Keywords
Placental bed biopsy; Retrospective analysis; Hypertension in pregnancy
Introduction
The term ‘placental bed’ was introduced by Dixon and Robertson around 60 years back and can be described as that part of decidua and adjoining myometrium which underlies the placenta and whose primary function is the maintenance of adequate blood supply to the intervillous space of the placenta [1].
Remodeling of maternal uterine spiral arteries is key to normal growth and development of the fetus and thus successful pregnancy. Remodeling of uteroplacental arteries, the so-called physiological changes of uteroplacental arteries, can be divided according to structural criteria into three stages:
1) Trophoblast invasion-independent vascular changes
2) Vascular remodeling induced by perivascularly located interstitial trophoblast
3) Trophoblast infiltration of vessel walls [2].
The spiral arteries of placental bed are invaded by the cytotrophoblasts which breaks down the endothelium, internal elastic lamina and the muscular coat of the vessel, which are largely replaced by fibrinoid. These physiological changes convert the vessels supplying the placenta from muscular end arteries to wide mouthed sinusoids, leading to around 5-10 fold dilatation of the vessel. These physiological changes lead to reduced vascular resistance and a considerable increase in blood supply to the fetus.
Early in normal pregnancy these physiological changes involve the myometrial segment of spiral arteries too, which are seen more at the centre of the placental bed. The mean external diameter of the myometrial spiral arteries in placental bed is approx 500 μ. These physiological changes are completed by 20 weeks.
In preeclampsia & IUGR, physiological changes are almost completely restricted to the decidual segment of the spiral arteries. Spiral arteries in the myometrial segment appears to have normal morphological structure as seen in normal non pregnant uterus, including the retention of internal elastic lamina. The mean external diameter is approx 200 μ. Lesions of thrombosis, acute atherosis with intramural foam cells occasionally occurs [3]. Many studies have described some characteristic features of vessels in placental bed in hypertensive pregnancies- i.e. hyalinization of true arterioles and intimal hyperplasia with medial degeneration and proliferative fibrosis of small arteries [4,5].
Absence of remodeling of spiral arteries has also been reported in IUGR cases, even in absence of hypertension [6].
Disorders of deep placentation are characterized by:
1) The degree of restriction of physiologic transformation of the spiral arteries
2) The presence of arterial lesions in the junctional zone myometrium of the placental bed [7]. This defective deep placentation is associated with a spectrum called The Great Obstetrical Syndromes that includes preeclampsia, IUGR, preterm labor with intact membranes, preterm PROM, abruptio placentae and spontaneous midtrimester abortion.
However, physiologic transformation of the spiral arteries is not an “all or none” phenomenon [8]. In severe preeclampsia also, a few spiral arteries in the center of the placental bed may show full transformation of the myometrial segment.
Also Vascular lesions do not occur in every spiral artery and they can be focal or segmental and therefore may be absent in random sections and biopsies [9].
Histological confirmation that the biopsy has been derived from the placental bed is based on the presence of trophoblast, adherent villi or transformed spiral arteries. However, the absence of these markers does not necessarily mean that the placental bed was not sampled [10,11].
Aims
• To assess the extent of dilatation of spiral arteries in placental bed in normotensive and hypertensive pregnancies
• To assess the extent of dilatation of spiral arteries in placental bed in relation to birthweight.
Methods
The data was collected from a hospital based cross-sectional survey of histological studies on biopsies from the placental bed, among women undergoing cesarean sections for various obstetrical reasons at Gauhati Medical College & Apollo hospitals, Guwahati in over a period of 2 years, a few years back. Singleton, live pregnancies who have crossed the period of viability were included. Now we have a retrospective analysis of the data collected in that period. Clearance from Ethical Committee has been taken clearly.
Women who were planned for an elective or emergency caesarean section during the study period were told that biopsy will be taken from placental bed for a study. Details of the procedure were discussed with the patient & husband. Those who gave written and informed consent were included in the study. Thus in 50 cases biopsy could be taken.
