Introduction
Infective or Mycotic Aneurysm (MA) is an infectious arterial wall degradation and dilation. The terminology was coined by Osler in 1885, for aneurysms originating due to heart valve infection [1]. The neurological complications arising can be potentially fatal including intracerebral bleeds, subarachnoid hemorrhages, ischemic stroke, seizures, and neuropathies [2-4]. Gastrointestinal complications include painful expanding mass, aortic aneurysms, gastrointestinal bleeding, acute mesenteric ischemia and hemoptysis [5,6]. Other complications include peripheral ischemia, coronary aneurysm, heart failure, abscess, and renal ischemia.
The Overall incidence of IIA is around 0.7-5.4% of all cerebral aneurysms worldwide and an incidence of 1.6 - 18% in developing countries [7-10]. Most of the IIA are caused due to bacterial infections (72.8%) followed by fungal infection (13.2%) [11,12]. Most IIA arise from infective endocarditis. The incidence of the IIA due to bacterial meningitis and subsequent intracranial hemorrhage is rare. Only a few cases of ruptured IIA secondary to bacterial meningitis have been reported [13-16]. Revised criteria regarding diagnosis and management have been put forth in this paper.
Case Report
A 75-year-old male patient with history of chronic smoking, hypertension, and type 2 diabetes mellitus presented with sudden onset altered sensorium and left eyelid drooping of 1-day duration. Patient had a blood pressure of 140/80 mm of Hg, pulse rate of 104/minute and temperature of 101.4°F (38.6°C). Physical examination revealed a lethargic elderly male, who was disoriented to time and place but oriented to person, following simple instructions and incoherent speech. The patient’s left pupil was 6 millimeter in size, round, and fixed. The right pupil was 4 millimeter, round, and reactive. Right lower limb strength of 3/5 Medical Research Council (MRC) grade and 5/5 MRC grade in all other limbs [17].
Blood investigation revealed leukocytosis with total count of 16,100 cells/mm3, with differential count favoring neutrophilia (84%), platelet count 340,000/mm3, blood urea - 99 mg%, serum creatinine - 2.0 mg%, serum electrolytes Sodium – 121 mmol/L, Potassium - 5.5 mmol/L, Chloride - 90 mmol/L, blood Glucose level – 456 mg/dL. Activated partial prothrombin time of 40.3 sec and control - 30 sec and glycated hemoglobin (Hb A1c) - 15.9%. Arterial blood gas analysis showed uncompensated metabolic acidosis with an anion gap. Multiple blood culture sets were obtained and were negative. Brain Magnetic Resonance Imaging (MRI) revealed a solitary tiny focus of restricted diffusion appearing hyperintense on diffusion-weighted imaging (DWI) with corresponding hypo intense Apparent Diffusion Coefficient (ADC) signal noted in the subcortical region of the left occipitotemporal lobe suggesting an acute infarct (Figure 1). Lumbar puncture demonstrated a white cell count of 195 with predominantly neutrophils (97%) and few lymphocyte (3%) with elevated glucose of 136 mg/dL, protein of 64.8 mg/dL, chloride of 118 mmol/L and adenosine deaminase (ADA) of 1.2 U/L. Transthoracic Echocardiography did not demonstrate any valvular lesion or vegetation and had an ejection fraction of 60%. Carotid Doppler study demonstrated mild atherosclerotic carotid vessel disease. Cerebral angiography was withheld due to underlying chronic kidney disease.
