Keywords
Endovascular thrombectomy; Anticoagulation; Haemorrhage; Acuteischaemic; Stroke; Safety meta analysis
Introduction
Acute ischaemic stroke (AIS) has a prevalence of approximately 1.7% in the Australian population, accounting for 3% of the total burden of disease and causes 5.2% of all deaths [1]. Around 40% of people who have had a stroke will be left with a disability, making it an extremely important health issue [1]. Atrial fibrillation (AF) is a major risk factor for the development of AIS, and is a contributor in up to 40% of cases. To reduce the risk of AIS in patients with AF, many are anticoagulated with medications such as vitamin K antagonists (VKAs) or new oral anticoagulants (NOACs) like apixaban and rivaroxaban or with direct-acting oral anticoagulants (DOACs) such as dabigatran [2]. Despite anticoagulation, up to 3.24% of patients with AF will still develop AIS annually [3].
The current standard of treatment for AIS is through thrombolysis via the use of an intravenous tissue plasminogen activator (IVtPA), such as alteplase [4]. This therapy is limited by a narrow therapeutic time window of less than 4.5 hours from the onset of stroke symptom, and contraindications, which include current anticoagulant use with an international normalised ratio (INR) higher than 1.7, therapeutic doses of low molecular weight heparin (LMWH) or current use of a NOAC or DOAC [5]. Approximately only 10% of AIS patients are eligible for IV-tPA [6]. Furthermore, IV-tPA carries up to a 7.7% risk of developing symptomatic intracranial haemorrhage (sICH) [7]. The development of sICH after IV-tPA may occur due to three major mechanisms: (1) recanalization of an occluded artery causing a reperfusion injury, (2) the direct impairment of haemostasis due to the thrombolytic and anticoagulant effect of IV-tPA, and (3) the direct disruption of the integrity of the blood-brain barrier [8]. Previous trials have shown parenteral anticoagulants to increase the risk of sICH from 0.4% with placebo or aspirin to 1.4% with anticoagulation [9].
An alternative treatment for AIS is endovascular thrombectomy (EVT), and recent data supports its benefits, with a number needed to treat to reduce disability of 2.6 [10]. Thrombectomy devices include stent retrievers and catheter aspiration devices and the procedure involves guiding a catheter to the internal carotid artery and further to the site of the occlusion, and then directly removing the thrombus. Endovascular thrombectomyis indicated for patients with AIS due to large artery occlusion in the anterior circulation [11]. This treatment should be undertaken when the procedure can be initiated between 6-24 hours after symptom onset [12]. Notable changes in AIS treatment have emerged since the evidence for the benefits of EVT in patients with major vessel (i.e., proximal middle cerebral artery [M1 segment], distal internal carotid artery, basilar artery) occlusion [13]. Five open-label multicentre randomised controlled trials (MR CLEAN, SWIFT PRIME, ESCAPE, REVASCAT and EXTEND-IA) have shown that the early use of EVT is safe and effective for reducing disability and is superior to standard therapy with IV-tPA alone. A recent meta-analysis has demonstrated that the earlier EVT is initiated after symptom onset, the better the functional outcomes that can be expected [14].
EVT provides an alternative treatment of AIS in patients who have contraindications for IV-tPA, such as taking anticoagulants or presenting after 4.5 hours after onset of stroke symptom onset. Data comparing EVT in the setting of anticoagulation using either aNOAC, DOAC or warfarin remains scarce and is limited to small, non-randomised studies [15]. It remains unclear if performing EVT in anticoagulated patient’s results in poorer outcomes than EVT performed in patients with normal haemostasis.
In light of this uncertainty, we conducted a systematic review and meta-analysis with the objective to evaluate the safety of EVT for AIS in patients taking anticoagulant therapy. To evaluate safety, the rates of sICH, good functional outcome at 90-days and mortality at 90-days will be compared between anticoagulated and non-anticoagulated patients undergoing EVT.
Literature Review
Our systematic review and meta-analysis was conducted according to the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) guideline [16].
Search strategy
Electronic searches were performed using PubMed, Ovid MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Review from 2010 up to December 2019. No language restriction was used in the original searches. Tomaximisethe search strategy sensitivity and to ensure all relevant studies were identified, keywords such as “endovascular thrombectomy”, and “anticoagulants” in combination with “stroke” were searched across databases. Moreover, we combined the terms (stroke OR cerebrovascular disorder) with (mechanical embolectomy OR thrombectomy) as keywords or MeSH terms (search strategy details are found in Appendix 1) (Figure 1).
