Aikaterini Dimitrouli1*, Lampis C. Stavrinou2, Maria Loufardaki3, Petros Galanis4, Theodosis Kalamatianos5, Damianos E. Sakas6, George Stranjalis7
1RN, MSc, Department of Neurosurgery, University of Athens, Evaggelismos Hospital, Athens, Greece
2MD, Department of Neurosurgery, Evangelisches Krankenhaus Bielefeld, Germany
3MA, Hellenic Center of Neurosurgical research (HCNR) “Professor Petros S. Kokkalis”, Athens, Greece
4RN, PhD, Center for Health Services Management and Evaluation, Faculty of Nursing, University of Athens, Athens, Greece
5PhD, Department of Neurosurgery, University of Athens, Evaggelismos Hospital, Hellenic Center of Neurosurgical research (HCNR) “Professor Petros S. Kokkalis”, Athens, Greece
6MD, Department of Neurosurgery, University of Athens, Evaggelismos Hospital, Hellenic Center of Neurosurgical research (HCNR) “Professor Petros S. Kokkalis”, Athens, Greece
7MD, Department of Neurosurgery, University of Athens, Evaggelismos Hospital, Hellenic Center of Neurosurgical research (HCNR) “Professor Petros S. Kokkalis”, Athens, Greece
- *Corresponding Author:
- Dimitrouli Aikaterini
Amfitritis 21-25, Zografou
Athens 15771, Greece
E-mail: katdim72@yahoo.gr
Phone number: 6936666093
Key words
Traumatic brain injury, outcome, rehabilitation, outcome measurement scales
Introduction
Traumatic brain injury (TBI) represents a leading cause of mortality and disability and thus a highly significant public health issue. The incidence of TBI in the U.S.A. is an estimated 175-200/100.000 injuries / year,[1] while even higher incidence rate estimates have been previously obtained for several European countries such as France (281 / 100 000 injuries/ year)[2] and Sweden (546/100.000 injuries / year).[3] Recent data derived from the Greek National Statistics Agency, indicate that TBI-associated road traffic accident mortality is as high as 146/1.000.000/year and thus significantly higher than the corresponding rates of 57/1.000.000/year for the U.K or 53/1.000.000/year for Sweden. Nevertheless, given the paucity of available epidemiological data on TBI in Greece, its overall economic and social impact remains unknown.[4-6]
Valid and reliable assessment of long term outcome in TBI survivors is a prerequisite for the evaluation of functional disability and appropriateness of healthcare and rehabilitation provision.[7] In this context, several scales have been previously used to evaluate outcome of TBI patients.[8]
The present study aimed in designing a simple outcome measurement scale for the evaluation of TBI on aspects of functionality, mobility, psychocognitive status as well as overall quality of life, in a time-sparing manner. The proposed scale incorporates selected elements of commonly used outcome scales8 and was tested on a cohort of 96 severe TBI patients, previously hospitalized in our department.
Methodology
Scale design
The proposed outcome scale questionnaire, named «Athens Disability Scale» (ADS; Appendix 1) was generated by selecting and combining items incorporated in the following internationally recognized outcome scales: the Glasgow Outcome Scale (GOS), the Functional Independence Measure + Functional Assessment Measure (system FIM + FAM), the Disability Rating Scale (DRS) and The Barthel Index (Barthel scale).[9-11] Feeding, personal hygiene, dressing, sphincter control, mobility, car transfer (not necessarily as the driver), verbal comprehension, verbal expression, emotional status and ability to work or study, are the 10 items that are examined with the new scale. Each of the 10 items incorporates 3 subcategories indicating total dependency (score =1), moderate dependency (score=2) or independency (score=3) with overall scale score ranging between 10-30 points. An ADS overall score of 26-30 indicates that the patient is independent, 15-25 indicates moderate dependency and 10-14 indicates total dependency.