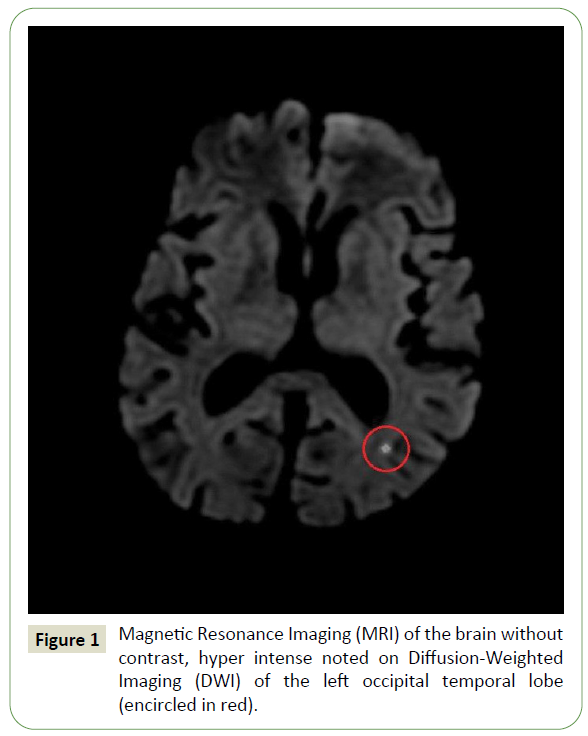
Figure 1: Magnetic Resonance Imaging (MRI) of the brain without contrast, hyper intense noted on Diffusion-Weighted Imaging (DWI) of the left occipital temporal lobe (encircled in red).
A provisional diagnosis of acute ischemic stroke secondary to vasculopathy from bacterial meningitis was made and treated accordingly. The patient’s mental status returned to baseline, hemodynamic stability was achieved and was transferred out of the Intensive Care Unit (ICU).
After 48hours of transferring out, the patient had a sudden onset of unresponsiveness. Examination showed decerebrate posturing, intubated for airway protection, and transferred to ICU. Noncontrast Computed Tomography (CT) of the head demonstrated a large parenchymal hematoma in the left temporal lobe with intraventricular extension and a significant mass effect (Figure 2). The rupture of the mycotic aneurysm secondary to meningitis was suspected. Urgent decompressive hemicraniectomy was planned but not performed due to family reluctance. The patient was terminally extubated.
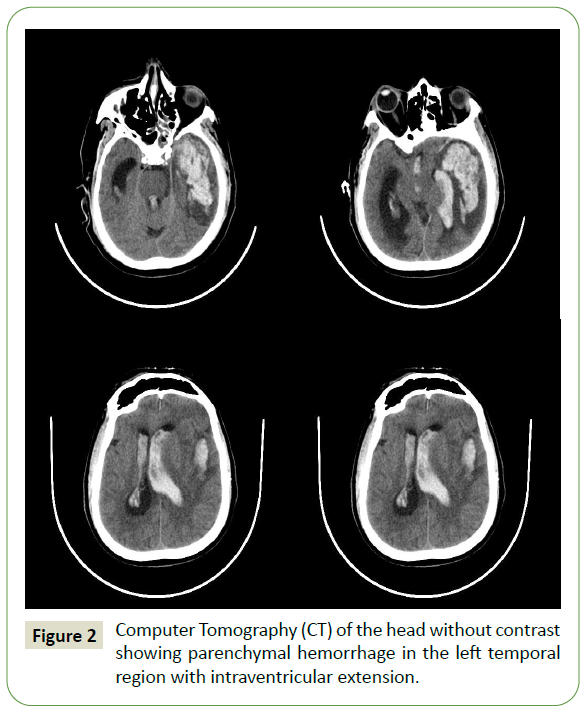
Figure 2: Computer Tomography (CT) of the head without contrast showing parenchymal hemorrhage in the left temporal region with intraventricular extension.
