Keywords
Milk; Protein; Co-precipitates
Introduction
Milk proteins have long been used as additives for processed food. Their nutritional and functional properties make them one of the best nutritional supplements [1]. Numerous markets are interested in functional proteins and in utilizing them in the food industry which has led to the increasing focus on improving production and separation methods of milk proteins of specific functions [2]. Producing co-precipitates proteins have a number of benefits such as increasing coagulum and functionality as it has higher nutritional value compared to Cazin [3]. Methods for producing milk protein concentrates have widely been developed with differences in structure and characteristics that can be used easily as a food component [4-6].
Several methods for the production and processing of coprecipitates proteins have already been developed and it is possible to discover new ones that allow greater returns for using co-precipitates proteins as commercial products in the food processing sector [7-15].
The industrial future tends to develop new consumer products and find alternatives to cow's milk. In this regard, camel's milk and goat's milk often turns viable candidates [1]. Camel's milk offers a unique advantage for regions where camels are found in large numbers. It urges the usage of nutrition techniques in the production and treatment of camel's milk. Interest for the variety ingoat's milk products has increased as well as researchers’ interest in goat's milk commercially as an alternative to cow milk products used in food rations or for children allergic to cow's milk [16-20].
Attention should be given to research by improving marketing of high-quality and of long life milk products. This will generate support from the private sector to advance the development of camels’ products in countries where camels are found in large numbers and to satisfy demand by offering camel's milk especially with the growth of the Arab community [1]. Now is the time for transformational food industries to have come to realize that milk proteins have considerable power to contribute different characteristics in order to enhance a variety of food products [21,22].
Objectives
Preparation of co-precipitates proteins for camels and goat’s milk in different technological methods, using heat, CaCl and acid or using ultrafiltration to concentrates the protein before precipitation.
Study the physiochemical and functional properties for the protein to identify and learn about its advantages.
To reach important recommendations for conducting research to manufacture some products that fit these characteristics properties.
Methods
Materials
1. Milk from healthy lactating camels and goats in Al-Qassim area, Saudi Arabia.
2. The chemicals were provided by the Postgraduate Laboratories at College of Design and Home Economics- Qassim University.
Methods
1. Preparation of milk: Camels and goats milk were separated at 40°C at pH 6.3
2. Preparing co-precipitates: The first method was by heating the milk until it reached 75°C for 5-20 minutes then adding Calcium 0.2% or HCL 0.1 N. The precipitation of the protein was at 85-95°C and pH 5.1. Collecting of proteins was done at room temperature and then filtered through Whatman No.1 paper. Washing the precipitate and removing water was later cared out. The drying process was carried out in a hot air oven at 55 and then finally, grinded the precipitate to a soft powder.
The second method was by using Ultrafiltration (UF). The concentration of milk was determined by Ultrafiltration, using (Ultrafiltration unit Reverse Osmosis–Model: ARMFILD C03962) to Fold-2. The milk was heated to reach 75°C and was hold for about 20-25 minutes. Next, steps were carried out as per the first method.
The gelatin flavor was prevented by adding 0.5-0.1 sodium sulfate and flavor stability was improved by heating the solution to encourage the volatilization of unwanted flavor components that can be removed during drying.
Methods of analysis
Chemical analysis: The chemical analysis for the skim milk was done for both camel and goat milk using (Lactostar (C) 2008 Funke Gerber Firmware 4.0.19#3510-r80403 Model (C) (2008). For (fat, protein, lactose, ash, non-fat solids, freezing point and PH)
Chemical analysis of co-precipitates components: Moisture, protein, fat, carbohydrate, ash and calcium.
Physical properties of co-precipitate: pH in 5% distilled water at 20°C, viscosity at 20°C and using LV spindle No (3) ± RPM=100 and color (b,a,L).
Functional properties of co-precipitate: Solubility, NSI, PDI, WHC, foaming capacity and stability, fat binding capacity, emulsification capacity and stability, proteins electrophoresis separation on the 7.5 alkaline native polyacrylamide gel PAGE with SDS, B-mercaptoethano and discontinuous buffer system.