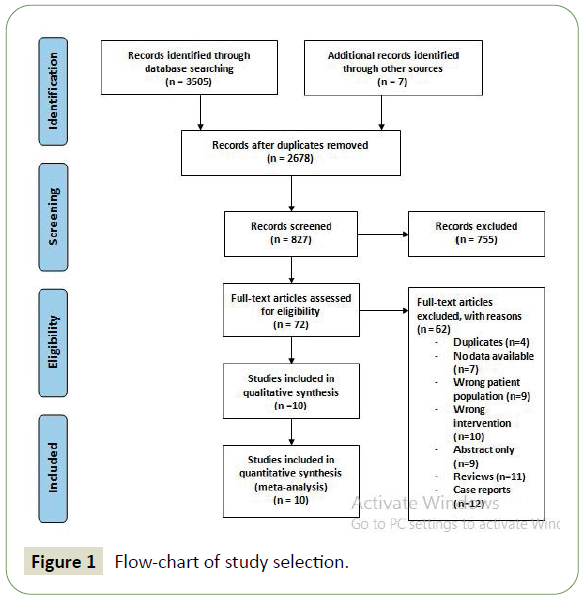
Figure 1: Flow-chart of study selection.
Selection process
We included both retrospective and prospective studies which reported the efficacy and other clinical outcomes of EVT for the treatment of AIS in adults ≥18 yearsreceiving anticoagulants or antiplatelet medication.
The inclusion criteria set for this systematic review are: (1) randomised control trials; (2) cohort studies which focus on AIS patients treated with EVT whilst therapeutically anticoagulated (defined as intravenous heparin with an elevated partial thromboplastin time, therapeutic dose of LMWH, VKA with INR > 1.7, a NOAC or DOAC regardless of coagulation test, or taking aspirin or other antiplatelet medication); (3) the study reports the modified Rankin Scale (mRS), National Institutes of Health Stroke Scale(NIHSS)scores or rates of sICH.
The exclusion criteria are: (1) case reports, editorials, systematic review, meta-analysis or pool-analysis, conference abstracts, letters to editors, animal studies, studies that are not in English; (2) repeated population or articles with overlapping data and (3) unable to extract relevant data.
Outcome measures
The rate of sICH was the primary outcome measure. Symptomatic intracranial haemorrhage was defined according to the criteria utilised in the original studies. Additional safety outcomes are mortality at 90-days defined as a mRS score of 6 at 90-days. The primary efficacy outcome was good functional outcome at 90-days, defined as a mRS score of 0-2 at 90-dayspost AIS. The modified Rankin Scale is a well-known method of measuring the degree of disability or dependence in the daily activities of people who have suffered from a stroke. The scale ranges from 0 (no symptoms) to 6 (death). A score of 2 means a patient has slight disability, however, is still able to look after their own affairs without assistance [17].
Data extraction and quality assessment
The titles and abstracts of retrieved reports were reviewed by two authors (J. Hindmarch and P. Vale) for potential eligibility. Disagreements were resolved by an additional reviewer. All data was extracted from article texts, figures and tables. The data was extracted using a standardised electronic form. Disagreements were resolved by consensus or with the help of an additional reviewer. Extracted data included patient characteristics such as age, gender, anticoagulant/ antiplatelet status, INR, sICH and mRS scores. In such cases where articles did not contain sufficient information, data was requested from the authors directly via email. Quality of the included studies was assessed using the GRADE scoring system (see Appendix 5).
Statistical analysis
Statistical analyses were carried out using STATA version 16 statistical software. Odds ratios and 95% confidence intervals (CIs) were extracted from the 10 studies included in the analysis using a fixed-effects model. Statistical heterogeneity was assessed using the Higgins’ and Thompson’s I2 index. An association between the use of anticoagulants and sICH was considered significant if P <0.05. Publication bias was assessed using a funnel plot and Egger's test statistics.