Discussion
Bacterial and fungal infections accounted for the majority of IIA. Most common organisms are Streptococcus species (30.7%), Staphylococcus species (19.4%), Aspergillus (7.5%), Candida (2.8%), Enterococcus (2.4%) and Pseudomonas (2.2%) interestingly, 12.7% of the patients were culture negative (Table 1) [12]. In fewer than 2% of cases, virus and parasites have been implicated [18,19]. In IIA where meningitis was proven to be the cause, most (75%) were due to bacterial and tuberculous infections and the rest (25%) was accounted for by fungal infections. Of the IIA caused by meningitis, 58% succumbed to the illness or its complication [20].
| |
Pathogen |
Percentage |
| Bacterial |
Streptococcus viridans |
25.5 |
| |
Staphylococcus aureus |
17.4 |
| |
Streptococcus pyogenes |
2.6 |
| |
Streptococcus pneumoniae |
2.6 |
| |
Enterococcus |
2.4 |
| |
Pseudomonas aeruginosa |
2.2 |
| |
Staphylococcus epidermidis |
1.8 |
| |
Other bacterial |
18.3 |
| Fungal |
Aspergillus |
7.5 |
| |
Candida |
2.8 |
| |
Mucormycetes |
1.3 |
| |
Other fungal |
1.7 |
| Viral |
-- |
0.6 |
| Parasitic |
-- |
0.7 |
| Culture negative |
-- |
12.7 |
Table 1 Percentage of pathogens reported in patients with IIA.
Similar to the non-infective aneurysm, pathology of IIA starts with the degradation of the arterial wall. Pathophysiology develops from either (1) the seeding of septic emboli into the vasa-vasorum, (2) systemic bacteremia infecting a preexisting intimal defect, (3) contiguous infection from adjacent structures, (4) inoculation due to iatrogenic or accidental trauma and or combination of above factors [21,22]. After inoculation, bacteria activate proinflammatory mediators which attract neutrophils to the arterial wall [23]. Bacteria release various proteases to activate human matrix metalloproteinases (proMMP 1, 8 and 9) and inactivate human plasma α1-proteinase inhibitor leading to disintegration of extracellular matrix and activation of neutrophil elastase [24,25]. Neutrophils release enzymes like elastin-degrading serine proteases and neutrophil gelatinase-associated lipocalin [26,27]. Activation of various bacterial and neutrophilic enzymes leads to the focal weakening of arterial wall and degradation of the surrounding extracellular matrix [21,28]. Infiltration of the vasa vasorum by neutrophil leads to weakening of the adventitia, muscularis, and then the internal elastic membrane [29,30]. Early dilation of the proximal and distal ends of the involved arterial wall is observed, followed by the consistent dilation of the complete segment Pulsation of the dilated and weakened arteries causes the formation of an aneurysm which can eventually rupture [29].
A point scoring based system was proposed by Korja M, et al. [31], which considered various clinical markers and investigative findings. The diagnostic criteria included the presence of one mandatory criterion and 12 supportive criteria (Table 2) [31]. A score of 3 or more is clinically definite diagnosis of IIA, a score of 2 was suggestive of a probable IIA and a score of 1 would be suggestive of possible IIA.
| Mandatory criterion: |
Supportive criteria: |
| Intracranial aneurysm on Neurovascular imaging |
- Infective endocarditis
- Meningitis
- Orbital cellulitis
- Cavernous sinus thrombophlebitis
- Multiplicity
- Distal location
- Fusiform shape
- Change is the shape and size of a new aneurysm on follow-up imaging
- Age less than 45y
- Fever/History of fever ≥ 7 days
- Recent lumbar puncture
- Intraparenchymal hemorrhage in brain imaging
|
| Diagnosis of IIA based on the above criteria. |
| Definitive |
Mandatory criterion plus three or more supportive criteria satisfied. |
| Probable |
Mandatory criterion plus two supportive criteria satisfied. |
| Possible |
Mandatory criterion plus one supportive criterion satisfied. |
Table 2 Proposed diagnostic criteria for infective intracerebral aneurysm.
In the case discussed above, due to abnormal baseline renal functions, carotid ultrasound Doppler was performed instead of angiography. Cerebral angiography (CA) is considered the gold standard for neurovascular imaging [32,33]. It can reliably detect small cerebral aneurysms up to 2 millimeters in size [34]. Alternatively, Computed tomography Angiography (CTA) or magnetic resonance angiography (MRA) can be used [35].