Statistical analysis: The data were statistically analyzed by ANOVA analysis with (SPSS) 24th Edition and the difference were significant at probability values (P<0.05).
Results
1. The highest percentage of the refinement of the inherent sediment for each camels’ and goats’ milk using HCl was 27.14%, 24.7% respectively. This process was noted by ultrafiltration while adding HCl to goat’s milk significantly higher than camel’s milk [6].
2. The total percent of solids in co-precipitates of camels’ milk that HCl was added is 60.6%, after it comes the one that was done by ultrafiltration with HCl. There were nonsignificant differences between the percentage of solids in co-precipitates in goats’ milk for both CaCl2 and HCl (Figure 1).
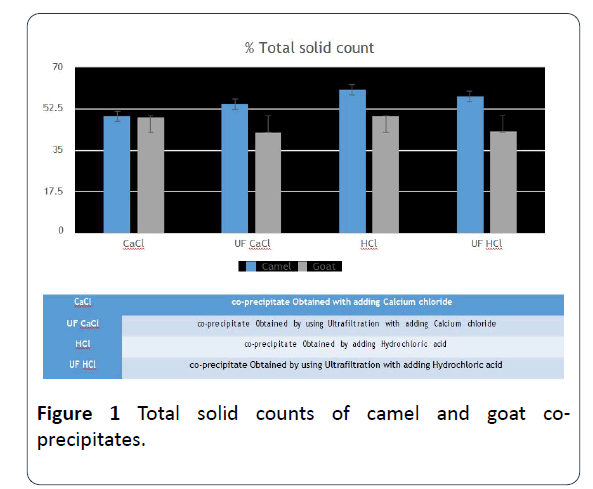
Figure 1: Total solid counts of camel and goat coprecipitates.
3. It has been found while studying the chemical composition of co-precipitates that there is a significant increase in goats’ milk moisture content at level 0.05 for that was precipitator with acid and then followed by the one that CaCl2 was used and the lowest is the one that has been done by ultrafiltration with HCl.
4. While in camels’ milk the highest significant percentage of co-precipitate moisture was when using CaCl2 and the lowest that was done by ultrafiltration with CaCl2 (Table 1).
Table 1: Chemical composition of camel and goat milk co-precipitates.
| Ash |
Lactos |
Fat |
Protein |
Mouster |
| Goat |
Camel |
Goat |
Camel |
Goat |
Camel |
Goat |
Camel |
Goat |
Camel |
|
| 6.03b,c ± 0.06 |
9.87a ± 0.03 |
5.85c ± 0.12 |
25.47b ± 0.11 |
3.52d ± 0.01 |
3.61c ± 0.02 |
82.67a ± 0.07 |
56.68c ± 0.2 |
11.58b ± 0.01 |
10.82a ± 0.03 |
CaCl |
| 8.06a ± 0.05 |
9.49b ± 0.03 |
11.88a ± 0.35 |
24.68c ± 0.08 |
5.18c ± 0.01 |
4.36a ± 0.02 |
71.69d ± 0.11 |
57.51b ± 0.1 |
10.68c ± 0.01 |
9.32b ± 0.01 |
UF CaCl |
| 5.21c ± 0.01 |
8.42c ± 0.02 |
5.72c ± 0.47 |
30.03a ± 0.33 |
7.07a ± 0.05 |
3.22d ± 0.02 |
79.16c ± 0.51 |
54.15d ± 0.33 |
13.87a ± 0.02 |
9.08c ± 0.01 |
HCl |
| 6.21b ± 0.01 |
8.25d ± 0.02 |
7.36b ± 0.18 |
24.76b,c ± 0.29 |
6.88b ± 0.01 |
4.06b ± 0.02 |
77.50b ± 0.29 |
59.49a ± 0.23 |
9.06d ± 0.03 |
8.48d ± 0.05 |
UF HCl |
aSignificance of co-precipitation, bSignificance of ultrafiltration with CaCl2, cSignificance of both co-precipitation and ultrafiltration, dComposition of CaCl2
5. Protein content in camels’ milk co-precipitate which was prepared by ultrafiltration while adding either HCl or CaCl2 was the highest one. Whereas the highest content of goats’ milk co-precipitate that was noted while using CaCl2 or HCl.