Results
Search results and study characteristics
A literature search from multiple databases yielded 3512 citations. After the screening process, 10 cohort studies [12,18-26] reported the risk of sICH following EVT in patients treated with either anticoagulant or antiplatelet therapy compared to those who were not. There were a total of 4881 patients included in the study, 758 of which were treated with anticoagulant or antiplatelet medication. In addition to medication used, patient characteristics such as gender, hypertension, diabetes, dyslipidaemia, AF and initial NIHSS score was also reported. Patients were from the Czech Republic, America, Switzerland, China, Germany, Austria and Madrid. Seven authors reported the rate of sICH after EVT in patients taking VKAs, four authors reported the same outcome in patients on heparin, three authors reported the outcomes of DOAC, one author reported the outcome of antiplatelet medications and one author reported the outcome of NOACs.
Table 1 shows patients in the anticoagulated group, were, on average older and had higher comorbidities such as hypertension, diabetes, AF and dyslipidaemia.
| Study |
Study design |
N (ITT) |
Types of anticoagulants used (n) |
Mean age (AC/N-AC) |
Hypertension (AC/ N-AC) |
Diabetes (AC/ N-AC) |
AF (AC/ N-AC) |
Dyslipidaemia (AC/ N-AC) |
| Krajickova et al. |
Cohort study |
285 |
VKA (21), DOAC (5) |
75/ 71 |
84.6%/ 72.2% |
46.2%/ 23.6% |
100%/ 44% |
61.5%/ 36.3% |
| De Marchis et al. |
Cohort study |
714 |
VKA (20) |
70.7/ 65.6 |
75%/ 59% |
21.4%/ 13.8% |
75%/ 28% |
50%/ 48.5% |
| Yang et al. |
Cohort study |
619 |
Heparin (269) |
63.6/ 64.1 |
50.6%/ 56.6% |
12.6%/ 17.7% |
24.5%/ 21.2% |
NR |
| Uphaus et al. |
Cohort study |
815 |
Heparin (269) |
76/ 69 |
81.2%/ 70% |
27.1%/ 19% |
81.2%/ 31.8% |
29.4%/ 22.6% |
| Nogueira et al. |
Cohort study |
305 |
VKA (19), IV heparin (10) |
67.5/ 67.6 |
69%/ 72% |
34%/ 18% |
66%/ 39% |
41%/ 33% |
| Cernik et al. |
Cohort study |
703 |
VKA (50), DOAC (15), LMWH (22) |
75.5/ 70 |
90%/ 75% |
36%/ 26% |
84%/ 38% |
48%/ 42% |
| Pandhi et al. |
Cohort study |
217 |
Aspirin (56), clopidogrel (2), aspirin+ clopidogrel (12), aspirin + dipyridamole (1) |
60/ 66 |
93%/ 68% |
52%/ 23% |
31%/ 22% |
65%/ 21% |
| Zapata-Wainberg et al. |
Cohort study |
502 |
VKA (104), DOAC (9), |
72.72/ 65.87 |
78.8%/ 59.6% |
28.3%/ 15.3% |
92%/ 24.7% |
50.4%/ 45.3% |
| Benavente et al. |
Cohort study |
117 |
VKA (12) |
72.8/ 67.07 |
80%/ 55.29% |
33.33%/ 22.35% |
NR |
36.66%/ 45.88% |
| Rebello et al. |
Cohort study |
604 |
VKA (29), dabigatran (11), rivaroxaban (4), apixaban (2) |
68.7/ 64 |
86%/ 75% |
41%/ 21% |
67%/ 24% |
43%/ 38% |
Abbreviations: NR: not reported
Table 1: Study designs and baseline patient characteristics.
Symptomatic intracranial haemorrhage
Pooled analysis of the included studies indicates there is no significant difference in the rate of sICH in anticoagulated patients or patients taking antiplatelet medication when compared to those with normal haemostasis (OR =1.21; 95% C.I.: 0.88, 1.67) (Figure 2). There was no underlying between-study variability (Higgins’ and Thompson; (I2<25%), and Heterogeneity chisquared = 8.35 (d.f. = 9) p = 0.5).
