Keywords
ADAMTS; Children; Idiopathic; Nephrotic
Introduction
Many predisposing factors for TE were reported in nephrotic patients, abnormalities in platelet activation and aggregation, activation of prothrombotic factors of coagulation system e.g. factors V,VII, VIII, X, von Willebrand factor (vWF), fibrinogen, and α 2-macroglobulin, decreased activity of fibrinolytic system such as plasminogen [1] and decreased endogenous anticoagulants,antithrombin III, protein C, protein S, and tissue factor pathway inhibitor resulting in local activation of the glomerular hemostasis system [2]. vWF mediates platelet adhesion and aggregation at sites of vascular injury [3]. It is released from the stimulated endothelium as unusually large (UL) multimer [4]. ULvWF favor platelet aggregation and formation of microvascular thrombi [5]. A disintegrin and metalloprotease with thrombospondin type-1 motif 13 (ADAMTS-13) can cleaves and thus converses ULvWF into a less active form [6]. Reduced ADAMTS-13 activity due to gene mutation or presence of autoimmune IgM and IgG inhibitors [7] results in deficient proteolysis of ULvWF with formation of disseminated plateletrich thrombi in the microcirculation seen in thrombotic microangiopathies (TMA) [8,9].
The aim of this study was to investigate ADAMTS 13 Activity levels in children with idiopathic nephrotic Syndrome and its relation to clinical and laboratory parameters.
Patients and Methods
This study was conducted on 120 children aged from two to seventeen years of both sexes selected from Pediatric Nephrology Units of Pediatric Departments of Tanta University Hospital (TUH) and Fayum University Hospital (FUH), The study was done from June 2016 till June 2017 after approval from Research Ethical Committees of TUH and FUH. A formal written consent of each child' parents was taken separately after explanation and assurance of them. All participants' names were hidden and replaced by code number to maintain privacy of the participant.
The children were divided in three groups Group (1) Forty patients with steroid sensitive nephrotic syndrome, Group (SSNS) (2) Forty patients with steroid resistant nephrotic syndrome (SRNS) and Group (3) Forty healthy controls matched age and sex.
Inclusion criteria
Steroid sensitive nephrotic syndrome (SSNS) and steroid resistant nephrotic syndrome (SRNS) groups were diagnosed according to definitions of Bagga and Strivastava [10].
Exclusion criteria
Other causes of generalized edema as renal, hepatic and heart failure, nutritional and allergic causes, congenital anomalies of the kidney, other causes of thrombo embolic disorders and auto immune diseases.
All children included in this study were subjected to the following:
1. Complete history taking: concerning past history of recurrence, responseveness to steroid therapy, thrombo embolism,
2. Full clinical examination: with special emphasis on Hypertension, peritonitis, thrombosis as a complication of NS.
3. Laborayory investigation including: Complete blood count (CBC), 24 hours collected Urine analysis for urine volume, urinary proteins,Total Serum protein and serum albumin and total serum cholesterol, PT, PTT and Serum ADAMTS 13 activity.
Specimen collection and handling
A 6 ml morning venous blood sample was collected under complete aseptic conditions for assessment of the level of serum ADAMTS 13. Morning urine samples were taken from the 60 children for complete urine analysis. We put 2 ml of the blood in EDITA tube for CBC and the remaining blood allowed for clotting, and the serum separated by centrifugation at room temperature then divided in two Eppendorf tubes. One for the routine examination which was done immediately and the other tube preserved and froze at -20°C prior to the assay.
Serum ADAMTS 13 activity levels
Measured by Human ADAMTS 13 ELISA Microplate Kit which based on sandwich enzyme-linked immune-sorbent assay (ELISA) technology in (ng/ml). Anti-human ADAMTS 13 antibody was pre-coated onto 96-well plates, the Biotin conjugated antihuman ADAMTS 13 antibody was used as detection antibodies. The standards, test samples and biotin conjugated detection antibody were added to the wells subsequently, and wash with wash buffer.
