Tushar Premraj Raut1*, Tarun Mathur1, Indoo Ambulkar2, Kanchan Gupta3, Khursheed Ansari4 and Srinivas Janga4
1Department of Neurology, Seven Hills Hospital, Andheri East Mumbai, India
2Department of Medical Oncology, Seven Hills Hospital, Andheri East Mumbai, India
3Department of Radiology, Seven Hills Hospital, Andheri East Mumbai, India
4Department of Neurosurgery, Seven Hills Hospital, Andheri East Mumbai, India
- Corresponding Author:
- Tushar Premraj Raut
Seven Hills Hospital, Andheri East, Mumbai, Maharashtra, India
Tel: +919930087434
E-mail: tushar.27r@gmail.com
Received date: 18 December 2016; Accepted date: 24 February 2017; Published date: 27 February 2017
Citation: Raut TP, Mathur T, Ambulkar I, et al. Severe Reversible form of Chemotherapy Induced Acute Disseminated Leuko-encephalopathy: A Case Report with Review of Literature. Ann Clin Lab Res. 2017, 5: 1.
Keywords
Leuko-encephalopathy; Chemotherapy; Magnetic resonance imaging
Introduction
Acute disseminated leukoencephalopathy (ADL) or toxin induced leukoencephalopathy is a term used to denote a broad clinico-radiological spectrum comprising a range of abnormalities from mild reversible clinical symptoms with MRI changes to profound encephalopathy resulting in death. This affection of the white matter tracts is also known as spongiform leukoencephalopathy [1]. Most common cause is use of chemotherapeutic agents. Clinical features can vary from a subtle encephalopathy to severe form including akinetic mute state and even death. Strong clinical suspicion and neuroimaging tools: Magnetic resonance imaging especially diffusion weighted sequence helps in early diagnosis of this potentially dreadful condition [2,3].
Few cases have been reported in literature. But it is very rare to have a patient with profound encephalopathy with florid changes on magnetic resonance imaging (MRI) show complete clinical and radiological recovery.
The authors here report a case of a gentleman with carcinoma rectum post operatively treated with 5 fluorouracil, oxaliplatin, calcium leucovorin regimen developing severe form of encephalopathy which recovered completely with complete reversal of changes on MRI.
Case Report
A 52-year-old male case of carcinoma rectum post abdominoperineal resection was undergoing chemotherapy (5 fluorouracil, oxaliplatin and calcium lecovorin) regimen. After Receiving 5th cycle of chemotherapy he developed acute onset altered sensorium and confusional state. He couldn’t recognize family members, talked irrelevantly, and next day developed drowsiness. Subsequently he developed dysphagia, tremors, and slowness of activities with unsteadiness and was ultimately bed bound. There was no associated fever, headache, or seizures. Clinically patient had rapidly progressive encephalopathy with its complications. On examination, his vitals were stable. General examination revealed mild pallor and icterus. He was conscious but drowsy, apathetic and mute. Clinically he was in a akinetic rigid state. Plantar response was extensor bilaterally.
Biochemical work up including thyroid stimulating hormone (TSH), plasma ammonia was sent. In view of rapidly developed encephalopathy with pyramidal and extrapyramidal features, Magnetic resonance imaging (MRI) brain was planned.
Investigations
Biochemical work revealed mildly elevated total bilirubin and liver enzymes. Hematological work up was normal.
MRI Brain revealed confluent T2 and FLAIR hyperintense signals involving bilateral subcortical white matter, genu, and splenium of corpus callosum. Diffusion weighted (DW) hyperintense signals with low value on Apparent diffusion coefficient (ADC) were observed in corresponding areas, suggestive of diffusion restriction. No abnormal parenchymal or leptomeningeal enhancement appreciated on contrast administration. Imaging features were suggestive of leukoencephalopathy.
Treatment
Patient had leukoencephalopathy immediately after chemotherapy administration hence possibility of toxin induced leukoencephalopathy was considered. Chemotherapy was stopped. He was initiated on Pulse methylprednisolone and High dose thiamine, vitamin B12 and folic acid. Next day, the patient showed considerable clinical improvement. Consciousness improved, bulbar dysfunction resolved, Ryle’s tube was removed. After 2 days patient became ambulant and was discharged after 3 days of pulse methylprednisolone on folic acid, methyl cobalamin tablets.