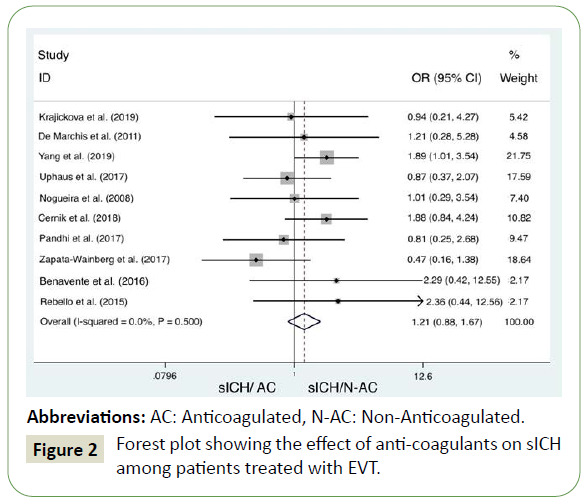
Figure 2: Forest plot showing the effect of anti-coagulants on sICH among patients treated with EVT.
Good functional outcome at 90-Days
The majority of authors report no significant difference in good functional outcome (mRS<2) at 90-days after AIS. Three authors did however report higher rates of good functional outcome in patients who were therapeutically anticoagulated prior to EVT. The greatest difference was reported by ?erník et al., [21] (9.4% in the anticoagulated group and 35.3% in the non-anticoagulated group, p = 0.002) (Table 2).
| Study |
mRS<2 (90 days) |
P |
90 Day Mortality |
P |
| AC |
N-AC |
AC |
N-AC |
| Krajickova et al. |
34.60% |
56.80% |
0.27 |
26.90% |
20.80% |
0.47 |
| De Marchis et al. |
NR |
NR |
NR |
17.90% |
21.60% |
0.58 |
| Yang et al. |
39.80% |
47.40% |
0.06 |
19.30% |
21.10% |
0.62 |
| Uphaus et al. |
25.90% |
39.20% |
0.017 |
44.70% |
25.60% |
0.0002 |
| Nogueira et al. |
9.40% |
35.30% |
0.002 |
40% |
37.90% |
0.854 |
| Cernik et al. |
36% |
49% |
0.03 |
35% |
27% |
0.127 |
| Pandhi et al. |
50% |
48% |
0.881 |
25% |
26% |
0.871 |
| Zapata-Wainberg et al. |
55.70% |
56.30% |
NS |
12.40% |
13.10% |
NS |
| Benavente et al. |
46.46% |
54.22% |
0.452 |
6.66% |
21.68% |
0.64 |
| Rebello et al. |
30% |
40% |
0.13 |
32% |
26% |
0.18 |
Abbreviations: NS: Not Significant, NR: Not Reported
Table 2: Comparison of mRS <2 and mortality at 90-days between AC and N-AC groups.
Mortality at 90-Days
The majority of authors report no significant difference between the mortality rate at 90-days in the anticoagulated and nonanticoagulated patient groups. Uphaus et al., [20], report a higher rate of mortality in the anticoagulated patient group compared to the non-anticoagulated group (44.7% vs. 25.6%, p = 0.0002) (Table 3).
| Study |
OR [95% Conf. Interval] |
% Weight |
| Krajickova et al. |
0.944 |
0.209 |
4.275 |
5.420 |
| De Marchis et al. |
1.210 |
0.278 |
5.276 |
4.580 |
| Yang et al. |
1.890 |
1.009 |
3.541 |
21.750 |
| Uphaus et al. |
0.870 |
0.365 |
2.073 |
17.590 |
| Nogueira et al. |
1.007 |
0.286 |
3.543 |
7.400 |
| Cernik et al. |
1.884 |
0.837 |
4.242 |
10.820 |
| Pandhi et al. |
0.812 |
0.246 |
2.685 |
9.470 |
| Zapata-Wainberg et al. |
0.473 |
0.162 |
1.378 |
18.640 |
| Benavente et al. |
2.286 |
0.416 |
12.555 |
2.170 |
| Rebello et al. |
2.364 |
0.445 |
12.564 |
2.170 |
| M-H pooled OR |
1.215 |
0.882 |
1.673 |
100.00 |
Heterogeneity Chi-squared= 8.35 (d.f.=9) p=0.500 I-squared (variation in OR attributable to heterogeneity)= 0.0% Test of OR=1 : z= 1.19 p=0.233
Table 3: A tabular summary of the meta-analysis.
Risk of Bias
Based on the Egger’s test, there was no significant risk of publication bias (p = 0.4851). Furthermore, the funnel plot indicates the studies are distributed approximately evenly around the effect size (Figure 3).