Application of ADS on Traumatic Brain Injury (TBI) patients-Inclusion criteria
A search on the departmental electronic database incorporating records of all previously hospitalized patients between February 1999 to June 2009, identified a total of 231 adult individuals that had suffered severe TBI (GCS ≤ 8 / 15), were aged ≤ 65 years and had left the hospital alive to either rehabilitation clinics or their homes. ADS was applied by means of telephone interviews involving patients (whenever possible), family members or caregivers that were in contact with the patients on a daily basis. Of the original 231 identified cases, 96 patients/family members/caregivers were contactable; 123 persons (53.2%) were not contactable due to no longer valid telephone numbers, whereas 12 of the contacted families indicated patient death. Prior to the application of ADS, descriptive data on demographic characteristics, mechanisms of injury, physiotherapy treatment, current accommodation standards, presence and treatment of seizures and continuous medical follow-up were gathered from our electronic database and/or during interviews (Appendix 2). Interviews were timed and were conducted by three authors-examiners.
Statistical analysis
Continuous variables are presented as mean (± standard deviation; SD) and median (interquartile range), while categorical variables are presented as absolute and relative frequencies. Kolmogorov-Smirnov test and histograms were used to evaluate normality for continuous variables. Continuous variables followed normal distribution and therefore parametric methods were used.
To investigate the relationship between categorical variables chi-square test was used, while the relationship between continuous and categorical variables was estimated with Student’s t-test. Pearson’s correlation coefficient was used to explore the relationship between continuous variables. A correlation matrix was assessed prior to conducting the multivariate linear regression analysis to check for collinearity among the independent variables. Pearson’s correlation coefficient was used to estimate correlations between variables. Variables with a value of p < 0.25 in the bivariate analysis were included in a multivariate linear regression model with backward stepwise selection method and adjusted beta coefficients with corresponding 95% confidence intervals were estimated.
One way analysis of variance (ANOVA) was used to compare mean values of interview completion times between examiners.