Results
Demographic data of studied groups are summarized in Table 1. There was highly significant decrease in serum ADAMTS 13 activity in SSNS and SRNS groups when compared to control group while there was no significant difference in serum ADAMTS 13 activity between SSNS and SRNS groups (Table 2). There was no significant difference in serum ADAMTS 13 activity between males and females in studied patients (Table 3). There was significant positive correlation between plasma ADAMTS 13 activity and total serum protien in patients with SSNS (Table 4). There was significant positive correlation between plasma ADAMTS 13 activities of patients with SSNS with serum albumin (Table 4). There was significant negative correlation between plasma ADAMTS 13 activity and 24 hours urine protein in patients with SSNS (Table 4). There was significant negative correlation between plasma ADAMTS 13 activities of patients with SRNS with serum cholesterol level (Table 4).
| |
Groups |
ANOVA or Chi-Square |
| SteroidSenstiveNephroticSyndrome (SSNS) |
SteroidResistantNephroticSyndrome (SRNS) |
Control |
F or X2 |
P-value |
Age
(Years) |
Range |
3.5-13 |
2.5 - 16 |
3 - 17 |
X2 =2.710 |
0.075 |
| Mean ±SD |
8.03±2.59 |
7.53 - 3.45 |
10 ± 4.4 |
| Weight (kg) |
Range |
14 - 35 |
15 - 54 |
12 - 59 |
X2=2.823 |
0.068 |
| Mean ±SD |
26.3 ± 6.127 |
25.73 ± 9.54 |
32.85 ± 14.28 |
| Sex |
Male |
22% |
32 - 80 % |
30 - 75 % |
F=3.333 |
0.189 |
| Female |
18- 45 % |
8 - 20 % |
10 - 25 % |
| SBP |
Range |
95- 140 |
90 - 120 % |
90 - 125 |
X2=0.896 |
0.414 |
| Mean ±SD |
107.75 ± 9.66 |
104.25 ± 7.48 |
107.25 ± 9.53 |
| DBP |
Range |
55 - 90 |
45 - 80 |
50- 80 |
X2=1.127 |
0.331 |
| Mean ±SD |
65.5 ± 8.75 |
63.5 - 9.05 |
67.75 ± 9.24 |
Table 1: Demographic data of studied groups.
| |
|
ANOVA |
TUKEY ‘S Test |
| SteroidsensitiveNephroticSyndrome |
SteroidResistantNephroticSyndrome |
Control |
F |
P- Value |
I & II |
I & III |
II & III |
| HB % (g/dl) |
Range |
10.1-12.4 |
8.9-13.9 |
9.5-13.2 |
0.482 |
0.620 |
|
|
|
| Mean ±SD |
11.230 ± 0 .264 |
11.060± 1.114 |
10.920 ± 1.170 |
WBc
x/cmm |
Range |
5-14 |
4-10 |
5.9-9.9 |
2.839 |
0.067 |
|
|
|
| Mean ±SD |
8.110 ± 2.191 |
6.770 ± 1.921 |
7.715 ± 1.237 |
| Platelet (/cmm) |
|
110-379 |
112-355 |
150-350 |
1.581 |
0.215 |
|
|
|
| |
219.250 ± 65.952 |
232.800 ± 57.948 |
254.500 ± 65.492 |
| TSP (G/dl) |
Range |
4-6 |
4-6.5 |
6-8 |
23.379 |
0.001* |
0.398 |
0.001* |
0.001* |
| Mean ±SD |
4.990 ± 0.829 |
5.315 ± 0.777 |
6.600 ± 0.754 |
Serumalbumin
(g/dl) |
Range |
2.1-3.5 |
2.1-3.5 |
3.5-5 |
77.742 |
0.001* |
0.665 |
0.001* |
0.001* |
| Mean ±SD |
2.720 ± 0.385 |
2.840 ± 0.402 |
4.275 ± 0.518 |
24 hrUnire PTN