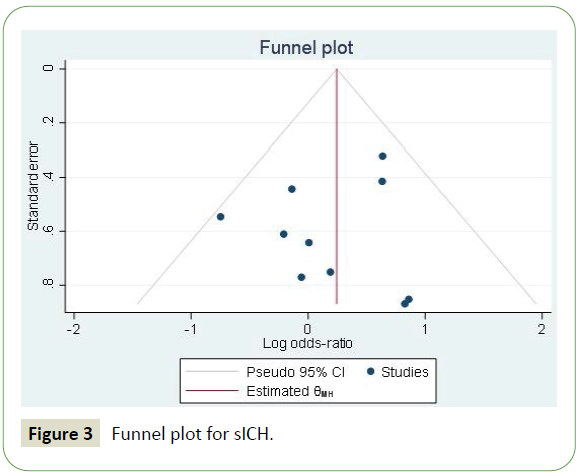
Figure 3: Funnel plot for sICH.
Discussion
Our study aimed to evaluate the safety of EVT in patients on therapeutic doses of anticoagulation medication. The results of our study suggest EVT in patients treated with prior anticoagulation or antiplatelet medication is safe. The rate of sICH among patients who were anticoagulated and patients with normal haemostasis did not differ significantly. This finding is extremely relevant, as therapeutic anticoagulation is a contraindication for IV-tPA. Endovascular thrombectomy may be considered as first-line treatment for AIS, regardless of anticoagulation status.
Previous randomised controlled trials have reported variability in the rates of sICH following EVT depending on the definition of sICH used. Rates range from 0% in the SWIFT-PRIME trial to 7.7% in the Multi-centre Randomized Clinical Trial of EVT for Acute ischemic stroke in the Netherlands (MR CLEAN). The mean rate of sICHin anticoagulated patients in our pooled analysis was 8.23%, a similar result to that reported in the MR CLEAN trial.
Among the studies included, the 90-day mortality was similar among anticoagulated and non-anticoagulated patients (27.03% vs. 24.98%). Nine authors reported no statistically significant (p<0.05) results between the two groups. Nogueira et al., [12], did however report that 90-day mortality was significantly greater in the non-anticoagulated population (p = 0.0002).
Good functional outcome (mRS ≤ 2) at 90-days was also similar between the two groups. Six authors reported no significant difference between patient groups. However, four authors reported those with abnormal haemostasis had a significantly lower chance of achieving good functional outcomes at 90-days. The greatest difference in good functional outcome was reported by Nogueira et al., [12] who compared patients treated with VKA and IV heparin compared to patients with normal haemostasis (9.4% vs. 35.3%; p = 0.002). Reasons for this difference may be attributed to the apparent increased age and lower health status of those treated with anticoagulants prior to their stroke, including higher prevalence of AF, diabetes and hypertension. Atrial fibrillation has been associated with an increased risk of sICH [26]. Other authors have suggested higher rates of good functional outcome with DOACs than with VKAs after EVT [26]. The inconsistency between studies may be explained by the use of various anticoagulants, and different degrees of anticoagulation control in those taking VKAs.
Endovascular thrombectomy in patients with elevated INR in the setting of warfarin therapy appears to be safe, and is supported by numerous authors included in the current metaanalysis. The studies included in this analysis report similar rates of sICH in patients with an INR >1.7 who were treated with EVT as is reported in the treatment arm of the PROACT II trial [26]. Benavente at al., [25], reported significantly higher mortality in patients with admission INR > 1.7. Heparin is often administered in the setting of EVT, however the optimal dosage is difficult to establish. Patients anticoagulated with heparin who were included in our analysis showed rates of sICH similar to nonanticoagulated patients.
Those studies which analysed the effects of DOACs show a trend of relatively low rates of sICH. This finding is supported by Seiffge et al., [27], who reports a superior safety profile of DOAC medication compared to warfarin. Ntaios et al., [28] also report a lower risk of sICH in those taking DOACs when compared to those taking warfarin. This finding highlights the possibility that in addition to DOACs having a lower baseline risk of sICH compared with VKA for long term stroke prophylaxis, this may extend to patients with AIS treated with EVT [29]. An alternative explanation is the difficulty of measuring the degree of anticoagulation in patients taking DOACs, as such, these patients may have been anticoagulated to a lesser degree than those taking VKAs. Furthermore, increasing the favourability of DOACs is the recent approval of an immediate reversal agent for dabigatran [22].