The reliability of the questionnaire was tested following previously described methods (test-retest reliability).[12] A correlation analysis (Pearson's r) was performed to evaluate the reliability of each question. On the results of the correlation analysis of the pilot study, the correlation rate was very high for all the questions included in the final version of the questionnaire (Pearson's r = 0.85–1.00). Cronbach’s alpha coefficient was 0.9 which indicates a high internal reliability of the questionnaire. A two-sided p < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 19.0 (Chicago, Illinois, USA).
Results
Mean scale and initial questionnaire application times did not significantly differ between the three individual examiners (Examiner 1 = 6.637 ± 0.995 min; Examiner 2 = 6.756 ± 1.057; Examiner 3 = 6.303 ± 0.889). The overall mean ± SD application time was 6,565 ± 0.235 min.
Demographic and clinical data of the participants are presented in Table 1. The mean ADS score was 24.5 (± 6.7), while median score was 27 (interquartile range 20). On the basis of the proposed ADS classification scheme (scores 26-30 = independent, scores 15-25 = moderately dependent and 10-14 = totally dependent), 58.5% (n = 56) of the sample was shown to be independent, 27.1% (n = 26) moderately dependent and 14.6% (n = 14) totally dependent. The absolute and relative frequencies of individuals within each of the ten ADS items are shown in Table 2.
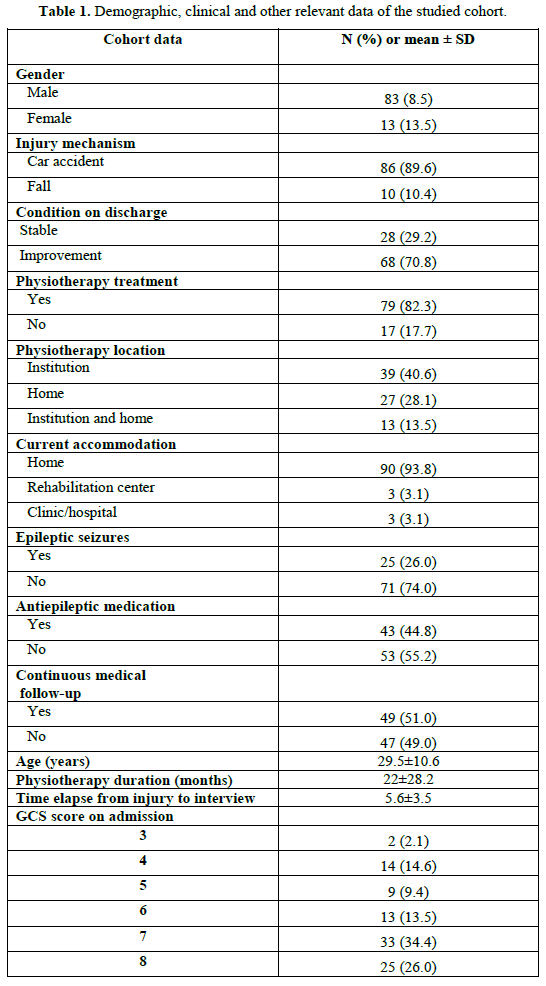

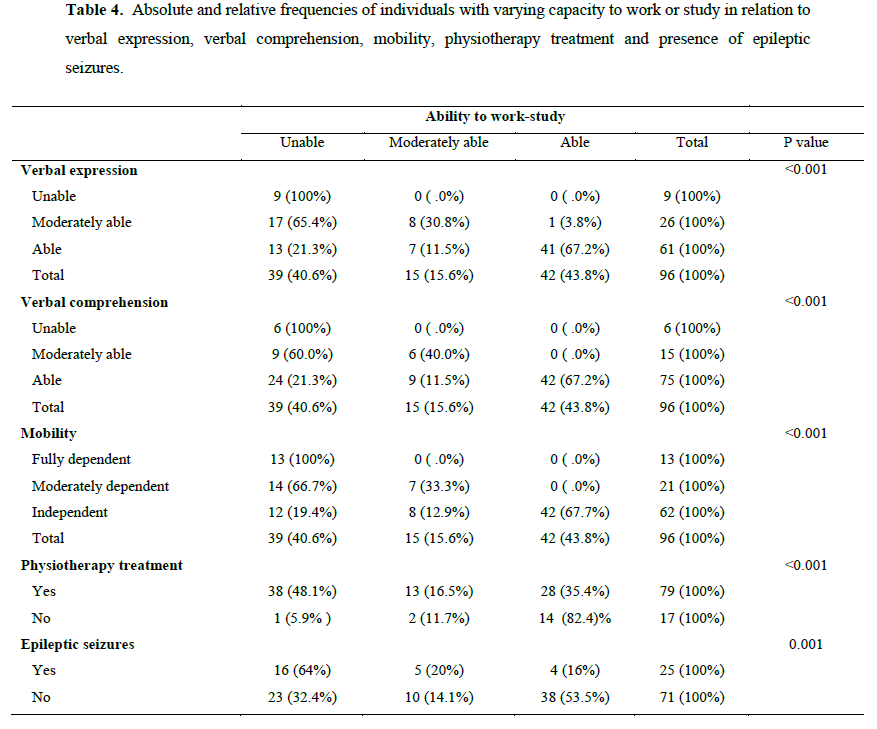
Bivariate analysis, identified statistically significant relations between the total ADS score and the following parameters: GCS score (r = 0.4; p<0.001), physiotherapy duration (r = - 0.5; p<0,001), physiotherapy treatment (t = -6.3, p < 0.001), presence of post-traumatic epileptic seizures (t = -3.2, p = 0.002) and use of antiepileptic medication (t = -3,7, p < 0.001). No statistically significant relations between the total ADS score and gender, age, injury mechanism or the elapsed time between admission and interviews, were identified. A correlation matrix was assessed prior to conducting the multivariate linear regression analysis to check for collinearity among the independent variables. Since the presence of post-traumatic epileptic seizures and the use of antiepileptic medication were strongly correlated (r = 0.66, p < 0.001), we chose to include only presence of post-traumatic epileptic seizures in the multivariate model. Multivariate linear regression method showed that increased GCS score, decreased physiotherapy duration and absence of post-traumatic epileptic seizures were independently associated with increased total ADS score (Table 3).
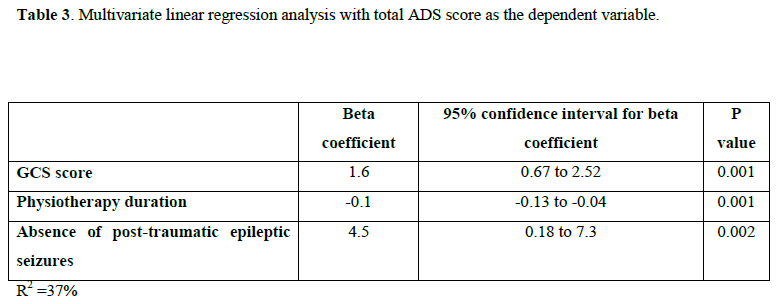
A statistically significant relation was found between the ability to work and verbal expression (x2=44, p<0.001), verbal comprehension (x2=27, p<0.001), mobility (x2=51, p<0.001), physiotherapy (x2=13.4, p<0.001) as well as presence of epileptic seizures (x2=11, p=0.001), as shown in Table 4.