6. Significant differences have been noticed in the percentage of fat in co-precipitates of camels’ milk that was prepared by ultrafiltration with adding either HCl or CaCl2. On the other hand, the highest percentage that was noticed in goats’ milk was the sample that was prepared with HCl [9].
7. There was a significant difference in the content of Lactose in the co-precipitates of camels’ milk that was higher than goats’ milk.
8. The co-precipitates that was prepared of camels’ milk using CaCl2 was the highest in ash content. Meanwhile, the highest sample of ash of goats’ milk was the one that was prepared by ultrafiltration with adding CaCl2. It’s important to say that there were some significant differences of the percentage of CaCl2, and the highest sample was the addition of CaCl2 (Figure 2).
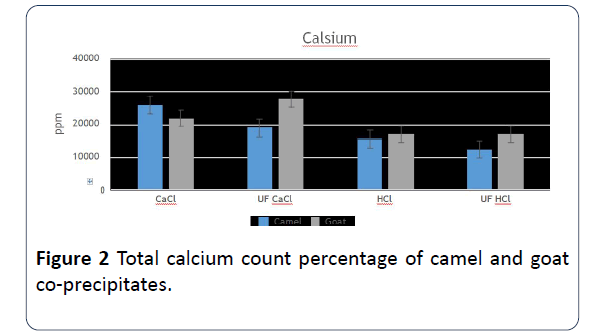
Figure 2: Total calcium count percentage of camel and goat co-precipitates.
9. There were non-significant differences between the samples of cows‘ and goats’ milk co-precipitates that were prepared using different methods in pH or acid number values [15].
10. While there were some significant differences at 0.05 in the solution’s viscosity 10% of co-precipitates between different samples. The highest viscosity value of camels’ milk co-precipitates was the sample that used CaCl2 or was done by ultrafiltration with CaCl2. Meanwhile, there were non-significant differences regarding goats’ milk, and its highest had HCl (Figure 3).
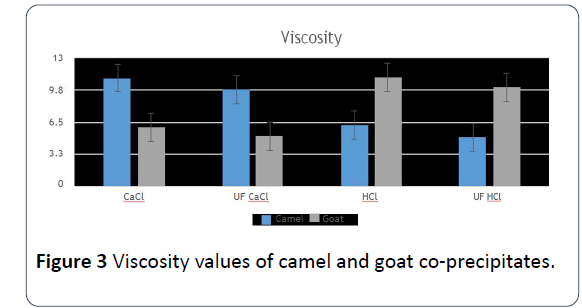
Figure 3: Viscosity values of camel and goat co-precipitates.
11. The precipitation substance had a noticeable effect on coprecipitates’ color degree. The whitest and the shiniest camels’ milk sample was the one that was precipitated with HCl, then the sample that was prepared by ultrafiltration either by using CaCl2 or HCl.
12. While the co-precipitates of goats’ milk were significant higher values when prepared by ultrafiltration with CaCl2.
13. As for the degree of greenness (a), there were significant differences between camels’ milk co-precipitates samples conversely to goats’ milk, except using ultrafiltration with HCl. As for the degree of yellowness, the highest value of camels’ milk co-precipitates was the sample that was prepared using ultrafiltration with HCl. It was of a more significant in goats’ milk than camels’ milk (Table 2).