The use of antiplatelet medications prior to EVT did not increased the risk of sICH, mortality at 90-days or good functional outcome at 90-days [15]. This finding contradicts that of a Japanese study which reported higher rates of sICH after EVT in patients treated with antiplatelet medication [22]. However, it is important to note that this study was considerably different in patient characteristics and the EVT devices used.
Our study is subject to a series of limitations which are worth mentioning. Firstly, although many authors used the European Co-operative Acute Stroke Study-II (ECASS-II) definition of sICH, this was not used across all studies included in the review. This makes comparison of absolute rates of sICH between each study impossible. Fortunately, however, each study included in the analysis reported similar results irrespective of the definition used. Secondly, all studies included were observational which increases the risk of various types of bias and confounding. Thirdly, there was variability between intervention and comparator groups. Patients in the intervention group were usually older, more patients had diabetes, hypertension, dyslipidaemia and AF, all of which may have influenced the outcome. These variables were not adjusted for by logistic regression analysis or multiple regression analysis. Finally, although authors who studied the effects of warfarin reported INR, those who included NOACs had no sensitive and specific makers of NOAC levels, which may have resulted in various degrees of anticoagulation among patients taking NOACs.
Conclusion
The findings of this analysis support the use of EVT in patients with AIS who are on therapeutic levels of anticoagulants or antiplatelet medications. Further large multicentre prospective studies comparing the rate of sICH in anticoagulated patients and patients with normal haemostasis treated with EVT are needed to confirm the generalizability of this study. Further studies are also warranted to clarify if the use of DOACs provide efficacy benefits compared to other anticoagulants.
Future Directions
This study offers practical information to select appropriate therapeutic strategies for patients with AIS who are therapeutically anticoagulated. Patients who have contraindications for thrombolysis such as therapeutic anticoagulation can be treated with EVT. Patients suffering from AIS now have a range of treatment options including thrombolysis, thrombectomy or a combination of both. Patients taking a NOAC, DOAC or a VKA with an INR>1.7 can be managed safely with EVT provided there are no other absolute contraindications.
Future research should focus on refining the coagulation parameters which could be considered safe to proceed with EVT, defining if there is an optimal period to perform EVT in regards to time of last dose of anticoagulation medication and also determine the benefits of using reversal therapy for DOAC medication. Future research could also focus on comparing EVT with and without combined thrombolysis in anticoagulated patients, those taking antiplatelet medications and those with normal haemostasis.
The risk of sICH with the use of various endovascular devices will continue to decline as new, smaller calibre devices are brought to market. The success of various endovascular devices in treating AIS usually requires a fine balance between ease of use, safety feasibility and efficacy. Devices which are easier to use and have a proven morbidity and mortality benefit continue to revolutionise the way we treat AIS.
Compliance with NHMRC Guidelines
NHMRC values and principles of ethical conduct were upheld during the completion of this studyas per the NHMRC 2007 National Statement of Ethical Conduct in Human Research (NHMRC, 2007). All data included in the present study was obtained from databases which are available to the public. This study posed negligible risk to the parties involved and did not require direct human participation, as such, ethics approval was not obtained. Potential benefits of the study include providing a more evidence based approach to the treatment of acute ischaemic stroke in anticoagulated patients. The study also demonstrates academic merit as well as the principle of beneficence in accordance with the NHMRC statement. Research performed was conducted in a non-discriminatory manner with maintenance of respect for autonomy of individuals involved in the studies reviewed. The results of the study were disseminated as reasonable for the type and scope of this study, maintaining the principle of justice.
Conflicts of Interest
The author has not declared any conflicts of interest to disclose.
38740
References
- Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, et al. (2015) 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46: 3020-3035.
- Alberts MJ, Eikelboom JW, Hankey GJ (2012) Antithrombotic therapy for stroke prevention in non-valvular atrial fibrillation. Lancet Neurol 11: 1066-1081.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, et al. (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50: e344-e418.
- Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS (2009) Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke 40: 3777-3783.
- De los Ríos la Rosa F, Khoury J, Kissela BM, Flaherty ML, Alwell K, et al. (2012) Eligibility for intravenous recombinant tissue-type plasminogen activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke 43: 1591-1595.
- Modrego PJ (2019) The risk of symptomatic intracranial hemorrhage after thrombolysis for acute stroke: current concepts and perspectives. Ann Indian Acad Neurol 22: 336.