Discussion
Valid and reliable assessment of long term outcome in TBI survivors is a prerequisite for the evaluation of functional disability and appropriateness of healthcare and rehabilitation provision. At present, assessment of TBI outcome is largely based on various well-established measurement scales, including GOS, FIM+FAM, DRS and the Barthel index. Nevertheless, the aforementioned outcome scales exhibit certain drawbacks in terms of usability and scope. Thus, GOS represents a crude functional assessment tool that does not examine aspects of disability-associated social burden.[8,9] While providing a more detailed investigation into various aspects of the patient’s motor, cognitive and psycosocial functions, FIM + FAM remains very time consuming to complete (approximately 35 min).[10] Similarly, while DRS represents a relatively thorough assessment tool, its application is time consuming (15-30 min) and the examiner’s familiarity with this scale is a prerequisite for timely completion.[11] The Barthel’s index scale while quick to administer, lacks sensitivity and focuses mainly on daily living aspects but excludes those related to cognition, emotionality and social functionality.[13-16]
In the present study a novel and relatively simple outcome measurement scale for the evaluation of TBI was designed and its suitability and usability was tested on a cohort of 96 severe TBI patients, previously hospitalized in our neurosurgery clinic. The proposed “Athens Disability Scale” (ADS) was generated by combining selected items from the aforementioned, well-established scales in a way that allows for the assessment of patient’s motor, psychocognitive as well as social abilities in a time sparing manner (< 7 min), thus representing a suitable tool for the needs of a neurosurgery clinic. Each of the 10 items in ADS incorporates 3 subcategories indicating total dependency (score =1), moderate dependency (score=2) or independency (score=3) with overall scale score ranging between 10-30 points. An ADS overall score of 26-30 indicates that the patient is independent, 15-25 indicates moderate dependency and 10-14 indicates total dependency. The classification scheme was derived following thorough examination of the ADS questionnaire and all possible score combinations. In this context, the classification “moderately dependent” is derived on the basis of the largest scoring range, since individuals that fall within this range, could neither be characterized as “fully dependent” even when they presented an ADS score of 15 points, nor “independent” even when they presented an ADS score of 25 points.
In the present study, putative correlations between overall ADS outcome scores and demographic/clinical data were investigated in order to test the prognostic value of the latter factors. Our analysis indicated that increased GCS scores, decreased physiotherapy duration and absence of post-traumatic epileptic seizures were independently associated with increased total ADS score. Ability to work or study was also shown to be associated with physiotherapy, presence of posttraumatic epileptic seizures, mobility levels, and psychocognitive status.
The predictive value of GCS scores in short and long-term TBI functional or occupational outcome assessed using various tools, is supported by the results of some early studies.[17-19] Interestingly, Balesteri et al. suggest that changing practices in TBI treatment over the past decade (incorporating aggressive early treatment) may impede GCS assessment on admission, leading to the loss of its predictive value. In this context it is noteworthy that several more recent studies indicate that GCS alone has limited prognostic value in long-term TBI functional and occupational outcome, which can be nevertheless improved when GCS score measures are combined together with pupillary reaction assessment as well as other injury severity scales and certain demographic data.[20-22]
Our results showed that decreased physiotherapy duration is independently associated with increased total ADS score. While this relationship is counterintuitive, a likely explanation for this finding is that severe disability following TBI does not improve with prolonged physical therapy, as indicated previously.[23]
Post-traumatic epilepsy (PTE) is a well-described consequence of TBI. Its incidence varies according to both TBI and PTE case definitions as well as the time elapsed between TBI and assessment.[24,25] The high frequency of PTE (26%) in the studied cohort appears consistent with previous epidemiological evidence indicating higher rates of PTE following severe, compared to mild and moderate TBI.[26-28] Moreover, the present results indicating poorer overall functional outcome in TBI survivors with PTE are in agreement with previous findings indicating a negative impact of early or late seizures on TBI functional outcome, assessed using various outcome scales.[29-32]
In the present study, while a trend towards decreased total ADS score with increasing age was apparent, this relationship did not reach statistical significance. Moreover, no apparent relation between gender and outcome was established. Evidence supporting an effect of age on severe TBI outcome has been previously obtained by numerous studies.[33-36] In contrast, an effect of gender on TBI outcome has been less well-characterized, with various studies presenting contradicting findings.[37-40] The extent to which the size and the uneven gender distribution of the studied sample influenced the present findings in relation to the putative effects of age and/or gender on outcome, warrants further investigation.