Materials were collected over a period of 2 years in the tertiary centres as mentioned above. After expulsion of placenta, biopsy material were taken by wedge resection of 1.5 × 1.5 cm tissue from the placental bed. Biopsy specimens were collected by direct visualization of the centre of the placental attachment site and taken from 2 randomly chosen placental sites. If bleeding occurred, hemostatic stitches were given.
Biopsy specimens were sent to the pathologist for histopathological examination in 10% formaldehyde. Sections of tissue were stained with hematoxylin-eosin stain.
The diameter of the arterial lumen was measured with the help of an ocular and objective micrometre. At first the slide was focused under objective (10X) and viewed with a 10X eyepiece. One vision of the ocular micrometre scale is equivalent to 13.6 μ at 100 times magnification. The arteries seen in the field were measured by placing the ocular scale over the arterial lumen and counting the number of small divisions occupied by the lumen. This number was multiplied by 13.6 to get the diameter in μ. The measurement of external diameter was taken at 3 or 4 different directions and its average has been taken.
Arteries which resembled like the non-pregnant state were considered non-dilated, which was approximately less than or equal to 272 micron. And arteries which showed feature of remodeling were considered normal. The placental beds which showed more than 50% non-dilated arteries or mean of arteries was less than 272 micron were considered to have NON-DILATED vessels. The beds in which more than 50% of arteries in the biopsy specimen were of normal caliber, were considered to have NORMAL vessels. Placental beds which showed equal number of non-dilated and normal caliber vessels, were considered to have MIXED morphology. Figure 1 shows a dilated spiral artery and Figure 2 shows a non-dilated spiral artery.
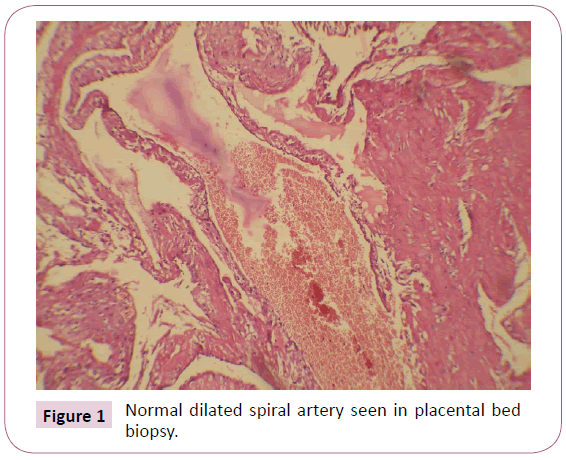
Figure 1: Normal dilated spiral artery seen in placental bed biopsy.
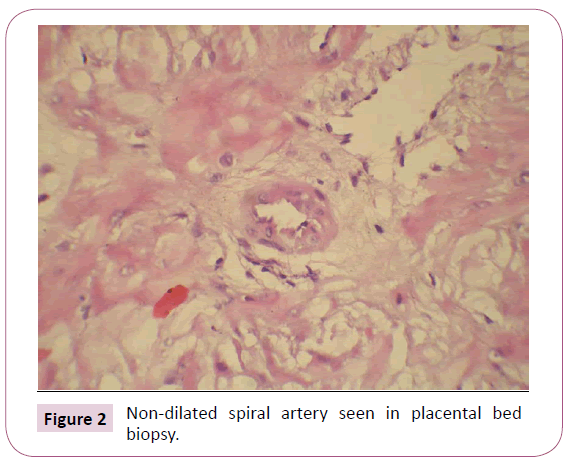
Figure 2: Non-dilated spiral artery seen in placental bed biopsy.
Out of 50 cases, 14 placental beds showed no vessels at all. Thus these cases were excluded from further studies.
The hypertensive women were subclassified as hypertension without proteinuria (gestational hypertension) & hypertension with proteinuria (preeclampsia). Gestational hypertension is defined as the onset of hypertension (blood pressure of 140/90 mmHg, 6 hours apart) and pre-eclampsia is hypertension with significant proteinuria (≥ 300 mg/day or ≥ 2+urinary protein) after 20 weeks of pregnancy. In addition, any woman with severe, uncontrolled HTN, cerebral or visual disturbances, epigastric pain, pulmonary oedema, oliguria (400 ml or less in 24 hours), or an abnormal platelet count and liver function profile were included in the pre-eclampsia group. The presence of proteinuria was screened using dipsticks, and the amount of protein was measured in a 24 hour urine sample.