(mg/24hr) |
Range |
206-2120 |
266-3162 |
100-200 |
31.932 |
0.001* |
0.822 |
0.001* |
0.001* |
| Mean ±SD |
1200.900 ± 503.304 |
1113.980 ± 617.465 |
153.000 ± 29.576 |
| Creatinine (mg/dl) |
Range |
0.3-1 |
0.5-2.5 |
0.2-1 |
5.923 |
0.005* |
0.392 |
0.100 |
0.003* |
| Mean ±SD |
0.710 ± 0.215 |
0.845 ± 0.429 |
0.495 ± 0.293 |
| Urea (mg/dl) |
Range |
21-42 |
15-41 |
15-25 |
21.916 |
0.001* |
0.020* |
0.001* |
0.001* |
| Mean ±SD |
31.350 ± 6.302 |
26.050 ± 7.749 |
18.800 ± 2.984 |
| GFR (ml/min/1.73 m2) |
Range |
100-165 |
18-165 |
100-165 |
2.998 |
0.058 |
|
|
|
| Mean ±SD |
136.000 ± 20.199 |
123.70 ± 35.861 |
143.20015.867 |
| PTT (sec) |
Range |
26-45 |
25-43 |
25-45 |
0.742 |
0.481 |
|
|
|
| Mean ±SD |
340650 ± 5.743 |
35.000 ± 6.164 |
32.700±7.299 |
| PT (sec) |
Range |
11-16 |
11-15 |
11-16 |
0.526 |
0.594 |
|
|
|
| Mean ±SD |
12.465 ± 1.399 |
12.395 ± 1.033 |
12.795 ± 1.476 |
| Serumcholesterol(mg/dl) |
Range |
200-384 |
266-510 |
140-200 |
74.983 |
0.001* |
|
|
|
| Mean ±SD |
321.200 ± 57.726 |
363.250 ± 67.942 |
169.500 ±19.050 |
Table 2: Routinelaboratory data of thestudiedgroups.
| Groups |
ADAM TS 13 (ng/ml) |
ANOVA |
| Range |
Mean ± SD |
F |
P-value |
| Group(1)SteroidSensitiveNephroticSyndrome |
0.07-4.62 |
1.03 ± 1.02 |
34.6 |
<0.001* |
| Group(2)SteroidResistantNephroticSyndrome |
0.06-1.79 |
0.95 ± 0.55 |
| Control Group(3) |
2.78-20.3 |
7.52 ± 4.83 |
| TUKEY'S Test |
| Resistant andSensitive |
Sensitive and Control |
Resistant and Control |
| 0.995 |
<0.001* |
<0.001* |
Table 3: Comparisonbetweenstudiedgroups as regardsto ADAMTS 13.
| Correlations |
| |
ADAM TS 13(ng/ml) |
| SteroidSenstiveNephroticSyndrome (SSNS) |
SteroidResistantNephroticSyndrome (SRNS) |
| r |
P- value |
r |
P- Value |
| HB % (g/dl) |
-0.032 |
0.892 |
0.224 |
0.342 |
| WBc (x/cmm) |
0.204 |
0.388 |
0.198 |
0.403 |
| Platelet (/cmm) |
-0.314 |
0.177 |
-0.228 |
0.334 |
| Total serumprotein (g/dl) |
0.618 |
0.044* |
-0.062 |
0.796 |
| Serumalbumin (g/dl) |
0.607 |
0.005* |
-0.082 |
0.73 |
| 24 hrUnire PTN (mg/24hr) |
-0.638 |
0.002* |
0.077 |
0.746 |
| SerumCreatinine (mg/dl) |
0.032 |
0.894 |
0.307 |
0.187 |
| Serum Urea (mg/dl) |
-0.199 |
0.399 |
-0.097 |
0.685 |
| GFR (ml/min/1.73 m2) |
0.242 |
0.304 |
-0.086 |
0.718 |
| PTT (sec) |
-0.062 |
0.794 |
-0.042 |
0.86 |
| PT (sec) |
-0.105 |
0.659 |
-0.21 |
0.374 |
| Serumcolesterol (mg/dl) |
-0.272 |
0.246 |
-0.498 |
0.025* |
| Age(Years) |
-0.309 |
0.184 |
0.303 |
0.194 |
| Weight (kg) |
-0.331 |
0.154 |
0.188 |
0.426 |
| SBP |
-0.141 |
0.553 |
0.46 |
0.041* |
| DBP |
-0.3 |
0.2 |
0.485 |
0.030* |
Table 4: Correlationsbetween ADAMTS 13 activity and laboratory data and of studiedPatients.