Follow-up
After 2 weeks of follow up, patient had recovered completely with normal neurological evaluation. Follow up MRI brain plain study was performed. Follow up MRI revealed complete resolution of diffusion weighted hyper intensities and resolution of T2 and FLAIR hyper intensities in subcortical white matter (Figures 1 - 3).
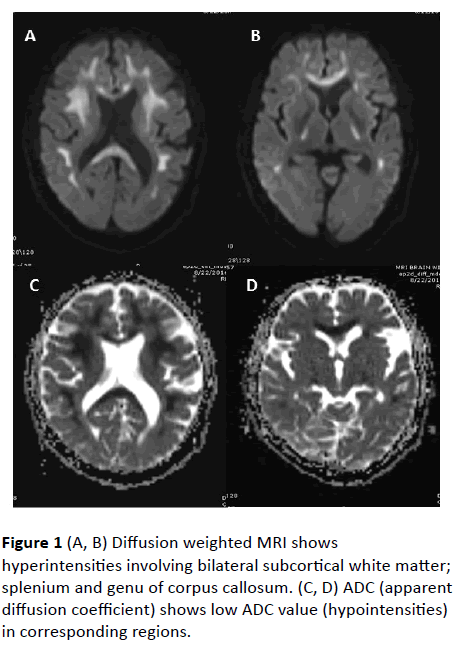
Figure 1 (A, B) Diffusion weighted MRI shows hyperintensities involving bilateral subcortical white matter; splenium and genu of corpus callosum. (C, D) ADC (apparent diffusion coefficient) shows low ADC value (hypointensities) in corresponding regions.
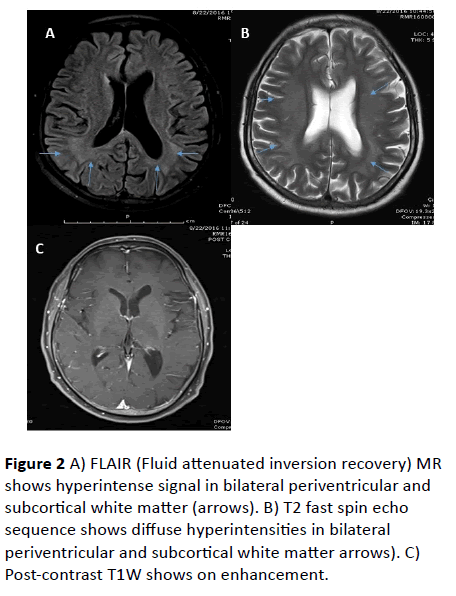
Figure 2 A) FLAIR (Fluid attenuated inversion recovery) MR shows hyperintense signal in bilateral periventricular and subcortical white matter (arrows). B) T2 fast spin echo sequence shows diffuse hyperintensities in bilateral periventricular and subcortical white matter arrows). C) Post-contrast T1W shows on enhancement.
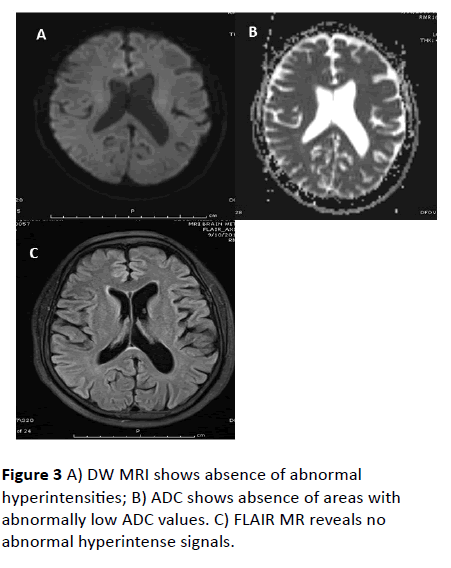
Figure 3 A) DW MRI shows absence of abnormal hyperintensities; B) ADC shows absence of areas with abnormally low ADC values. C) FLAIR MR reveals no abnormal hyperintense signals.
