Keywords
Sulforaphane; Nrf2; Liver cancer; Angiogenesis
Abbreviations
ALT: Alanine Aminotransferase; ARE: Antioxidant Response Element; DEN: Diethyl Nitrosamine; HCC: Hepatocellular Carcinoma; ITCs: Isothiocyanates; Keap1: Kelch-like ECH-Associated Protein 1; Nrf2: The Nuclear Factor Erythroid 2–Related Factor 2; ROS: Reactive Oxygen Species; RT-qPCR: Reverse Transcription Quantitative Polymerase Chain Reaction; SFN: Sulforaphane; WST-1: Water-Soluble Tetrazolium Salt-1
Introduction
Hepatocellular carcinoma (HCC) is a highly aggressive form of solid malignancy and is the third cause of cancer-related deaths [1]. The incidence of HCC is rising globally at an accelerated rate [2], making it the fifth most common cancer in men and the seventh most common cancer in women [1,3]. HCC can be treated curatively via surgical resection or liver transplantation if diagnosed during the early stage; however, majority of the HCC patients are diagnosed during the advanced stage; therefore, their median survival times are generally lower than one year, resulting in poor prognosis. A primary reason for poor prognosis in HCC patients is the absence of effective therapies, particularly for the advanced stages. Sorafenib, a multi-kinase inhibitor, is the first agent that has demonstrated survival benefits in patients with unresectable advanced HCC [4]. Sorafenib has showed overall survival benefit; however, the response rate is not acceptable in clinical practice because given the several adverse effects of this drug, only few patients were able to continue it. In patients with conditions, such as hand-foot syndrome, severe hypertension, and acute liver injury, more effective therapies are required to improve the prognosis of advanced HCC patients. In addition to a molecular targeted therapy, the potent antitumor activity exerted by certain natural product-derived drugs has been reported for several types of cancers [5,6].
Sulforaphane (SFN), a naturally occurring isothiocyanate derived from cruciferous vegetables, especially broccoli, has been widely used for treating inflammatory diseases. This substance is regarded safe for oral intake because it is not artificially produced and is rarely associated with liver injury. In addition, SFN is a proven, important cancer preventive agent with high activity in some clinical neoplasms, including colorectal cancer [7], urinary bladder cancer [8,9], prostatic cancer [10,11], breast cancer [12,13], thyroid cancer [14,15], and leukemia [16,17]. However, the inhibitory effect of SFN on liver cancer, mainly HCC, remains unknown. In this study, we used two different strains of the human liver cancer cell line in both in vitro and in vivo models and attempted to explore the therapeutic potential of SFN on liver cancer and understand its molecular mechanisms. To our knowledge, this is the first report to clearly demonstrate the efficacy of SFN as an inhibitory agent for liver cancer in “in vivo” model without any remarkable adverse effects.
Materials and Methods
Liver cancer cell lines and reagents
Human liver cancer cell lines of HepG2 and Huh7 were procured from the RIKEN BRC CELL BANK (Ibaraki, Japan). They were maintained as monolayer cultures in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Both Invitrogen, Waltham, MA, USA) and 1% penicillin or streptomycin in an incubator at 37°C, with exposure to a humidified atmosphere of 5% CO2. SFN was purchased from Toronto Research Chemicals Inc. (Toronto, ON, Canada).