Table 2: Color values of camel and goat milk co-precipitates.
| Color value |
Type of treatment |
| b |
a |
L |
| Goat |
Camel |
Goat |
Camel |
Goat |
Camel |
|
| 12.75a ± 0.003 |
4.85d ± 0.028 |
-2.23a ± 0.005 |
-2.2b ± 0.028 |
74.10b ± 0.057 |
66.23d ± 0.057 |
CaCl |
| 6.87d ± 0.005 |
7.33b ± 0.005 |
-2.10a ± 0.17 |
-2.48c ± 0.018 |
74.84a ± 0.011 |
81.00c ± 0.005 |
UF CaCl |
| 8.91c ± 0.006 |
6.85c ± 0.028 |
-2.26a ± 0.005 |
-2.95d ± 0.028 |
68.29c ± 0.005 |
84.12a ± 0.011 |
HCl |
| 10.55b ± 0.01 |
8.72a ± 0.011 |
-2.55b ± 0.02 |
-1.94a ± 0.005 |
66.83d ± 0.005 |
81.89b ± 0.005 |
UF HCl |
aSignificance of co-precipitation, bSignificance of ultrafiltration with HCl, cSignificance of both co-precipitation and ultrafiltration, dComposition of HCl
14. Concerning the solubility of co-precipitates, the highest solubility was at 25°C and on pH=7 for the samples that were done by ultrafiltration with adding either HCl or CaCl2 to camels’ milk. The solubility has increased with the increase of protein concentration (Figure 4) [19].
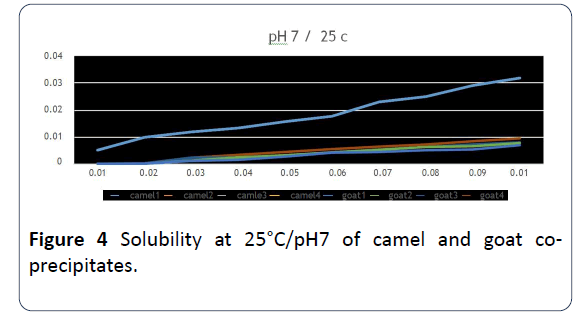
Figure 4: Solubility at 25°C/pH7 of camel and goat coprecipitates.
15. When studying the solubility on a different pH and on 25°C it was pH=2 the highest parameters while using ultrafiltration with HCl in case of camels’ milk, on pH=8 the parameters were by ultrafiltration either by adding CaCl2 or HCI, the best one was on PH=10, at pH=4 there were non-significant differences between the parameters (Figure 5 and Table 3). As for goats’ milk, the parameters that were done by ultrafiltration either with adding CaCl2 or HCl on pH=4, 8 and 10 were the best of them. While on pH=2 there were non- significant differences between the parameters [20].
Table 3: C o-precipitation of camel and goat by using CaCl and Hcl.
| Camel 1 |
Goat 1 |
Camel 2 |
Goat 2 |
Camel 3 |
Goat 3 |
Camel 4 |
Goat 4 |
| CaCl |
UF CaCl |
HCl |
UF HCl |
| Co-precipitate obtained with adding calcium chloride |
Co-precipitate obtained by using ultrafiltration with adding calcium chloride |
Co-precipitate obtained by adding Hydrochloric acid |
Co-precipitate obtained by using ultrafiltration with adding Hydrochloric acid |
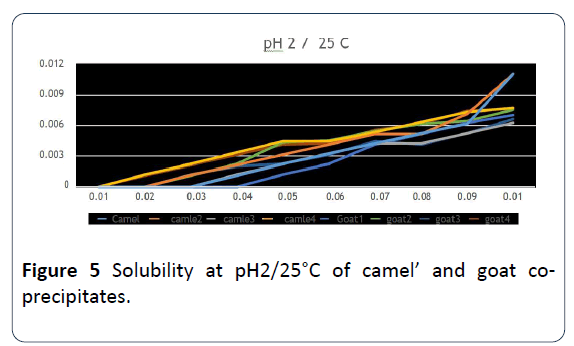
Figure 5: Solubility at pH2/25°C of camel’ and goat coprecipitates.
16. The parameters that used ultrafiltration with HCl for both camels’ and goats’ milk that HCI was added to, and to goats’ milk that HCl was added to only, significant differences than the rest of the parameters in both protein distribution and soluble nitrogen Index. This shows the effect that ultrafiltration parameter has on protein spread and on soluble nitrogen Index (Figures 6 and 7).