Discussion
Toxic leukoencephalopathy [1] is a condition affecting the cerebral white matter as a result of many agents: endogenous or exogenous like chemotherapeutic agents, immunosuppressive therapy, antimicrobial agents, environmental toxins, drug abuse, organ failure (liver/kidneys), etc. Various chemotherapeutic agents result in this condition like methotrexate, vincristine, ifosfamide, fludarabine, cytarabine, 5-FU, cisplatin, and the interferons [4]. Vis a vis these agents can result in affection of central nervous system in various ways like Encephalopathy: Acute, subacute, chronic, multifocal, Posterior reversible encephalopathy (PRES). Also, they can result in seizures, cerebellar dysfunction, aseptic meningitis, and myelopathy [5]. 5-FU has been frequently reported as a causative agent of leukoencephalopathy. However, the reported incidence is less than 5% and the cause is multifactorial [6].
Clinical features can vary from mild confusion, disorientation, memory disturbances, dysarthria, gait ataxia and in some cases florid extrapyramidal features resulting in an akinetic mute state and even coma and death [1]. Our case presented with severe encephalopathy and pyramidal, extrapyramidal features resulting in akinetic rigid state.
5-FU is a fluorine substituted analogue of pyrimidine uracil used in the treatment of various malignancies especially gastrointestinal, breast etc. It mainly acts by blocking the DNA synthesis by reducing the formation of thymidine monophosphate via the inhibition of the thymidylate synthetase and incorporation into RNA. Despite the fact that this drug readily penetrates the blood-brain barrier, neurotoxicity is still uncommon with 5-FU. Various factors like female gender, malnutrition, or liver dysfunction and dihydropyrimidine dehydrogenase (DPD) deficiency are associated with risk of developing 5-FU-induced leukoencephalopathy [7]. Encephalopathy is dose and schedule dependent and is usually reversible after stopping the offending agent however at times it can result in permanent damage [8,9]. Encephalopathy can be acute to subacute. Our patient had an acute encephalopathy.
The exact pathogenesis of drug induced leukoencephalopathy is not known. The characteristic histopathologic features include myelin pallor, myelin edema, axonopathy, macrophage infiltration and vacuolation of neuropil. These findings are analogous to those described in an animal model of acute methotrexate intoxication [10]. There is another entity named delayed necrotizing encephalopathy which is also associated with chemotherapy use but with entirely different pathogenesis, clinical and radiological presentation.
The common radiological imaging findings of leukoencephalopathy include symmetrical periventricular hypoattenuation on CT scan and diffuse hyper intensity in the white matter and corpus callosum on T2 weighted MRI and DW-MRI which is more sensitive. There is relative sparing of basal ganglia, cortex and subcortical region [11]. There is no contrast enhancement unlike that seen in diffuse necrotizing leukoencephalopathy. Our case had imaging features consistent with those mentioned in literature. Diffusion abnormalities suggest restriction of movement of water molecules. This restriction diffusion could be due to intramyelinic edema (myelin vacuolation), cytotoxicity due to endothelial damage, or due to direct toxic demyelination that makes this region susceptible to the accumulation of fluid in the extracellular spaces. Diffusion abnormalities occurring in other clinical condition like stroke are irreversible, however very few cases in the literature have been described showing complete reversibility after such extensive changes. Paul et al. [12] demonstrated complete clinical reversibility whereas Siva Subramanian et al. [13] demonstrated complete clinical and radiological reversibility in 2 cases. Few cases of methotrexate or 5 FU induced reversible diffusion abnormalities have been described [14-17]. Mcckinney et al. [18] described 19 cases of acute toxic leukoencephalopathy. Our case had 5-FU and oxaliplatin induced severe reversible encephalopathy which is rare.
No definitive treatment has been proposed as the most important step is to withdraw the offending agent. One study mentions treatment modalities ranging from supportive measures to steroids, plasma exchange and thiamine [19]. Inflammatory changes can respond to steroid. Although there is no evidence to support use of steroids, it can be tried in severe encephalopathy with florid MRI changes. In view of scan literature on treatment, the most important thing that holds true is withdrawing the responsible drug stopping from disease to progress. In our case, we used pulse methylprednisolone in view of severe encephalopathy resulting in a good clinical response.
Conclusion
Acute toxin induced leukoencephalopathy is a rare and unique clinical entity with characteristic radiological features well depicted on MRI study especially diffusion weighted imaging.