Water-Soluble Tetrazolium salt (WST)-1 assay
In order to evaluate the direct effect of SFN on the human liver cancer cell lines, we compared cell proliferation with or without SFN treatment. The human liver cancer cell lines of HepG2 and Huh7 were respectively seeded on uncoated plastic dishes at a density of 1.5 × 104 cells/mL. Following overnight culture, the cells were treated for 24 h with different SFN concentrations (0, 5, 10, 20, and 40 μM). Cell proliferation was measured using the WST-1 assay as per the manufacturer’s protocol.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tumor tissue samples using acid guanidinium thiocyanate-phenol-chloroform extraction. The mRNA levels of HMOX1, ABCC2, NQO1, and cell cycle genes were measured using qPCR with the Applied Biosystems Step One Plus™ Real-Time PCR (Applied Biosystems, Foster City, CA, USA), as described previously [18,19]. Primer sequences were as follows:
• HMOX1, forward 5’-AGTTCCTGATGTTGCCCACCAGGCT-3’ and reverse 5’-TTGCTCTGAGCAGCGCTGCCTCCCA-3’;
• ABCC2, forward 5’-GAGCACCAGCAGCGATTTCT-3’ and reverse 5’-AGCCAACAGTGTCCCCACTT-3’;
• NQO1, forward 5’-CAGCTCACCGAGAGAATAGT-3’ and reverse 5’-GAGTGAGCCAGTACGATCAGTG-3’;
• CCND1, forward 5’-CCGTCCATGCGGAAGATC-3’ and reserve 5’-GAATCTCCAGGGAATAGGGC-3’;
• CDK2, forward 5’-TGGTACCGAGCTCCTGAAAT-3’ and reverse 5’-GAATCTCCAGGGAATAGGGC-3’;
• CCNB1, forward 5’-GAACCTGAGCCAGAACCTGA-3’ and reverse 5’-ACAGGTCTTCTTCTGCAGGG-3’;
• CDK1, forward 5’-TTGGATTCTATCCCTCCTGG-3’ and reverse 5’-CTGGAGTTGAGTAACGAGCTGA-3’;
• CDKN1A, forward 5’-ACCTGTCACTGTCTTGTACC-3’ and reverse 5’-GTAGAAATCTGTCATGCTGGTC-3’.
The cycling conditions were as follows: initial holding stage at 95°C for 20 sec; followed by 40 cycles at 95°C for 3 s and at 60°C for 30 s, followed by the melting curve stage at 95°C for 15 s, at 60°C for 1 min, and at 95°C for 15 s.
Xenograft model
All the animal procedures were performed as per the recommendations of the Guide for Care and Use of Laboratory Animals (National Research Council, USA). The study was approved by the animal facility committee of Nara Medical University (Authorization number: #11639). Six-week-old athymic nude mice (BALB/cSlc-nu/nu) were purchased from Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan) and housed in stainless steel mesh cages under controlled conditions (temperature: 23 ± 3°C, relative humidity: 50 ± 20%, 10–15 air changes/hour, illumination: 12 h/d). The animals were allowed tap water access ad libitum throughout the study period.
For tumor inoculation, 3 × 106 cells were suspended in 200 μL medium and Matrigel High Concentration (Corning, Tewksbury, MA, USA; 1:1) and subcutaneously injected into the bilateral flanks of the mice. The tumor dimensions were measured weekly using a caliper, and volumes were calculated using the following formula: 1/2 (W2 × L), where W and L are the measured width and length, respectively. Seven days prior to the inoculation, the mice were given oral administration of SFN at a dose of 50 mg/ kg in 0.1 ml saline containing 0.5% DMSO; the mice in the vehicle group were administered an equivalent volume of saline solution containing 0.5% DMSO. The administered SFN dose in this study was adjusted according to previous reports [20-22]. All the mice were sacrificed 5 weeks following the inoculations, and the size of each collected tumor was recorded.
Immunohistochemistry
The cell proliferation ability was assessed by performing immunohistochemical quantification of the Ki-67 [23]. Moreover, tumor angiogenesis was estimated using CD 34 immunohistochemistry. In addition, a quantitative analysis of the immunopositive area was performed using Adobe Photoshop software (Adobe Systems Inc., USA).
Statistical analyses
Results are presented as mean ± standard deviation values and analysed using Student’s t -test for the unpaired data (SPSS version 22; IBA, Armonk, NY, USA). p<0.05 was considered statistically significant.