Also to see the correlation of birthweight and spiral arterial remodeling, the birthweights were divided into the following groups- <2 kg, 2-2.5 kg, 2.6-3 kg and >3 kg. ≤ 2.5 kg birthweights were considered low birth weights.
For statistical analysis, t-test and chi square test was used. We used statistical software Stat Pac version 4.0 for Microsoft Excel for Windows.
Results
Out of 50 cases that were included, 14(28%) were excluded because no vessel was seen in the biopsy specimen. Out of the remaining 36 cases, 16 were normotensive and 20 were hypertensive (11 gestational hypertension, 9 Preeclampsia). Number of cases with non-dilated, mixed or normal vessels in relation to hypertension are shown in Figure 3 and Table 1. The p value calculated is 0.0230, which is statistically significant, i.e. non-dilated vessels in gestational hypertension and PE is statistically significant. Out of 20 cases of GH and PE, 14(70%) have non-dilated vessels, 2 mixed and 4(20%) normal vessels. Out of 16 normotensive cases, 6 i.e 35% had non-dilated vessels.

Figure 3: Vessel dilatation in relation to hypertension in pregnancy.
| |
Normal vessels |
Mixed |
Non-dilated |
Total |
| Normotensive |
9 |
1 |
6 |
16 |
| Gestational Hypertension(GH) |
3 |
1 |
7 |
11 |
| PE (preeclampsia) |
1 |
1 |
7 |
9 |
| Total |
13 |
3 |
20 |
36 |
Table 1 Spiral vessel dilatation in relation to hypertension in pregnancy.
Out of 36 cases, in 4 cases birthweight was <2 kg, 13 cases had birthweight 2-2.5 kg, 15 cases had 2.6-3 kg and in 4 cases birthweight was >3 kg. Number of non-dilated and normal vessels in relation to the birthweights are shown in Figure 4 and Table 2. The P value calculated is 0.0113, which is statistically significant, i.e. non-dilated vessels in lower birthweights is statistically significant. Out of the 17 IUGR cases (weight ≤ 2.5 kg), 15 cases (88.23%) had non-dilated vessels.
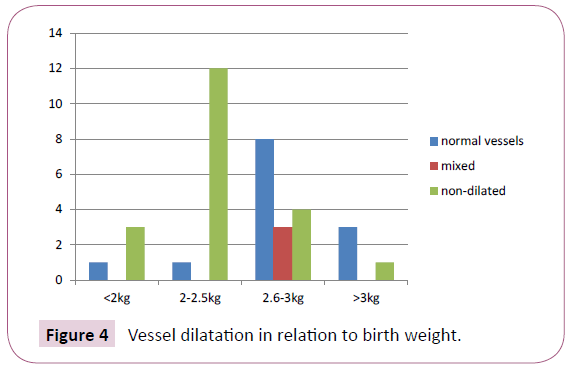
Figure 4: Vessel dilatation in relation to birth weight.
| |
Normal vessels |
Mixed |
Non-dilated |
Total |
| <2kg |
1 |
0 |
3 |
4 |
| 2-2.5kg |
1 |
0 |
12 |
13 |
| 2.6-3kg |
8 |
3 |
4 |
15 |
| >3kg |
3 |
0 |
1 |
4 |
| Total |
13 |
3 |
20 |
36 |
Table 2 Spiral vessel dilatation in relation to birth weights.