The management can be divided into conservative, endovascular, and surgical depending on the risk of rupture [11,12]. Risk of aneurysm rupture is high with any of the following, (1) size > 7 millimeter, (2) patient age > 60 years, (3) female gender, (4) posterior circulation location, (5) symptomatic (6) ethnicity (e.g. Finnish and Japanese), (7) comorbidities (hypertension, active smoking) (8) previous history of subarachnoid hemorrhage, and (9) history of ruptured aneurysm [36-38]. A guideline has been proposed for the treatment of IIA (Figure 3). All patients should be started on conservative management and planned for endovascular or surgical intervention. A patient with a low risk of rupture or those that are unable to undergo surgical or endovascular intervention can be managed conservatively [39].
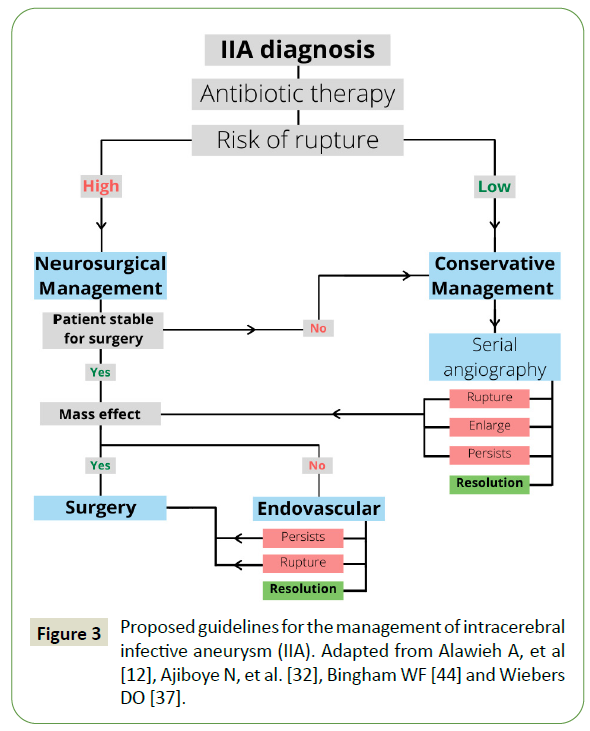
Figure 3: Proposed guidelines for the management of intracerebral infective aneurysm (IIA). Adapted from Alawieh A, et al [12], Ajiboye N, et al. [32], Bingham WF [44] and Wiebers DO [37].
The conservative management consists of long-term antibiotic course and serial angiographic examinations [11,40]. Ideally, the choice of antibiotic is based on the culture sensitivity reports. However, no delay should be made in treating bacterial meningitis empirically with 3rd or 4th generation cephalosporin, ceftriaxone (2 gram intravenous every 12h hourly) or cefepime (2 gram intravenous every 8th hourly) and vancomycin (15-25 milligram/kilogram body weight intravenous every 12th hourly). Addition of ampicillin (2 gram intravenous every 4th hourly) for patients aged above 50 years (Figure 4) [41-43]. Intravenous dexamethasone (10 milligram every 6th hourly) to be started along with antibiotics and continued for 3 to 4 days [44]. 4 to 6 weeks duration of antibiotics has been supported in existing literature [40]. Serial cerebral angiography should be started 1-2 weeks after initiation of antibiotic therapy and followed up with angiography every 2 to 3 weeks. Supportive care and treatment of complications should be done according to protocols.