Results
SFN suppresses HepG2 cell growth by causing cell cycle arrest
In order to explore the inhibitory effect of SFN on the human liver cancer cell lines, WST assay was performed. The WST assay examined the effect of SFN on cell proliferation of the HepG2 cells. SFN significantly inhibited cell proliferation of the HepG2 cells in a dose-dependent manner (Figure 1A). Thereafter, we evaluated the gene expression of HMO1, ABCC2, and NQO1 in the HepG2 cells to determine whether Nrf2 partly mediated the inhibiting effect of SFN, a known Nrf2 agonist. All these were Nrf2 target genes and were significantly upregulated in the SFN-treated group (Figure 1B). Given that growth inhibition of cancer cells is usually associated with cell cycle arrest, we investigated the effect of SFN on the expression of the cell cycle-related genes, CCND1, CCNB1, CDK1, CDK2, and CDKN1A in the HepG2 cell. Compared with those of the controls, the mRNA expression levels of CCND1, CCNB1, CDK1 and CDK2 in the HepG2 cells were distinctly lower in the SFN-treated group (Figure 2). Similarly, SFN significantly inhibited cell proliferation of the Huh7 cells in a dose-dependent manner. The Nrf2 target genes were significantly upregulated in the SFN-treated group. The mRNA expression levels of CCND1, CCNB1, CDK1, and CDK2 in the Huh7 cells were substantially lower in the SFN-treated group (Supplementary Figure S1).
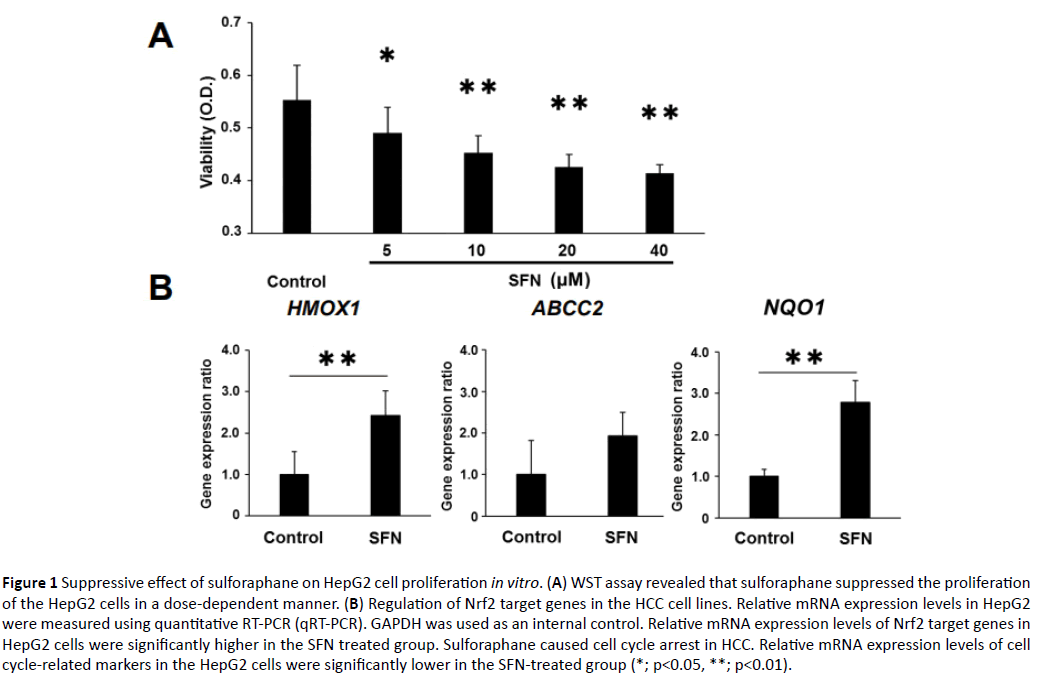
Figure 1: Suppressive effect of sulforaphane on HepG2 cell proliferation in vitro. (A) WST assay revealed that sulforaphane suppressed the proliferation of the HepG2 cells in a dose-dependent manner. (B) Regulation of Nrf2 target genes in the HCC cell lines. Relative mRNA expression levels in HepG2 were measured using quantitative RT-PCR (qRT-PCR). GAPDH was used as an internal control. Relative mRNA expression levels of Nrf2 target genes in HepG2 cells were significantly higher in the SFN treated group. Sulforaphane caused cell cycle arrest in HCC. Relative mRNA expression levels of cell cycle-related markers in the HepG2 cells were significantly lower in the SFN-treated group (*; p<0.05, **; p<0.01).