Table 3 shows the number of cases with or without hypertension in relation to birthweight. P value for relation between low birthweight and Hypertension is 0.5072, which is not very significant in our study. Thus it can be assumed that nondilated vessels in relation to low birthweight is independent of hypertension.
| |
Normotensive |
Hypertensive |
Total |
| <2kg |
2 |
2 |
4 |
| 2-2.5kg |
6 |
7 |
13 |
| 2.6-3kg |
5 |
10 |
15 |
| >3kg |
3 |
1 |
4 |
| Total |
16 |
20 |
36 |
Table 3 Relation of birthweight to hypertension in pregnancy in this study.
Out of 36 cases, 19 cases were primigravida and 17 cases were multigravida. 12 cases of primigravida i.e 63.16% have narrow vessels as shown in Figure 5 and Table 4. Though the p value is not statistically significant (p=0.4232), yet the percentage of narrow vessels is higher in primigravida.
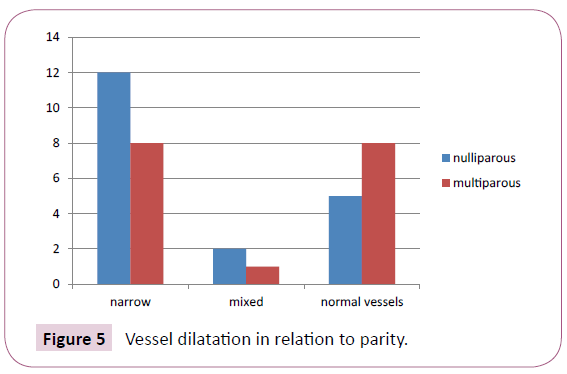
Figure 5: Vessel dilatation in relation to parity.
| |
Non-dilated |
mixed |
Normal vessels |
| Nulliparous |
12 |
2 |
5 |
| Multiparous |
8 |
1 |
8 |
Table 4 Spiral vessel dilatation in relation to parity.
Out of 50 cases included, there were 5 preterm cases (<37 weeks), out of which 1 case had no vessel, 3 cases had non-dilated vessels and 1 case had normal vessel. Thus it is seen that in preterm cases also, percentage of non-dilated vessels is high.
To assess relation of maternal age with extent of spiral arterial dilatation, age groups were divided into ≤ 20 years, 21-25, 26- 30 and >30 years. Number of cases with normal, mixed and non-dilated spiral arteries in relation to maternal age have been shown in Table 5. The p value calculated is 1.992, which is not significant. Thus no relation was seen of maternal age to spiral arterial dilatation.
| |
Normal |
Mixed |
Non-dilated |
Total |
| ≤ 20 years |
1 |
1 |
1 |
3 |
| 21-25 years |
4 |
1 |
7 |
12 |
| 26-30 years |
4 |
1 |
7 |
12 |
| > 30 years |
4 |
- |
5 |
9 |
| Total |
13 |
3 |
20 |
36 |
Table 5 Spiral arterial dilatation in relation to maternal age.
Discussion
In many studies of placental bed biopsies, some specimens had no vessels. This percentage range from 40-70%. [12,13]. In our study, in 28% cases, no vessels were seen in the biopsy specimens.
vessels or defective physiological changes in myometrial spiral arteries in PIH and preeclampsia. Hanssens et al. reported these changes in 74% cases of preeclampsia [14]. Sagol et al. also reported 71% [15]. Guzin et al. reported 65% cases had defective spiral arteries i.e. non-dilated vessels in hypertensive pregnancies [16]. In these studies the percentage of non-dilated spiral arteries in normotensive pregnancies is also very similar to our study.
Studies have shown that defective deep placentation can occur in growth restriction even in normotensive women [17,18]. In the present study also we found a significant co-relation between low birth weights and non-dilated spiral vessels. (p= 0.0113), which is independent of the effects of hypertension. Out of the IUGR cases in our study (with or without hypertension), 88% had nondilated vessels.
Though there are just 4 cases of preterm labour/premature rupture of membranes, out of these 3 have non-dilated vessels. Thus defective deep placentation may have a role in preterm labour, as highlighted by Kim et al. [19].
In this study, higher percentage of primigravida cases had nondilated vessels. This point has been highlighted in the chapter 2 of book Placental bed disorders [20]. Such results may indicate a process of pre-conditioning and inflammation in pregnancy, which is more important in primigravidas. Interestingly percentage of nulliparous women is higher in preeclampsia and preterm labor group. Thus defective deep placentation in this group may be related to other factors also.
