Ewa Kotula1,2, Léonard Jagot-Lacoussiere1,2, Heriberto Bruzzoni-Giovanelli1,2,3 and Jean-Luc
Poyet1,2,4
1INSERM UMRS1160, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, Paris, France
2Université Paris Diderot, Sorbonne Paris Cité, Paris, France
3Centre d’Investigations Cliniques 9504 INSERM-AP-HP, Hôpital Saint-Louis, Paris, France
4C-Dithem, Inserm Consortium for Discovery and Innovation in Therapy and Medicine, France
- Corresponding Author:
- Jean-Luc Poyet
INSERM UMRS1160, Université Paris Diderot
Sorbonne Paris Cité, Institut Universitaire d’Hématologie
Hôpital Saint-Louis, 1 rue Claude Vellefaux, 75010 Paris, France
Tel: +33 1 42499263
E-mail: jean-luc.poyet@inserm.fr
Received date: 21 July 2016; Accepted date: 25 July 2016; Published date: 30 July 2016
Citation: Kotula E, Jagot-Lacoussiere L, Bruzzoni-Giovanelli H, et al. Specific Accumulation of a Cell Penetrating Peptide Targeting AAC-11 in
Melanoma Tumors. Arch Can Res. 2016, 4:3.
Melanoma is the most serious and potentially dangerous form of skin cancer with a rapid increase in prevalence, especially among western countries. The prognosis for patients with the more advanced form of the disease, metastatic melanoma, remains poor with survival rates ranging from 6.7% to 8% at 5 years, and a median survival of 6 to 9 months. Therefore, there is a clear unmet clinical need to identify new drugs to combat melanoma.
Brief Report
Melanoma is the most serious and potentially dangerous form of skin cancer with a rapid increase in prevalence, especially among western countries. The prognosis for patients with the more advanced form of the disease, metastatic melanoma, remains poor with survival rates ranging from 6.7% to 8% at 5 years, and a median survival of 6 to 9 months [1]. Therefore, there is a clear unmet clinical need to identify new drugs to combat melanoma.
The survival protein AAC-11 has previously been shown to be overexpressed in multiple cancer cells, including melanoma, and contribute to tumor invasion and metastases [2]. We and others have shown that AAC-11 is critically involved in tumor survival, through inhibition of apoptosis, by inhibiting Acinus–mediated DNA fragmentation and in an E2Fdependent manner [3,4]. Silencing of AAC-11 sensitizes tumor cells to chemotherapeutic drugs whereas its overexpression drastically increases tumor cells migration and invasiveness [3,5]. Recently, AAC-11 has been identified as a novel immune escape gene in tumors that promotes immune resistance to antigen-specific T cells [6]. Thus, inactivation of AAC-11 might constitute an attractive approach for developing cancer AAC-11 is involved in multiple protein-protein interactions [3,7,8]. Recent data indicate that the heptad leucine repeat domain of AAC-11 is crucial for its biological functions, probably by acting as a protein-protein interaction module [3,9-11]. Therefore, targeting the heptad leucine repeat domain of AAC-11 might prevent its binding to its protein partners, thus inhibiting AAC-11 function as signal transducer. therapeutics.
We have developed a cell penetrating peptide, called RT53, spanning the heptad leucine repeat region domain of AAC-11 (residues 363-399) fused at the N-terminus to the transmembrane-penetrating sequence penetratin that exhibits selective cytotoxicity toward cancer cells, but not normal cells. RT53 can induce membranolysis of cancer cells, leading to rapid, necrotic death, probably through its selective interaction and accumulation in cancer cells plasma membranes, suggesting binding with a membrane partner(s) that is absent or minimally present in the membranes of untransformed cells [12]. Moreover, RT53 was able to inhibit tumor growth in vivo in human melanoma xenograft models, BRAF wild type and V600E mutant, with limited to nonexistent off-target toxicity [12].
To better understand the in vivo performance of RT53, we studied the bio-distribution of rhodamine-labelled wild-type RT53 and a control peptide in which in which two conserved leucines were substituted by glycines [12] in nude mice bearing subcutaneous C8161 human melanoma tumors with an IVIS imaging system (Xenogen). Very interestingly, efficient homing and accumulation of the fluorescent conjugate in the tumor was clearly evident at 1h postadministration for the wild-type peptide, but not the control peptide (Figure 1). These results indicate that RT53 is able to target and accumulate in melanoma tumors in vivo.