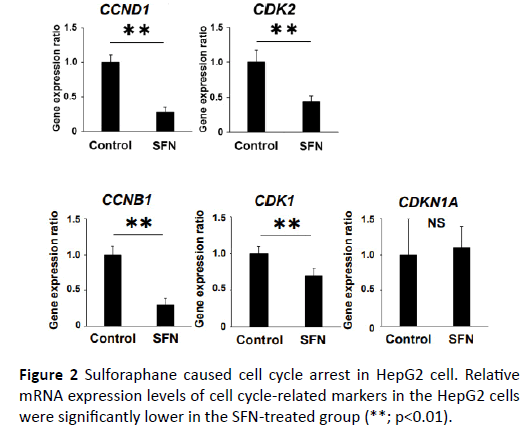
Figure 2: Sulforaphane caused cell cycle arrest in HepG2 cell. Relative mRNA expression levels of cell cycle-related markers in the HepG2 cells were significantly lower in the SFN-treated group (**; p<0.01).
SFN suppresses liver cancer growth in in vivo xenografted model
Given the in vitro inhibitory effect of SFN on human liver cancer cell growth, we further evaluated the suppressive effect of SFN on in vivo tumor growth of xenografted liver cancer in athymic nude mice. At the end of the experiments, both the mean tumor volumes and weights were significantly lower in the SFNtreated mice than in the control mice (Figure 3A). HepG2 cellderived xenograft tumors of the control mice aggressively grew following inoculation, while the tumors in the mice treated with SFN exhibited modest growth (Figures 3B and 3C). However, body weight, an indicator of the general health of animals, did not show a significant difference between these two groups at the sacrifices (Supplementary Figures S2 and S3). In addition, there were no significant changes of the serum level of alanine aminotransferase (ALT), albumin, and total bilirubin in the course of this experiment in each group (Supplementary Table S1). These observations implicate that the indicated dose of SFN would rarely cause any liver injury in mice, supporting that it could be a safe and effective agent for liver cancer therapy at least in the xenograft tumor model. Similar results were shown in the experiment of the Huh7 cell lines (Supplementary Figure S4).
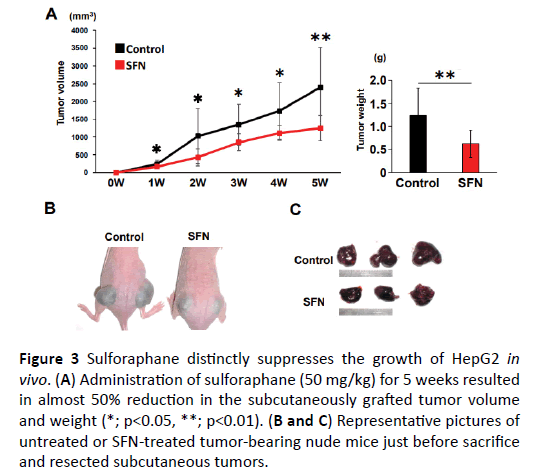
Figure 3: Sulforaphane distinctly suppresses the growth of HepG2 in vivo. (A) Administration of sulforaphane (50 mg/kg) for 5 weeks resulted in almost 50% reduction in the subcutaneously grafted tumor volume and weight (*; p<0.05, **; p<0.01). (B and C) Representative pictures of untreated or SFN-treated tumor-bearing nude mice just before sacrifice and resected subcutaneous tumors.