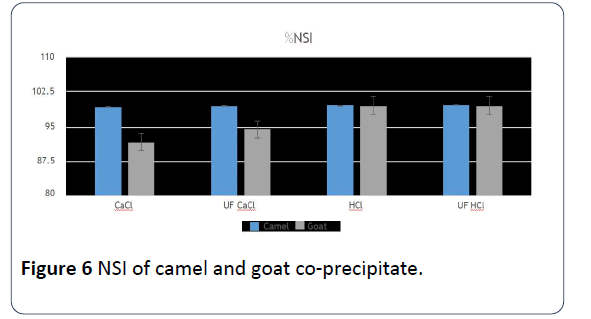
Figure 6: NSI of camel and goat co-precipitate.
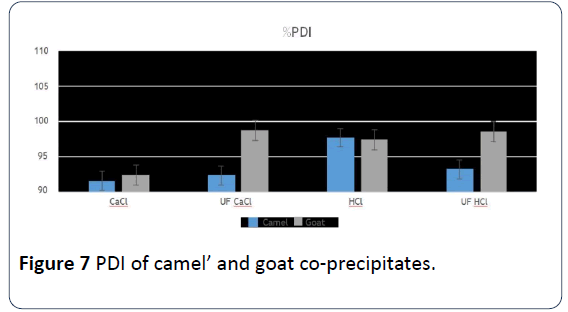
Figure 7: PDI of camel’ and goat co-precipitates.
17. The ability to hold water for co-precipitates in camels’ milk parameter that was prepared by ultrafiltration with adding either HCl or CaCl2 is the highest in contrast to goats’ milk co-precipitates that was precipitated in CaCl2 or HCI only (Figure 8).
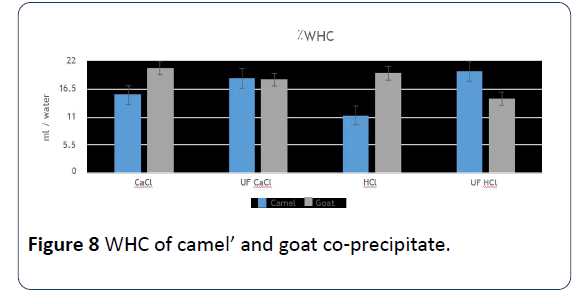
Figure 8: WHC of camel’ and goat co-precipitate.
18. When measuring foam formation, it turned out that the highest capacity was found in goats’ milk precipitate with CaCl2 and in camels’ milk with HCl compared to eggs’ albumin. With more condensation, intangibility differences increased and foam size got better. With using 10 g camels’ milk co-precipitates has the biggest foam size, using CaCl2 then HCl, as it reached 64.7%-65.7% compared to eggs’ albumin. Whereas ultra-filtrated, CaCl2 goats’ milk co-precipitates was 61.8% compared to eggs’ albumin [1].
19. The highest record of co-precipitates goats’ milk foam stability was with using HCI only or CaCl2 only and with using the latter there was little detaching of liquid from foam. While the lowest record was of co-precipitates camels’ milk foam that was prepared with ultrafiltration, whether with or without adding CaCl2 or HCI (Figure 9).
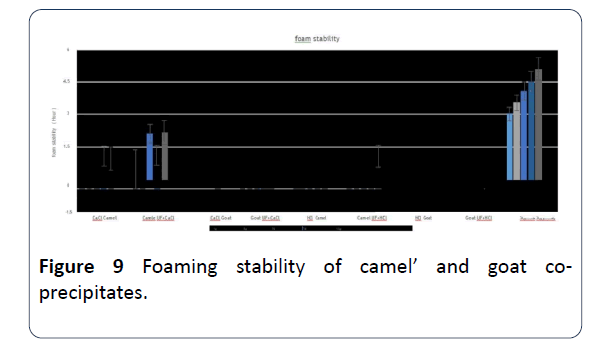
Figure 9: Foaming stability of camel’ and goat coprecipitates.