Return to work (or study) represents an important outcome index for severe TBI survivors.[41] The present findings indicated that 43.8% of the studied cohort returned to prior full-time occupation, 15.6% returned to work under specific conditions (part-time, supervisor or specialized device-assisted occupations or within a modified occupational/study environment), while 40.6% was unable to return to work/study. The relatively high rates of return to work/study following severe TBI shown herein appear in accord with the results of a recent meta-analysis.[42]
Previous studies indicate that several factors, including demographic characteristics,[43,44] TBI severity,[44, 45] as well as cognitive and motor status[46-48] can influence return to work/study. As a corollary, the results presented herein show a significant relation between return to work/study and verbal expression, verbal comprehension, mobility, physiotherapy treatment and presence of epileptic seizures. Moreover, consistent with previous findings,[49,50] the present study highlights several impeding factors for return to work/study, including motor deficits, tremor, fatigue and difficulties in verbal expression and comprehension.
A limitation of the present pilot study concerns its retrospective nature and the small number of participants. Further studies on larger cohorts incorporating survivors of varying TBI severity or other neurological injuries, as well as comparative studies using other well-established scales are warranted to extend the present findings and provide indices of sensitivity, usefulness and reliability of the proposed novel scale.
Conclusions
The present study investigated the suitability and usability of a novel and simple outcome measurement scale on a cohort of 96 severe TBI patients, previously hospitalized in our department. The proposed “Athens Disability Scale” (ADS) combines selected elements of commonly used outcome scales and allows for the assessment of TBI outcome on motor, psychocognitive and social aspects, in a time-sparing manner (<7min). Outcome scores were correlated with demographic and clinical data. The analysis indicated that increased GCS score, decreased physiotherapy duration and absence of posttraumatic epileptic seizures were independently associated with increased total ADS score. Ability to work or study was also shown to be associated with physiotherapy, presence of posttraumatic epileptic seizures, mobility levels, cognitive and psychosocial status. The present results indicate that our novel and relatively simple scale (ADS) may represent a useful outcome assessment tool that allows for the rapid evaluation of functional disability and quality of life following TBI.
2759
References
- Kraus JF, Mc Arthur DL. Epidemiologic aspects of brain injury. Neurol Clin 1996; 14:435-450.
- Tiret L, Hausher E, Thicoipe M, Garros B, Maurette P, Castel JP. The epidemiology of head trauma in Aquitine (France) 1986. Community based study of hospital admission and deaths. Int J Epidemiol 1990; 19(1):133-140.
- Anderson EH, Bjorklund R, Emanuelson I, Stalbammar D. Epidemiology of traumatic brain injury: a population based study in western Sweden. Acta Neurol Scand 2003; 107(4):256-259.
- Stranjalis G, Sakas D, Marmarou A. Difficulties in implementing a standardized transfer policy in severe head injuries in Greece. J Restorative Neurosci Neurorehabil 2000; 6: 24.
- Stranjalis G, Singounas E. Development of neurosurgery in Greece: past, present and future. J Neurosurg 1998;88(4):782-785.
- Stranjalis G, Bouras T, Korfias S, Andrianakis I, Pitaridis M, Tsamandouraki K. Outcome in 1.000 head injury hospital admissions: The Athens Head Trauma Registry. J Trauma 2008;65(4): 789-793.
- Lindsay K, Bone I. Outcome after brain damage. In: Lindsay K, Bone I., et al, eds. Neurology and Neurosurgery Illustrated. 4th ed. Edinburgh: Churchill Livingstone eds; 2004:214.