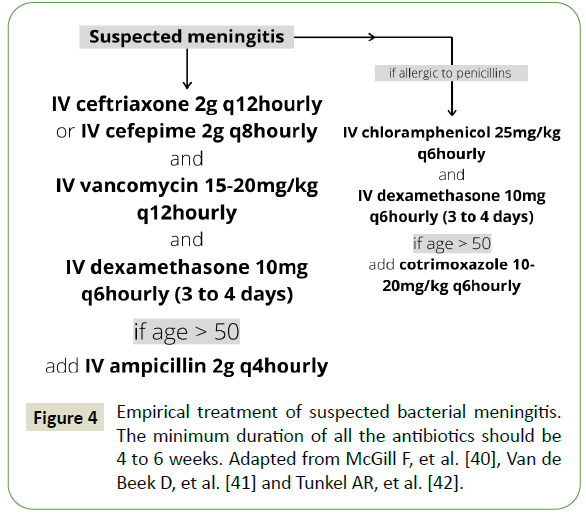
Figure 4: Empirical treatment of suspected bacterial meningitis. The minimum duration of all the antibiotics should be 4 to 6 weeks. Adapted from McGill F, et al. [40], Van de Beek D, et al. [41] and Tunkel AR, et al. [42].
Surgical intervention involves craniotomy and clipping of the aneurysm. Other surgical or microsurgical techniques include excision, trapping, bypass, and wrapping. Endovascular techniques include coiling, liquid embolic agents, ballooning, and flow-diverting stents [12].
Ducruet AF, et al. [11] and Serra R, et al. [20] in a comprehensive review of IIA concluded that 62% of patients had a better outcome after the endovascular or surgical intervention, 20% had a progressive neurological decline, 5% died due to the complication of the aneurysm before the intervention, and 12% died immediately after intervention was performed [11,12]. Overall mortality from IIA has significantly reduced from 36.5% prior to the year 2000 to 12.1% in the last decade [12]. IIA are associated with a very high mortality risk (60-90%) in ruptured cases and a comparatively lower mortality risk (10-30%) in un-ruptured cases. Endovascular interventions for intracerebral aneurysms have a total morbidity and mortality of 9.8% as compared to 12.6% for open surgical procedures [37]. Cumulative 1 and 5-year survival rates with open surgical and endovascular interventions were 78% vs. 71-75% and 69% to 53-41% respectively [38-51].
Conclusion
Because of the nature of the disease, the early diagnosis and management of IIA is challenging. Patients who present with features suggestive of meningitis and neurological deficit must undergo urgent brain imaging along with neurovascular studies to evaluate for IIA. No delay in initiation of empiric antibiotics along with early surgical or endovascular management based on the proposed guideline can be potentially lifesaving. Serial angiographic monitoring of the lesion and timely adjustments in management should be done.
39690
References
- Osler W (1885) The Gulstonian lectures on malignant endocarditis. Br Med J. 1(1263):522.
- Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, et al. (2017) Challenges in infective endocarditis. J Am Coll Cardiol. 69(3):325-344.
- Sotero FD, Rosário M, Fonseca AC, Ferro JM (2019) Neurological complications of infective endocarditis. Curr Neurol Neurosci Rep. 5:1-8.
- Sörelius K, Wanhainen A, Wahlgren CM, Langenskiöld M, Roos H, et al. (2019) Nationwide study on treatment of mycotic thoracic aortic aneurysms. J Vasc Endovasc Surg. 2:239-246.
- Benhassen LL, Højsgaard A, Terp KA, de Paoli FV (2018) Surgical approach to a mycotic aneurysm of the pulmonary artery presenting with haemoptysis: A case report and a review of the literature. Int J Surg Case Rep. 50:92-96.
- Schmidt F, Dinkel HP (2002) Entwicklung eines mykotischen Aneurysmas der A. mesenterica superior nach septischer Embolie. Der Radiologe. 7:564-567.
- Frazee JG, Cahan LD, Winter J (1980) Bacterial intracranial aneurysms. J of neuro surg. 5:633-641.
- Patra P, Ricco JB, Costargent A, Goueffic Y, Pillet JC, et al. (2001) Infected aneurysms of neck and limb arteries: A retrospective multicentre study. Ann Vasc Surg. 2:197-205.