20. Using a concentration of 3 g of co-precipitates, the highest foam stability was of goats’ milk that was ultrafiltered with added HCl or prepared with added CaCl2. As for camels’ milk the highest was the one prepared with ultrafiltration and added CaCl2 [5].
21. When examining fat binding ability of co-precipitate, it was found that goats' co-precipitate milk that was prepared using CaCl2 is more capable of binding with fat at a significant of 0.05 compared to the co-precipitate that was done by ultrafiltration with added HCI, whereas in camels' milk, the co-precipitate that was prepared with ultrafiltration with HCI was the highest in fat binding ability compared to other samples (Figure 10).
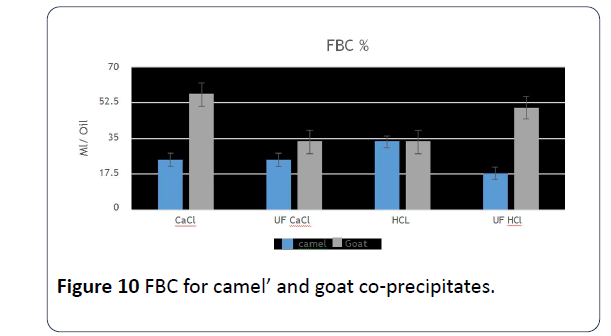
Figure 10: FBC for camel’ and goat co-precipitates.
22. In examining fat emulsion stability, CaCl2 goats’ and camels' milk co-precipitate were the best. Followed by
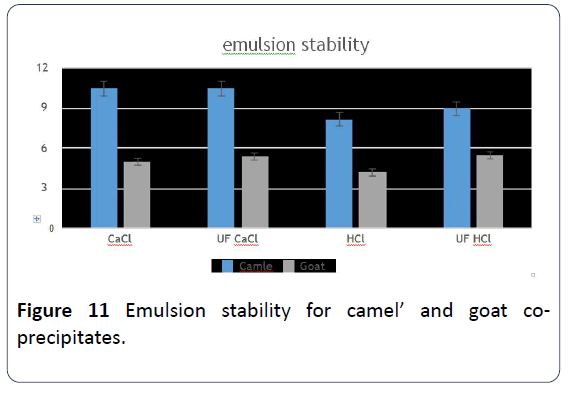
Figure 11: Emulsion stability for camel’ and goat coprecipitates.
23. An electrophoresis (SDS) separation was done to the coprecipitates proteins of both goat’s and camel’s milk and there weren’t any intangible differences in the casein particles nor in the whey protein whether in regards to electrophoresis rates or the molecular weight for each protein except for case number 1 in which CaCl2 was added to it, as a decrease of the special patterns density has been noted as well as a huge decrease in betalactoglobulin. As for goats’ milk precipitated, there hasn’t been any intangible difference in any of its proteins whether in electrophoresis rates or the molecular weight, the density or the concentration of protein has been noticed to be increasing when treated with ultrafiltration and with added HCl, and it was observed also that the whey proteins disappeared significantly (Figures 12 and 13) [16].
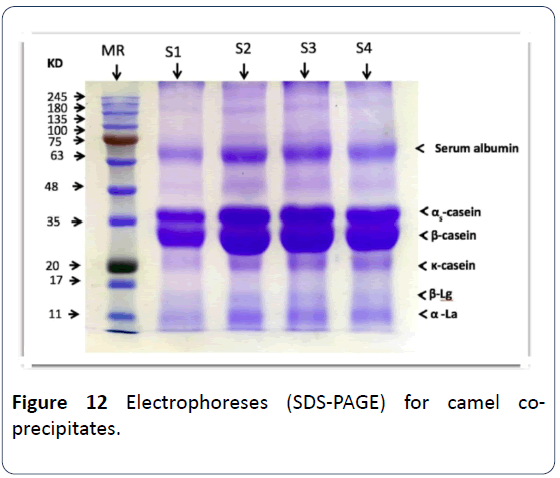
Figure 12: Electrophoreses (SDS-PAGE) for camel coprecipitates.