- Nichol AD, Higgins AM, Gabbe BJ, Murray LJ, Cooper DJ, Cameron PA. Measuring functional and quality of life outcomes following major head injury: common scales and checklists. Injury 2011; 42(3):281-287.
- Wright J. (2000). The Glasgow Outcome Scale. The Center for Outcome Measurement in Brain Injury. Available from : https://www.tbims.org/combi/gos/index.html (access 21/1/2011)
- Wright J. (2000). The Functional Assessment Measure. The Center for Outcome Measurement in Brain Injury. Available from: https://www.tbims.org/combi/FAM/index.html (access 21/1/2011)
- Wright J. (2000). The Disability Rating Scale. The Center for Outcome Measurement in Brain Injury. Available from : https://www.tbims.org/combi/drs/index.html (access 21/1/2011)
- Stranjalis G, Kalamatianos T, Stavrinou LC, Tsamandouraki K, Alamanos Y. Neck pain in a sample of Greek urban population (fifteen to sixty-five years): analysis according to personal and socioeconomic characteristics. Spine (Phila Pa 1976) 2011; 36(16).
- Mahoney F, Barthel D. ?Functional evaluation: The Barthel Index?. Md Med J 1965; 14:61-65.
- Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. ?Stroke rehabilitation: Analysis of repeated Barthel index measures?. Arch Phys Med Rehabil 1979; 60(1):14-17.
- Shah S, Vanclay F, Cooper B. ?Improving the sensitivity of Barthel Index for stroke rehabilitation?. J Clin Epidemiol 1989; 42(8):703-709.
- Sulter G, Steen C, De Keyser J. ?Use of the Barthel Index and modified Rankin scale in acute stroke trials?. Stroke 1999; 30(8):1538-1541.
- Zafonte R, Hammond F, Mann N, Wood D, Black K, Millis S. Relationship between Glasgow Coma Scale and functional outcome. Am J Phys Med Rehabil 1999; 75(5):364-369.
- Asikainen I, Kaste M, Sarna S. Predicting late outcome for patients with traumatic brain injury referred to a rehabilitation programme: a study out of 508 Finnish patients 5 years or more after injury. Brain Inj 1998; 12(2): 95-107.
- Balesteri M, Csoznyka M, Chatfield D, Steiner L, Schmidt E, Smielewski P, et al. Predictive value of Glasgow Coma Scale after brain trauma: change in trend over the past ten years. J Neurol Neurosurg Psyciatry 2004; 75(1):161-162.
- Wagner A, Hammond F, Sasser H, Wiercisiewski D, Norton H. Use of injury severity variables in determining disability and community integration after traumatic brain injury. Journal of Trauma-Injury-Infection and Critical Care 2000; 49(3):411-419.
- Mc Nett M. A review of the predictive ability of Glasgow Coma Scale scores in head-injured patients. J Neurosci Nurs 2007; 39(2):68-75.
- Foreman B, Caesar R, Parks J, Madden C, Gentilello L, Shafi S, et al. Usefulness of the Abbreviated Injury Score and the Injury Severity Score in comparison to the Glasgow Coma Scale in the predicting outcome after traumatic brain injury. Journal of Trauma-Injury-Infection and Critical Care 2007; 62(4):946-950.
- Walker W, Picket T. Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J Rehab Res 2007; 44(7):975-982.
- Frey L. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia 2003; 44(10):11-17.
- Ferguson P, Smith G, Wannamaker B, Thurman D, Pickelsimer E, Selassie A. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia 2010; 51(5):891-898.
- Annegers J, Hauser W, Coan S, Rocca W. A population-based study of seizures after traumatic brain injuries. N Engl J Med 1998; 338(1):20-24.
- D?Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol 2004; 17(6):731-735.
- Tempkin N. Risk factors for posttraumatic seizures in adults. Epilepsia 2003; 44(10):18-20.
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia 1999; 40(5):584-589.
- Mazzini L, Cossa FM, Angelino E, Campini R, Pastore I, Monaco F. Posttraumatic epilepsy: neuroradiologic and neuropsychological assessment of long-term outcome. Epilepsia 2003; 44(4):569-574.
- Andelic N, Hammergren N, Bautz-Holter E, Sveen U, Brunborg C, R?e C. Functional outcome and health-related quality of life 10 years after moderate-to-severe traumatic brain injury. Acta Neurol Scand 2009; 120(1):16-23.