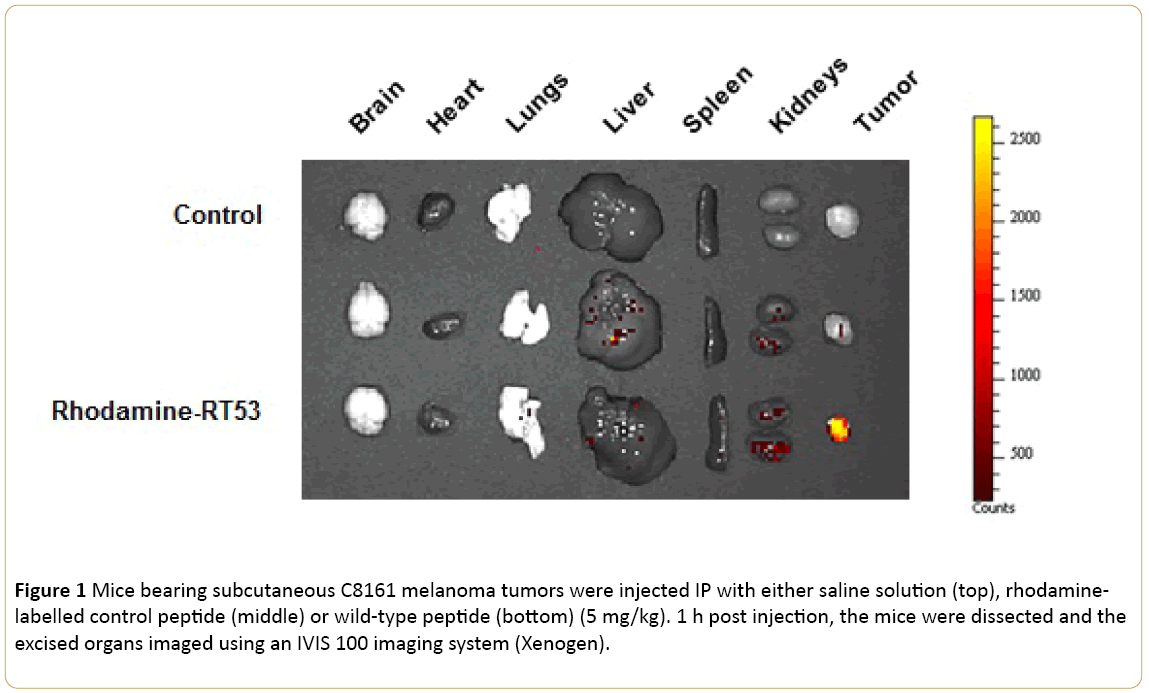
Figure 1: Mice bearing subcutaneous C8161 melanoma tumors were injected IP with either saline solution (top), rhodaminelabelled control peptide (middle) or wild-type peptide (bottom) (5 mg/kg). 1 h post injection, the mice were dissected and the excised organs imaged using an IVIS 100 imaging system (Xenogen).
In conclusion, our data demonstrate that the RT53 exhibits potentially desirable features as novel anticancer class of drug in melanoma and constitutes a promising anti-cancer molecule worthy of further evaluation and development.
Acknowledgement
This work was supported by the INSERM and a grant from INSERM Transfert and SATT IDF Innov.
9958
References
- Garbe C (2010) Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer46: 270-283.
- Faye A, Poyet JL (2010) Targeting AAC-11 in cancer therapy. Expert OpinTher Targets14: 57-65.
- Rigou P (2009) The anti-apoptotic protein AAC-11 interacts with and regulates Acinus-mediated DNA fragmentation. Embo J 28: 1576-1588.
- Morris EJ (2006) Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo. PLoS Genet 2: e196.
- Kim JW (2000) AAC-11 overexpression induces invasion and protects cervical cancer cells from apoptosis. Lab Invest80: 587-594.
- Noh KH (2014) API5 confers tumoral immune escape through FGF2-dependent cell survival pathway. Cancer Res74(13):3556-3566.
- Ahel D (2009) Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science325: 1240-1243.
- Van den Berghe L (2000) FIF [fibroblast growth factor-2 (FGF-2)-interacting-factor], a nuclear putatively anti-apoptotic factor, interacts specifically with FGF-2. MolEndocrinol 14: 1709-1724.
- Tewari (1997) AAC-11, a novel cDNA that inhibits apoptosis after growth factor withdrawal. Cancer Res 57: 4063-4069.
- Song KH (2015) Apoptosis inhibitor 5 increases metastasis via Erk-mediated MMP expression. BMB Rep48: 330-335.
- Han BG (2012) Helical repeat structure of apoptosis inhibitor 5 reveals protein-protein interaction modules. J BiolChem287: 10727-10737.
- Jagot-Lacoussiere L (2016) A cell penetrating peptide targeting AAC-11 specifically induces cancer cells death. Cancer Res.