Limitations of the study- Although the distribution of interstitial trophoblast in the junctional zone myometrium is not homogeneous (and varies not only between patients, but also between biopsy specimens from the same patient), placental bed biopsy specimens have limitations because they only provide information about a small segment of the placental bed. It is possible that areas close to the nonbiopsy site may have a completely different degree of vascular transformation [10]. Moreover the number of cases in this study is quite less.
Conclusion
Gestational hypertension, preeclampsia, IUGR, preterm labour are associated with defective deep placentation, which may be associated with different degrees of restricted remodeling and obstructive lesions of the spiral arteries in the junctional zone or inner myometrium. All the conditions included in ‘Great Obstetrics Syndrome’ are likely to share same pattern of defective deep placentation. This concept may be used to improve the characterization of the disorders of the placental bed.
Understanding the molecular processes that occur during implantation and the process of endometrial decidualization and angiogenesis is a target for pre-pregnancy diagnosis and therapy. Characterization of angiogenesis and the development of biomarkers in early placental development are likely to provide diagnostic and hopefully noninvasive predictive markers to identify mothers at risk for defective deep placentation syndromes such as preeclampsia, IUGR, preterm birth and other adverse pregnancy outcomes.
26398
References
- Dixon HG, Robertson WB (1958) A study of vessels of the placental bed in normotensive and hypertensive women. J Obstet Gynaecol Br Emp 65: 803-809.
- Kaufmann P (2003) Endovascular Trophoblast Invasion: Implications for the Pathogenesis of Intrauterine Growth Retardation and Preeclampsia. Biology of Reproduction 69: 1–7.
- Brosens I (1972) the role of spiral arteries in pathogenesis of preeclampsia. Obstet Gynecol Annu 1: 91-177.
- Dixon HG, Robertson WB (1958) A study of vessels of the placental bed in normotensive and hypertensive women. J Obstet Gynaecol Br Emp 65: 803-9.
- Brosens I (1964) A study of the spiral arteries in the deciduas basais in normotensive and hypertensive pregnancies. J Obstet Gynaec Br Cwth 71: 222-30.
- Khong TY (1986) Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and small-for-gestational age infants. Br J Obstet Gynaecol 93: 1049-59.
- Brosens P (2011) The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 204: 193–201.
- Espinoza J, Romero R, Yeon MK, Kusanovic JP, Hassan S, et al. (2006) Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med 34: 447– 458.
- Meekins JW (1994) A study of placental bed spiral arteries and trohoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 101: 669-674.
- Renaer M, Brosens I (1963) Spiral arterioles in the decidua basalis in hypertensive complications of pregnancy. Ned Tijdschr Verloskd Gynaecol 63: 103–118.
- Pijnenborg R, Bland JM, Robertson WB (1981) The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta 2: 303–316.
- Brosens I (1977) Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol 84: 656-663.
- Robson SC (2002) Punch biopsy of the human placental bed. Am J Obstet Gynecol 187: 1349-1355.
- Hanssens M (1998) Renin like immunoreactivity in uterus and placenta from normotensive and hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol 81: 177-184.
- Sagol S (1995) Comparison of uterine artery Doppler velocimetry in relation to trophoblast migration into the myometrium of the placental bed. Obstet Gynecol 85: 760-765.
- Guzin K (2005) The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in preeclamptic pregnancies. Arch Gynecol Obstet 272: 283-288.
- Khong TY (1986) Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and small-for-gestational age infants. Br J Obstet Gynaecol 93: 1049-1059.
- De Wolf (1980) The human placental bed: electron microscopic study of trophoblastic invasion of spiral arteries. Am J Obstet Gynecol 137: 58-70.
- Kim YM (2003) Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 189: 1063-1069.
- Robert P (2010). Placental Bed Disorders. Cambridge University Press. 1st South Asian edn, 18.