- Morawetz RB, Karp RB (1984) Evolution and resolution of intracranial bacterial (mycotic) aneurysms. Neurosurg. 1:43-49.
- Meena AK, Sitajayalakshmi S, Prasad VS. Murthy JM (2000) Mycotic aneurysm on posterior cerebral artery: Resolution with medical therapy. 3:276.
- Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, et al. (2010) Intracranial infectious aneurysms: A comprehensive review. Neuro Rev. 1:37-46.
- Alawieh A, Chaudry MI, Turner RD, Turk AS, Spiotta AM (2018) Infectious intracranial aneurysms: a systematic review of epidemiology, management, and outcomes. J neuro interv surg. 7:708-716.
- Choi KH, Park MS, Kim JT, Nam TS, Choi SM, et al. (2012) Infectious intracranial aneurysm presenting with series of strokes as a complication of pneumococcal meningitis. Eur neuro. 4:260.
- Cuesta AP, Llorente AM, Ordóñez O, de Aragón AM (2017) Aneurisma micótico intracraneal en lactante con meningitis neumocócica. Enfermedades infecciosas y microbiología clínica. 4:267-269.
- Griffiths SJ, Sgouros S, James G, John P (2000) Intraventricular haemorrhage due to ruptured posterior inferior cerebellar artery aneurysm in tuberculous meningitis. Childs Nerv Syst. 12:872-874.
- Heidelberger KP, Layton WM, Fisher RG (1968) Multiple cerebral mycotic aneurysms complicating posttraumatic pseudomonas meningitis: Case report. J neurosurg. 6:631-635.
- Compston, Alastair (2010) Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. Figures and diagrams with aids to the examination of the peripheral nervous system. By Michael O’Brien for the Guarantors of Brain, Saunders Elsevier (2010) Figures. 133:2838-2844.
- Mahadevan A, Tagore R, Siddappa NB, Santosh V, Yasha TC, et al. (2008) Giant serpentine aneurysm of vertebrobasilar artery mimicking dolichoectasia: An unusual complication of paediatric AIDS. Report of a case with review of the literature. Clin. Neuropathol. 1:37-52.
- Modi G, Ranchod K, Modi M, Mochan A (2008) Human immunodeficiency virus associated intracranial aneurysms: Report of three adult patients with an overview of the literature. 1:44-46.
- Kannoth S, Iyer R, Thomas SV, Furtado SV, Rajesh BJ, et al. (2007) Intracranial infectious aneurysm: Presentation, management and outcome. J Neurol Sci. (1-2):3-9.
- Deipolyi AR, Rho J, Khademhosseini A, Oklu R (2016) Diagnosis and management of mycotic aneurysms. Clin Imaging. 2:256-262.
- Lee WK, Mossop PJ, Little AF, Fitt GJ, Vrazas JI, et al. (2008) Infected (mycotic) aneurysms: Spectrum of imaging appearances and management. Radiographics. 7:1853-1868.
- Parkhurst GF, Decker JP (1995) Bacterial aortitis and mycotic aneurysm of the aorta: A report of twelve cases. Am. J. Pathol.5:821.
- Okamoto T, Akaike T, Suga M, Tanase S, Horie H, et al. (1997) Activation of human matrix metalloproteinases by various bacterial proteinases. J Biol Chem. 9:6059-6066.
- Potempa J, Watorek W, Travis J (1986) The inactivation of human plasma alpha 1-proteinase inhibitor by proteinases from Staphylococcus aureus. J Biol Chem. 30:14330-14334.
- Buckmaster MJ, Curci JA, Murray PR, Liao S, Allen BT, et al. (1999) Source of elastin-degrading enzymes in mycotic aortic aneurysms: bacteria or host inflammatory response. Cardiovasc Surg. 1:16-26.