In our study, there was no patients with thrombotic episodes in different studied patient groups. There was significant positive correlation between plasma ADAMTS 13 activity and both systolic and diastolic blood pressure in patients with SRNS with Figures 1 and 2.
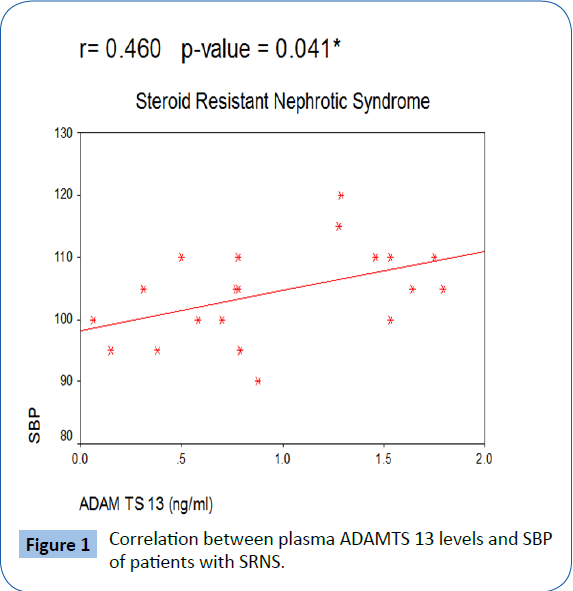
Figure 1: Correlation between plasma ADAMTS 13 levels and SBP of patients with SRNS.
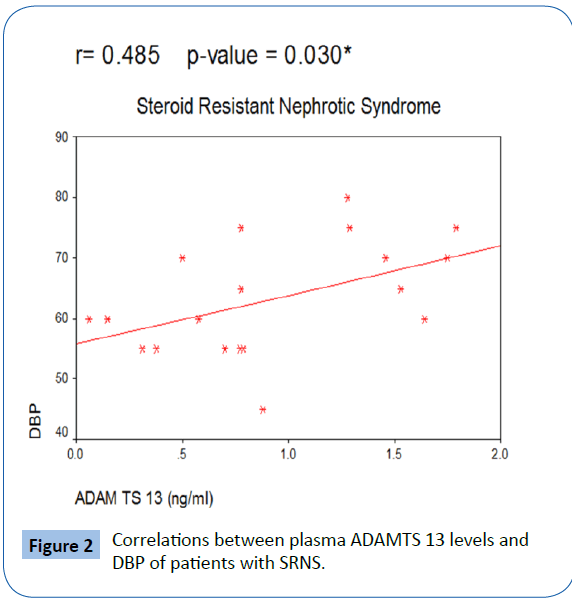
Figure 2: Correlations between plasma ADAMTS 13 levels and DBP of patients with SRNS.
Discussion
Elevated plasma vWF antigen (vWF: Ag) and/or decreased ADAMTS 13 activity are associated with negative outcomes of several disorders [11]. An imbalance between the circulating levels of vWF and ADAMTS 13 has been reported in a number of acquired diseases in adults such as coronary artery disease and myocardial infarction, peripheral arterial disease, ischemic stroke, preeclampsia, inflammatory bowel disease, and liver cirrhosis [12,13] and these findings have been reported also in different diseases of pediatric age such as type 1 diabetes mellitus [14] and ESRD [15].