- Skandsen T, Ivar Lund T, Fredriksli O, Vik A. Global outcome, productivity and epilepsy 3-8 years after severe head injury. The impact of injury severity. Clin Rehabil 2008; 22(7):653-662.
- Marquez de la Plata C, Hart T, Hammond F, Frol A, Hudak A, Harper C, et al. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil 2008; 89(5):896-903.
- Hukkelhoven C, Steyerberg E, Rampen A, Farace E, Habbema D, Marshal L, et al. Patient age and outcome following severe traumatic brain injury: an analysis of 5.600 patients. J Neurosurg 2003; 99(4):666-673.
- Mosenthal A, Livingston D, Lavery R, Knudson MM, Lee S, Morabito D, et al. The effect of age on functional outcome in mild traumatic brain injury: 6 month report of a retrospective multicentered trial. J Trauma 2004; 56(5):1042-1048.
- Livingston D, Lavery R, Mosenthal A, Knudson MM, Lee S, Morabito D, et al. Recovery at one year following isolated traumatic brain injury: a Western Trauma Association prospective multicenter trial. J Trauma 2005; 59(6):1298-1304.
- Slewa-Younan S, Green A, Baguley I, Gurka J, Marosszeky J. Sex differences in injury severity and outcome measures after traumatic brain injury. Arch Phys Med Rehabil 2004; 85(3):376-379.
- Slewa-Younan S, Baguley I, Heriseanu R, Cameron I, Pitsiavas V, Mudaliar Y, et al. Do men and women differ in their course following traumatic brain injury? A preliminary prospective investigation of early outcome. Brain Inj 2008; 22(2):183-191.
- Kirkness C, Burr R, Mitchel P Newell D. Is there a sex difference in the course following traumatic brain injury? Biol Res Nurs 2004; 5(4):299-310.
- Moore D, Ashman T, Cantor J, Kriknick R, Spielman L. Does gender influence cognitive outcome after traumatic brain injury? Neuropsychol Rehabil 2010; 20(3):340-354.
- Walker W, Marwitz J, Kreutzer J, Hart T, Novack T. Occupational categories and return to work after traumatic brain injury: A multicenter study. Arch Phys Med Rehabil 2006; 87(12):1576-1582.
- Shames J, Treger I, Ring H, Giaquinto S. Return to work following traumatic brain injury: trends and challenges. Disabil Rehabil 2007; 29 (17):1387-1395.
- Ponsford JL, Olver JH, Curran C, Ng K. Prediction of employment status 2 years after traumatic brain injury. Brain Inj 1995; 9(1):11-20.
- Gollaher K, High WM Jr, Sherer M, Bergloff P, Boake C, Young ME, et al. Prediction of employment outcome one to three years following traumatic brain injury. Brain Inj 1998; 12(4):255-263.
- Wagner AK, Hammond FM, Sasser HC, Wiercisiewski D. Return to productive activity after traumatic brain injury: relationship with measures of disability, handicap and community integration. Arch Phys Med Rehabil 2002; 83(1):107-114.
- Sherer M, Sander AM, Nick TG, High WM Jr, Malek JF, Rosenthal M. Early cognitive status and productivity outcome after traumatic brain injury: findings from the TBI Model Systems. Arch Phys Med Rehabil 2002; 83(2):183-192.
- Greenspan AI, Wrigley JM, Kresnow M, Branche-Dorsey CM, Fine PR. Factors influencing failure to return to work due to traumatic brain injury. Brain Inj 1996; 10(3):207-218.
- Cifu DX, Keyser-Marcus L, Lopez E, Wehman P, Kreutzer JS, Englander J, et al. Acute predictors of successful return to work one year after traumatic brain injury: A multicenter analysis. Arch Phys Med Rehabil 1997; 78(2):125-131.
- Mc Namee S, Walker W, Cifu DX, Wehman PH. Minimizing the effect of TBI-related physical sequelae on vocational return. JRRD 2009; 46 (6):893-908.
- Hawley CA, Ward AB, Magnay AR, Mychalkiw W. Return to school after brain injury. Arch Dis Child 2004; 89(2):136-142.









