavioural
comorbidities who, at the same time, show the poorest
long-term outcome. In contrast, it is surprising that parents
of children with multiple severe disabilities (CP, mean
GMFCS 4.5) perceive such a positive outcome and high
satisfaction with previous inpatient treatment, despite the
poor long-term outcome with respect to age-appropriate
eating behaviour. Moreover, parents of children with
severe CP described normal life satisfaction. This may
be interpreted that these parents have developed good
coping strategies and appropriate long-term expectations
with respect to the limited ability of improvement in their
severely handicapped children. On the other hand, we
suggest that the caregivers of children with behavioural
comorbidities and to some extent also parents of children
with developmental disabilities, mainly caused by genetic
syndromes may need more coaching in order to work on
realistic goals and coping with the eating problems of their
children.
Limitations
The strength of this study is the large sample size
with a wide range of comorbidities and the long followup
period. Certainly, the sample shows a bias towards
neurodevelopmental disorders and disabilities with a fairly
low prevalence of gastroenterological and surgical cases.
These comorbidities have usually been excluded or been
treated in regional paediatric hospitals before admission
to the special centre of developmental paediatrics. Based
on these findings, a prospective longitudinal study would
be useful to examine more precisely the influence of
comorbidities on the prognosis of eating disorders in
childhood.
Conclusion
Comorbidities are crucial parameters for the longterm
outcome of early eating and feeding disorders as
perceived by parents and should be taken into account in
the treatment. A multidimensional ICF-based description
of eating disorders in childhood could be useful both in
clinical practice and in further studies. A training and
coaching of caregivers concerning problem perception
and coping strategies together with realistic long-term
expectations seems to be necessary. Especially, high
burden and stress in parents of children with behavioural
comorbidities may need more attention than expected over
a longer period of time.
Highlights
• Normal development and absence of neurological
and behavioural disorders predict good outcome in
infant eating disorders
• Prematurity alone shows no negative impact on the
long-term outcome
• Severe feeding and eating disorders in children with
neurodevelopmental and behavioural disorders
persist over several years
• In spite of low long term improvements of the
eating disorders, caregivers of children with
neurological impairments are much more satisfied
with the previous treatment
• Caregivers of children with behavioural comorbidities are in need of realistic long-term
expectations, ie. achievable goals and coping
strategies.
Conflicts of Interest
The authors declare that they have no relevant or
material financial interests that relate to the research
described in this paper.
The authors confirm that they had no pharmaceutical
or industry support for this study that require
acknowledgement.
REFERENCES
- Diagnostic and Statistical Manual of Mental Disorders DSM-5. Washington, D.C: American Psychiatric Association; 2013.
- Goday PS, Huh SY, Silverman A, et al. Pediatric feeding disorder: Consensus definition and conceptual framework. J Pediatr Gastroenterol Nutr. 2019;68(1):124-129.
Google Scholar, Crossref, Indexed at
- Marshall J, Ware R, Ziviani J, et al. Efficacy of interventions to improve feeding difficulties in children with autism spectrum disorders: a systematic review and meta-analysis. Child Care Health Dev. 2015;41(2):278-302.
Google Scholar, Crossref, Indexed at
- Marinschek S, Pahsini K, Scheer PJ, et al. Long-term outcomes of an interdisciplinary tube weaning program: A quantitative study. J Pediatr Gastroenterol Nutr. 2019;68(4):591-594.
Google Scholar, Crossref, Indexed at
- Schadler G, Suss-Burghart H, Toschke AM, et al. Feeding disorders in ex-prematures: causes - response to therapy - long term outcome. Eur J Pediatr. 2007;166(8):803-808.
Google Scholar, Crossref, Indexed at
- Sharp WG, Berry RC, McCracken C, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. 2013;43(9):2159-2173.
Google Scholar, Crossref, Indexed at
- Gosa MM, Carden HT, Jacks CC, et al. Evidence to support treatment options for children with swallowing and feeding disorders: A systematic review. J Pediatr Rehabil Med. 2017;10:107-136.
Google Scholar, Crossref, Indexed at
- Three Zt. DC:0-3R: Diagnostic classification of mental health and developmental disorders of infancy and early childhood. Washington, DC: Zero To Three; 2005.
- Kerzner B, Milano K, MacLean WC, et al. A practical approach to classifying and managing feeding difficulties. Pediatr. 2015;135(2):344-353.
Google Scholar, Crossref, Indexed at
- Burklow KA, Phelps AN, Schultz JR, et al. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998;27(2):143-147.
Google Scholar, Crossref, Indexed at
- Rommel N, De Meyer AM, Feenstra L, et al. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003;37(1):75-84.
Google Scholar, Crossref, Indexed at
- Krom H, Meij TGJd, Benninga MA, et al. Long-term efficacy of clinical hunger provocation to wean feeding tube dependent children. Clin Nutr. 2020;39(9):2863-2871.
Google Scholar, Crossref, Indexed at
- Sharp WG, Volkert VM, Scahill L, et al. A systematic review and meta-analysis of intensive multidisciplinary intervention for pediatric feeding disorders: How standard is the standard of care?. J Pediatr. 2017;181:116-124.
Google Scholar, Crossref, Indexed at
- Organization WH. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. 1992.
- Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. Perzentile für den body-mass-index für das Kindes- und jugendalter unter heranziehung verschiedener deutscher stichproben. Monatsschrift Kinderheilkunde. 2001;149:807-818.
Google Scholar, Indexed at
- Wilken M, Jotzo M. Ersterhebungsbogen bei Therapien von Fütterstörungen oder Sondenentwöhnung. In: Bolten M, Möhler E, Gontard Av editors. Psychische Störungen im Säuglings- und Kleinkindalter. Göttingen; Bern; Wien [u.a.]: Hogrefe; 2013:134-138.
- Burklow KA, McGrath AM, Valerius KS, et al. Relationship between feeding difficulties, medical complexity, and gestational age. Nutr Clin Pract. 2002;17(6):373-378.
Google Scholar, Crossref, Indexed at
- Trabi T, Dunitz-Scheer M, Kratky E, et al. Inpatient tube weaning in children with long-term feeding tube dependency: A retrospective analysis. Infant Ment Health J. 2010;31(6):664-681.
Google Scholar, Crossref, Indexed at
- Sharp WG, Stubbs KH, Adams H, et al. Intensive, manual-based intervention for pediatric feeding disorders: results from a randomized pilot trial. J Pediatr Gastroenterol Nutr. 2016;62(4):658-663.
Google Scholar, Crossref, Indexed at
- Sharp WG, Volkert VM, Stubbs KH, et al. Intensive multidisciplinary intervention for young children with feeding tube dependence and chronic food refusal: An electronic health record review. J Pediatr. 2020;223:73-80.
Google Scholar, Crossref, Indexed at