Our results showed that there is no significant difference between studied groups regarding platelet count. Eneman B. et al found that NS is associated with a significantly increased risk of thrombotic events. Alterations in plasma levels of pro- and anticoagulant factors are involved in the pathophysiology of venous thrombosis in NS. However, the fact that the risk of both venous and arterial thrombosis is elevated in NS points to an additional role for blood platelets. Increased platelet counts and platelet hyperactivity have been observed in nephrotic children. Platelet hyperaggregability, increased release of active substances, and elevated surface expression of activation-dependent platelet markers have been documented. The mechanisms underlying platelet alterations are probably due to changes in plasma levels of platelet-interfering proteins and lipid changes, as a consequence of nephrosis [16].
Anand et al. [17] clarifies the importance of coagulation profile in nephrotic syndrome as a high index of suspicion for thromboembolic complications especially in patients with thrombocytosis.
Our results showed significant decrease in total serum protein and serum albumin in both steroid sensitive, steroid resistant NS when compared to control group. This is in agreement with U.S. National library of medicine that defined nephrotic syndrome [18] and Mulukala et al. [19] who stated that NS is manifested by hyperproteinuria, low total serum protein, hypoalbuminemia and edema. While there is no difference in serum albumin and total serum protein between SSNS and SRNS groups.
In our study, there is no significant difference in prothrombin time (PT) and partial thromboplastin time (PTT) between studied groups. This is in agreement with Yalçinkaya et al. [20] who stated that PT, PTT as well as platelet count and mean plasma Protein C activity were similar in the NS group when compared with the control group and in addition no remarkable difference was found in the mean plasma Protein C activity between the steroid sensitive and resistant NS groups. In contrast, the mean plasma Anti thrombin III (AT III) activity was significantly reduced in patients with NS when compared to controls correspondingly, it was directly correlated with serum albumin and inversely correlated with proteinuria.
But our findings regarding PT and PTT are not in agreement with Anand NK, et al. [17] who stated that thromboembolic complications of NS should be suspected in patients with thrombocytosis, hyper fibrinogenemia, prolonged APTT and in children with low levels of AntithrombinT-III, protein C and protein S.
Limited studies of ADAMTS 13 have been done on pediatric nephritic patients. Our results showed that there was highly significant decrease in serum ADAMTS 13 activity in SSNS and SRNS studied groups when compared to control group. This is in agreement with JiangLiQion, who stated that ADAMTS 13 activities of both steroid sensitive group and steroid resistant group are decreased when compared with normal control group [21].
In this work, there was no significant difference in serum ADAMTS 13 activity between studied SSNS and SRNS groups. This is in agreement with JiangLiQion, stated that no differences are observed in ADAMTS 13 activity among Steroid Resistant nephrotic Syndrome group when compared to Steroid Sensitive nephrotic Syndrome group [21].
Our results showed that there was no significant difference between studied males and females as regard ADAMTS 13 activity in studied patients.
Correlation analysis of our study showed that there that there is a significant positive correlation between plasma ADAMTS13 activity of patients and total serum protien and serum albumin in patients with SSNS while there is a significant negative correlation between plasma ADAMTS 13 activity of patients and serum cholesterol level in SRNS group. This correlation results are in agreement with JiangLiQion, who stated that plasma ADAMTS 13 antigen of patients with NS is positively correlated with serum albumin (r=0.385, P<0.01) and negatively correlated with total blood cholesterol (r=-0.317, P<0.01) [21].
Correlation analysis of our study showed that there is significant negative correlation between plasma ADAMTS 13 activity and 24 hours urine protien of patients with SSNS. This is in agreement with LiQion J [21], who stated that ADAMTS 13 activity of patients with nephrotic syndrome is negatively correlated with the quantitative measurement of 24 hours urinary protein (r=- 0.242, P<0.05).
Conclusion
It is recommended for regular follow-up of children with nephrotic syndrome and early estimation of reduced serum ADAMTS 13 level to control this risk factor of thrombosis. It may be possible to re-engineer ADAMTS 13 protease to improve specific activity, which may offer preventive and therapeutic benefits to nephrotic patients in pediatric age with thromboembolic complications.