They can present with subtle to profound encephalopathy, coma and even death.
These changes even if extensive and severe are usually reversible both clinically and radiologically on withdrawing the offending drug at the earliest.
More research needs to be done in terms of treatment for this condition however pulse methylprednisolone if not contra indicated can be tried in severe forms of encephalopathy as we did in our case.
18491
References
- Filley CM, Kleinschmidt-DeMasters BK (2001) Toxic leukoencephalopathy. N Engl J Med 345: 425-432.
- Cheung WY, Fralick RA, Cheng S (2008) The confused cancer patient: A case of 5-fluorouracil-induced encephalopathy. Curr Oncol 15: 234-236.
- Fujikawa A, Tsuchiya K, Katase S, Kurosaki Y, Hachiya J (2001) Diffusion weighted MR imaging of Calmofur-induced leukoencephalopathy. Eur Radiol 11: 2602-2606.
- Sioka C, Kyritsis AP (2009) Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol 63: 761-767.
- Schlegel U (2011) Central nervous system toxicity of chemotherapy European association of neurooncology magazine 1: 25-29.
- Yeh KH, Cheng AL (1997) High-dose 5-fluorouracil infusional therapy is associated with hyperammonaemia, lactic acidosis and encephalopathy. Br J Cancer 75: 464-465.
- Reddy N, Garacch M, Reddy MJ (2016) A case of 5 fluorouracil induced leukoencephalopathy in a patient on treatment for carcinoma rectum. IJSR 5: 126-127.
- Wu AN, Yin JH, Lo HC, Chang JL, Lee WH (2001) Leukoencephalopathy induced by chemotherapy with 5-fluorouracil: A case report. Acta Neurol Taiwan 10: 134-140.
- Lucato LT, McKinney AM, Short J, Teksam M, Truwit CL (2006) Reversible findings of restricted diffusion in 5-Flourouracil Neurotoxicity. Australas Radiol 50: 364-368.
- Shibutani M, Okeda R (1989) Experimental study on subacute neurotoxicity of methotrexate in cats. Acta Neuropathol (Berl) 78: 291-300.
- Akitake R, Miyamoto S, Nakamura F, Horimatsu T, Ezoe Y, et al. (2011) Early detection of 5-FU-induced acute leukoencephalopathy on diffusion-weighted MRI. Jpn J Clin Oncol 41: 121-124.
- Paul BS, Singh G, Bansal R, Paul G (2013) Diffusion weighted MR imaging of 5-fluorouracil and oxaliplatin induced leukoencephalopathy. J Postgrad Med 59: 135-137.
- Sivasubramanian S, Moorthy S, Sreekumar KP, Kannan RR (2010) Diffusion-weighted magnetic resonance imaging in acute reversible toxic leukoencephalopathy: A report of two cases. Ind J Radiol Imaging 20: 192-194.
- Kuker W, Bader P, Herrlinger U, Heckl S, Nagele T (2005) Transient encephalopathy after intrathecal methotrexate chemotherapy: Diffusion-weighted MRI. J Neurooncol 73: 47-49.
- Rollins N, Winick N, Bash R, Booth T (2004) Acute methotrexate neurotoxicity: Findings on diffusion-weighted imaging and correlation with clinical outcome. AJNR Am J Neuroradiol 25: 1688-1695.
- Sandoval C, Kutscher M, Jayabose S, Tenner M (2003) Neurotoxicity of intrathecal methotrexate: MR imaging findings. AJNR Am J Neuroradiol 24: 1887-1890.
- Tha KK, Terae S, Sugiura M, Nishioka T, Oka M, et al. (2002) Diffusion-weighted magnetic resonance imaging in early stage of 5-fluorouracil-induced leukoencephalopathy. Acta Neurol Scand 106: 379-386.
- McKinney AM, Kieffer SA, Paylor RT, Santa-Cruz KS, Kendi A, et al. (2009) Acute toxic leukoencephalopathy: Potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am J Roentgenol 193: 192-120.
- Pirzada NA, Ali II, Dafer RM (2000) Fluorouracil-induced neurotoxicity. Ann Pharmacother 34: 35-38.