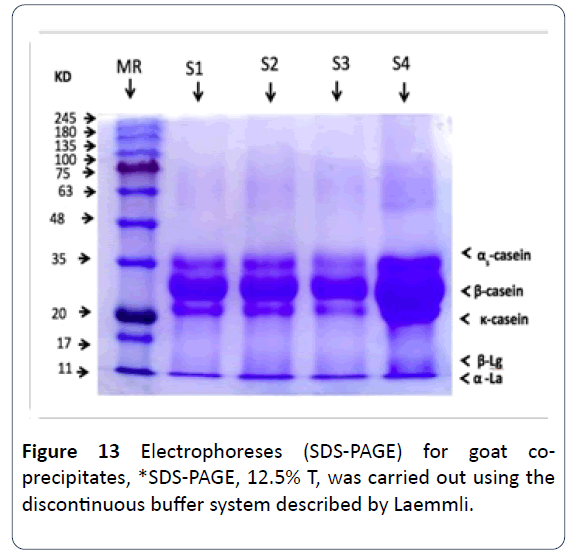
Figure 13: Electrophoreses (SDS-PAGE) for goat coprecipitates, *SDS-PAGE, 12.5% T, was carried out using the discontinuous buffer system described by Laemmli.`
Discussion and Conclusion
The co-precipitate can be used directly to supply or strengthen food sources of low quality or low protein content and can also be used in different foods to raise nutritional value. This requires further Interest to be taken to be used in many goods.
1. Research the minimum protein recovery including extraction and precipitation conditions.
2. Research the preparation of co-precipitate using more than one source of proteins.
3. Evaluation of the effect of supplementation of coprecipitate protein nutritionally and biochemical in both laboratory and experimental animals on chronic diseases such as diabetes, high blood pressure.
4. Evaluation the effect of co-precipitate protein sediment on people who are allergic to bovine s Casin.
5. Research the effect of co-precipitate treatment on food products.
6. Research the effect of co-precipitate protein on the sensory properties of food products.
7. Evaluation of co-precipitate effect on the functional properties in food products.
• Suggested studies on its use in the following foods are:
• Ice-cream and web cream using the co-precipitate of goat milk prepared by adding calcium chloride, hydrochloric acid or camel milk using ultra-filtration with both.
• Milk flavored drinks, juices and fermented milk using coprecipitate of camel and goat milk prepared by the ultrafiltration method either by adding hydrochloric acid or calcium chloride.
• Meat products using co-precipitate prepared by ultrafiltration method with the addition of calcium chloride or hydrochloric acid to both camel and goat milk.
• Baked goods using co-precipitate prepared by ultrafiltration method with the addition of hydrochloric acid or calcium chloride to camel milk, calcium chloride or hydrochloric acid for goats' milk.
Recommendation
It’s recommend to keep studying the application of these different types of co-precipitates in different kinds of food, especially the ones treated with ultrafiltration with added HCl or CaCl2 like juice or soft dairy products or ice cream and using goats’ milk co-precipitates with added HCl or CaCl2 in making baked goods and meat products and its effect on their functional, organoleptic properties. It’s also recommended to research the effect of added co-precipitates protein on the nutritional and biological value, whether by laboratory experiments or animal testing (those used for testing chronic diseases like hypertension or diabetes).
It’s recommend to keep studying the application of these different types of co-precipitates in different kinds of food, especially the ones treated with ultrafiltration with added HCl or CaCl2 like juice or soft dairy products or ice cream and using goats’ milk co-precipitates with added HCl or CaCl2 in making baked goods and meat products and its effect on their functional, organoleptic properties. It’s also recommended to research the effect of added co-precipitates protein on the nutritional and biological value, whether by laboratory experiments or animal testing (those used for testing chronic diseases like hypertension or diabetes).
23548
References
- Mati A, Senoussi-Ghezali C, Zennia SSA, Almi-Sebbane D, El-Hatmi H, et al. (2017) Dromedary camel milk proteins, a source of peptides having biological activitiese: A review. Int Dairy J 73: 25e37.