- Serra R, Grande R, Montemurro R, Butrico L, Caliò FG, et al. ( 2015) The role of matrix metalloproteinases and neutrophil gelatinase-associated lipocalin in central and peripheral arterial aneurysms. Surg. 1:155-162.
- Dobrin PB, Mrkvicka R (1994) Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 4:484-488.
- Molinari GF, Smith L, Goldstein MN, Satran R (1973) Pathogenesis of cerebral mycotic aneurysms. Neurol. 4:325.
- Nakata Y, Shionoya S, Kamiya K (1968) Pathogenesis of mycotic aneurysm. Angio. 10:593-601.
- Kannoth S, Thomas SV, Nair S, Sarma PS (2008) Proposed diagnostic criteria for intracranial infectious aneurysms. J Neurol Neurosurg Psychiatry. 8:943-946.
- Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R (2015) Unruptured cerebral aneurysms: evaluation and management. The Sci World J.
- Rinkel GJ (2005) Intra-cranial aneurysm screening: indications and advice for practice. Lancet Neurol. 2:122-128.
- White PM, Wardlaw JM, Easton V (2000) Can non-invasive imaging accurately depicts intracranial aneurysms? A systematic review. Radiology. 2:361-370.
- White PM, Teasdale EM, Wardlaw JM, Easton V (2001) Intra-cranial aneurysms: CT angiography and MR angiography for detection—prospective blinded comparison in a large patient cohort. Radiology. 3:739-749.
- Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ (2007) Risk of rupture of un-ruptured intracranial aneurysms in relation to patient and aneurysm characteristics: An updated meta-analysis. Stroke. 4:1404-1410.
- Wiebers DO (2003) International study of un-ruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet. 362(9378):103-110.
- Korja M, Lehto H, Juvela S (2014) Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study. Stroke. 7:1958-1963.
- Phuong LK, Link M, Wijdicks E (2002) Management of intracranial infectious aneurysms: A series of 16 cases. Neurosurg. 51(5):1145-1152.
- McGill F, Heyderman RS, Michael BD, Defres S, Beeching NJ, et al. The UK joint specialist society’s guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent Adults. 72(6):768-769.
- Van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, et al. (2016) ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clinical microbiology and infection. 22:S37-S62.
- Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, et al. (2017) Infectious diseases society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 64(6):e34-e65.
- Mai NT, Chau TT, Thwaites G, Chuong LV, Sinh DX, et al. (2007) Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. New Eng J Med. 357(24):2431-2440.
- Bingham WF (1977) Treatment of mycotic intracranial aneurysms. J neurosurg. 46(4):428-437.
- Asai T, Usui A, Miyachi S, Ueda Y (2002) Endovascular treatment for intracranial mycotic aneurysms prior to cardiac surgery. Eur J Cardiothorac Surg. 21(5):948-950.
- Clare CE, Barrow DL (1992) Infectious intracranial aneurysms. Neurosurg Clin N Am. 3(3):551-566.
- Moskowitz Ma, Rosenbaum Ae, Tyler Hr (1974) Angiographically monitored resolution of cerebral mycotic aneurysms. Neurol. 24(12):1103.
- Tunkel AR, Kaye D (1993) Neurologic complications of infective endocarditis. Neurol clin. 11(2):419-440.
- John S, Walsh KM, Hui FK, Sundararajan S, Silverman S, et al. (2016) Dynamic angiographic nature of cerebral mycotic aneurysms in patients with infective endocarditis. Stroke. 47(1):e8-e10.
- Luo CM, Chan CY, Chen YS, Wang SS, Chi NH, et al. (2017) Long-term outcome of endovascular treatment for mycotic aortic aneurysm. Eur J Vasc Endovasc Surg. 54(4):464-471.
- Liu MY, Jiao Y, Yang Y, Li Q, Zhang X, et al. (2020) Open surgery and endovascular repair for mycotic aortic aneurysms: Benefits beyond survival. J Thorac Cardiovasc Surg. 159(5):1708-1717.