Recommendations
Regular follow-up of nephrotic patients and estimation of serum ADAMTS 13 level as its decresed level is a risk factors of thrombosis.
20148
References
- ZumbergM, Kitchens CS (2007) Consultativehemostasis and thrombosis. 2nd edn., Saunders Elsevier, Philadelphia.
- Karim F, Adil SN, Afaq B(2013) Deficiency of ADAMTS-13in pediatricpatientswithsevere sepsis and impacton in-hospitalmortality. Medicine 95: e3374.
- Arya M, Anvari B, Romo GM (2002) Ultralargemultimers of von Willebrand factor formspontaneoushigh-strengthbondswiththeplateletglycoproteinIb-IX complex: Studiesusingopticaltweezers. Blood 99: 3971-3977.
- Austin SK, Starke RD, Lawrie AS (2008) The VWF/ADAMTS13 axis in theantiphospholipidsyndrome: ADAMTS13 antibodies and ADAMTS13 dysfunction. Br J Haematol 141: 536-544.
- Mannucci PM, Peyvandi F (2006) TTP and ADAMTS13: WhenIsTestingAppropriate? Hematology Am SocHematolEducProgram2007:121-126.
- Tsai HM (2006) ADAMTS13 and microvascularthrombosis. ExpertRevCardiovascTher4: 813-825.
- Turner N, Nolasco L, Tao Z (2006) Human endothelialcellssynthesize and releaseADAMTS-13. J ThrombHaemost4: 13.
- Claus RA, Bockmeyer CL, Budde U(2009)Variations in the ratio between von Willebrand factor and its cleaving protease duringsystemic inflammation and association with severity and prognosis of organ failure. ThrombHaemost101: 239-247.
- BaggaA,Srivastava RN (2005) Nephroticsyndrome. In: Srivastava RN, Bagga A,4th edn., PediatricNephrology, Jaypee, New Delhi, pp: 159-200.
- Hyun J, Kim HK, Kim JE (2009) Correlation between plasma activity of ADAMTS-13 and coagulopathy, and prognosis in disseminated intravascular coagulation. Thromb Res 124: 75-79.
- Bongers TN, de Bruijne EL, Dippel DW (2009) Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis 207: 250-254.
- Molvarec A, Rigo J Jr, Boze T (2009) Increased plasma von Willebrand factor antigen levels but normal von Willebrand factor cleaving protease (ADAMTS13) activity in preeclampsia. ThrombHaemost 101:305-311.
- Skeppholm M, Kallner A, Kalani M (2009) ADAMTS13 and von Willebrand factor concentrations in patientswith diabetes mellitus.BloodCoagulFibrinolysis 20:619-926.
- Shen L, Lu G, Dong N (2011) Von Willebrand factor, ADAMTS13 activity, TNF-α and theirrelationships in patientswithchronickidneydisease.ExpTherMed 3: 530-534.
- Eneman B, Levtchenko E, van den Heuvel B (2015)Plateletabnormalities in nephroticsyndrome.PediatrNephrol 31: 1267-1279.
- Anand NK, Chand G, Talib VH (1996) Hemostaticprofile in nephroticsyndrome. IndianPediatr33:1005-1012.
- Charles Silberberg (2013) Nephroticsyndrome. US National Library of Medicine. USA.
- Mulukala SK, Nishad R, Kolligundla LP (2016) In silicoStructuralcharacterization of podocin and assessment of nephroticsyndrome-associatedpodocinmutants. IUBMB Life 68: 578-588.
- Yalçinkaya F, Tümer N, Gorgani AN (1995) Haemostaticparameters in childhoodnephroticsyndrome. IntUrolNephrol27: 643-647.
- LiQiong J (2011) Variationof von Willebrand factor cleavingprotease in plasma of patientswithnephroticsyndrome and expression as well as influencingfactors in human renal tubular epithelialcells. Internal Medicine.