- Al-Shamsi, Kholoud A, Mudgil P, Hassan HM, Maqsood S (2018) Camel milk protein hydrolysates with improved techno functional properties and enhanced antioxidant potential in invitro and in food model systems. J Dairy Sci 101: 47-60.
- Brodkorb A, Croguennec T, Bouhallab S, Kehoe J (2016) Heat induced denaturation, aggregation and gelation of whey proteins. In: PLH McSweeney, JA O’Mahony editors. (4th edn.). Proteins: Applied aspects: Advanced dairy chemistry. New York, NY, USA: Springer Science Business Media; 1: 155-174.
- Dichinson E (2010) Food emulsions and forums: stabilization by particles. J Colloid Interface Sci 15: 40-49.
- Ereifej KI, Alu’datt MH, Alkhalidy HA, Alli I, Rababah T (2011) Comparison and characterization of fat and protein composition for camel milk from eight Jordanin locations. Food Chem 127: 282-289.
- Farah Z (2011) Camel milk. Swiss Federal Institute of Technology, Zurich, Switzerland Elsevier Ltd., pp: 512-517.
- Garju S, Gogate PR (2017) Review on approaches for efficient recovery of whey proteins from dairy industry effluents. J Food Eng 215: 84-96.
- Ham J, Lee G, Jeong, G, Oh M, Kim D, et al. (2010) Characteristics of Korean-Saanen goat milk caseins and somatic cell counts in comparison with Holstein cow milk counterparts. Small Ruminant Res 93: 202-205.
- Abril I, García-Montoya, Cendón TS, Arévalo-Gallegos S, Rascón-Cruz Q (2012) Lactoferrin a multiple bioactive protein: An overview. Biochimica et Biophysica Acta 1820: 226-236.
- Kasipathy K (2016) Chemical composition, physical, and functional properties of milk and milk ingredients. School of Science and Health, University of Western Sydney, Penrith, Locked Bag 2751, NSW, 1797, Australia.
- Li A, Zhao Z (2017) Formation of lactoferrin/sodium caseinate complexes and their adsorption behaviour of the air/water interface. Food Chem 232: 697-703.
- Martin D, Walte HG, Lorenzen P (2016) Ribonucleosides in raw milk and heat-treated milk samples from cows, sheep, goats and camels and their potential biotechnological application. Small Ruminant Res 137: 162-168.
- Naeim MA, Sakr HS, Hatem HE, Mehana NM (2007) Some functional properties of caseins and whey proteins prepared from cow's and goat's milk. Egyptian J Dairy Sci 35: 15-20.
- O’Mahony JA, Fox PF (2013) Milk proteins: Introduction and historical aspects, Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, (4th edn.).
- Phelam M, Kerins D (2011) The potential role of milk-derived peptides in cardio vassals disease. Food Funct 2: 153-167.
- Roman JA, Sgarbieri VC (2006) The hydrophilic, foaming and emulsifying properties of casein concentrates produced by various methods. Int J Food Sci Tech 41: 609-617.
- Salem SA, Meead GH, El-Rashody, Fardous MM (2017) Physicochemical and sensory properties of ice cream made from camel milk and fortified with dates products. Int J Humanities, Arts, Med Sci 5: 29-40.
- Thomar P, Gonzalez-Jordan A, Dittmer J, Nicolai T (2017) Effect of orthophosphate and calcium caseinate solutions. Int Dairy J 64: 1-8.
- Raikos V (2010) Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll 24: 259-265.
- Weiss J, Takhistov P, McClements D (2006) Functional materials in food nanotechnology. J Food Sci 71: 107-116.
- Yang Y, Zheng NN, Yang J, Bu D, Wang J, et al. (2014) Animal species milk identification by comparison of two-dimensional gel map profile and mass spectrometry approach. Int Dairy J 35: 15-20.
- Atameri PZ, Schubert T, Holder A, Boom R, Hinrichs J (2017) Bovine β- casein: Isolation properties and functionality: A review. Int Dairy J 66: 115-125.


