- Zhang Y, Wang Y, Zuo Z, Wang Z, Roy J, et al. (2014) Effects of tissue plasminogen activator timing on blood–brain barrier permeability and hemorrhagic transformation in rats with transient ischemic stroke. J Neurol Sci 347: 148-154.
- Counsell C, Sandercock P (1999) Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev 1999: 2.
- Kurowski D, Jonczak K, Shah Q, Yaghi S, Marshall RS, et al. (2017) Safety of endovascular intervention for stroke on therapeutic anticoagulation: multicenter cohort study and meta-analysis. J Stroke Cerebrovasc Dis 26: 1104-1109.
- Campbell BC, Donnan GA, Lees KR, Hacke W, Khatri P, et al. (2015) Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol 14: 846-854.
- Nogueira RG, Smith WS (2009) Safety and efficacy of endovascular thrombectomy in patients with abnormal hemostasis: Pooled analysis of the MERCI and multi MERCI trials. Stroke 40: 516-522.
- Koga M, Iguchi Y, Ohara T, Tahara Y, Fukuda T, et al. (2018) Acute ischemic stroke as a complication of Stanford type A acute aortic dissection: a review and proposed clinical recommendations for urgent diagnosis. General thoracic and cardiovascular surgery 66: 439-445.
- Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, et al. (2018) Association of intracerebral hemorrhage among patients taking non–vitamin K antagonist vs. vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA 319: 463-473.
- Sugiura Y, Yamagami H, Sakai N, Yoshimura S (2017) Predictors of symptomatic intracranial hemorrhage after endovascular therapy in acute ischemic stroke with large vessel occlusion. J Stroke Cerebrovasc Dis 26: 766-771.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8: 336-341.
- Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19: 604-607.
- Krajíčková D, Krajina A, Herzig R, Vítková E, Halúsková S, et al. (2019) Safety and efficacy of mechanical thrombectomy with stent-retrievers in anticoagulated patients with anterior circulation stroke. Clin Radiol 74: e165-e211.
- Yang M, Huo X, Gao F, Wang A, Ma N, et al. (2019) Safety and efficacy of heparinization during mechanical thrombectomy in acute ischemic stroke. Front Neurol 10: 299.
- Uphaus T, Singer OC, Berkefeld J, Nolte CH, Bohner G, et al. (2017) Safety of endovascular treatment in acute stroke patients taking oral anticoagulants. Int J Stroke 2017: 412-415.
- Černík D, Šaňák D, Divišová P, Köcher M, Cihlář F, et al. (2018) Mechanical thrombectomy in patients with acute ischemic stroke on anticoagulation therapy. CardioVasc Interv Radiol 41: 706-711.
- Pandhi A, Tsivgoulis G, Krishnan R, Ishfaq MF, Singh S, et al. (2018) Antiplatelet pretreatment and outcomes following mechanical thrombectomy for emergent large vessel occlusion strokes. J Neurointerv Surg 10: 828-833.
- Zapata-Wainberg G, Ximenez-Carrillo A, Trillo S, Fuentes B, Cruz-Culebras A, et al. (2018) Mechanical thrombectomy in orally anticoagulated patients with acute ischemic stroke. J Neurointerv Surg 10: 834-838.
- Benavente L, Larrosa D, García-Cabo C, Pérez ÁI, Rico M, et al. (2016) Safety and efficacy of mechanical thrombectomy in acute ischemic stroke of anticoagulated patients-a prospective observational study. J Stroke Cerebrovasc Dis 25: 2093-2098.
- Rebello LC, Haussen DC, Belagaje S, Anderson A, Frankel M, et al. (2015) Endovascular treatment for acute ischemic stroke in the setting of anticoagulation. Stroke 46: 3536-3539.
- Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, et al. (1999) Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: A randomized controlled trial. JAMA 282: 2003-2011.
- Seiffge DJ, Hooff RJ, Nolte CH, Béjot Y, Turc G, et al. (2015) Recanalization therapies in acute ischemic stroke patients: impact of prior treatment with novel oral anticoagulants on bleeding complications and outcome. Circulation 132: 1261-1269.
- Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P (2012) Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: A systematic review and meta-analysis of randomized controlled trials. Stroke 43: 3298-3304.
- Pollack Jr CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, et al. (2015) Idarucizumab for dabigatran reversal. N Engl J Med 373: 511-520.