SFN diminishes the proliferation of the xenografted tumor cells
We assessed the tumor cell viability using Ki-67 immunohistochemistry. The number of Ki-67 immuno-positive cells in the tumor of the SFN-treated group was significantly lower than that in the tumor of the control group (Figures 4A-4C). Using this quantitative assessment, we found that SFN effectively diminished Ki-67 immunopositive cell proliferation in the xenograft tumors. Along with these suppressed features of cell proliferation, the mRNA expression levels of CCND1, CCNB1, CDK1 and CDK2, cell cycle regulators, were considerably down regulated in the subcutaneous tumors of SFN-treated mice. Additionally, to determine the effect of SFN on tumor cell apoptosis, we performed immunohistochemical evaluation of caspase 3. The number of caspases 3 immuno-positive cells in the tumor of the SFN-treated group was significantly larger than that of the control group (Supplementary Figure S5).
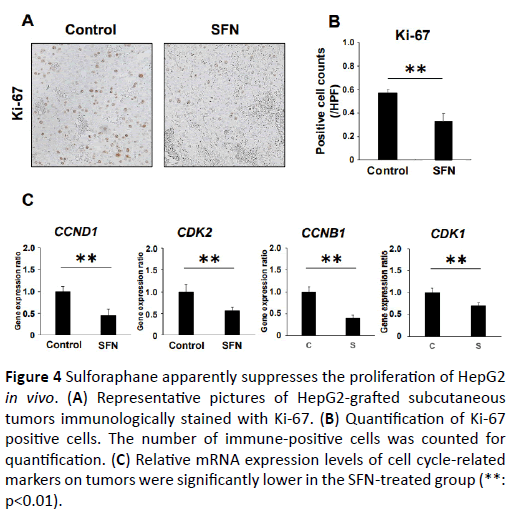
Figure 4: Sulforaphane distinctly suppresses the growth of HepG2 in vivo. (A) Administration of sulforaphane (50 mg/kg) for 5 weeks resulted in almost 50% reduction in the subcutaneously grafted tumor volume and weight (*; p<0.05, **; p<0.01). (B and C) Representative pictures of untreated or SFN-treated tumor-bearing nude mice just before sacrifice and resected subcutaneous tumors.
SFN regulates the Nrf2 target genes
In order to determine the antitumor effect of SFN directly through the Nrf2 signaling cascade, we further evaluated the Nrf2 target gene expression levels in the xenograft tumors, such as HMOX1, ABCC2, and NQO1. ABCC2 and NQO1 showed significant upregulation in the SFN-treated group, suggesting that SFN actually regulated the Nrf2 target genes in the subcutaneously grafted liver cancer (Figure 5).
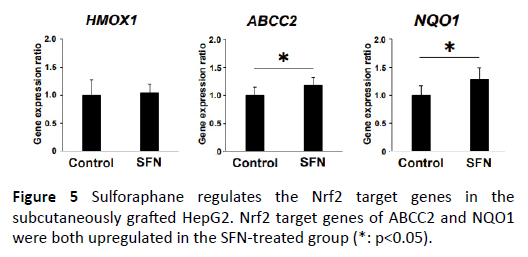
Figure 5: Sulforaphane regulates the Nrf2 target genes in the subcutaneously grafted HepG2. Nrf2 target genes of ABCC2 and NQO1 were both upregulated in the SFN-treated group (*: p<0.05).
SFN suppresses intra-tumoral angiogenesis
Angiogenesis is a physiologic process that is important for tissue growth, remodeling, and wound healing; however, it is also a prerequisite for tumor growth and metastasis [24]. We evaluated the intratumoral cell angiogenesis using CD34 immunohistochemistry. The immunopositive area in the tumor of the SFN-treated group was lower than that of the tumor in the control group (Figure 6A). Qualificative assessment showed that the CD34 immunopositive area in the tumors of the SFN treated group was significantly smaller than that in the tumors of the control group (Figure 6B).
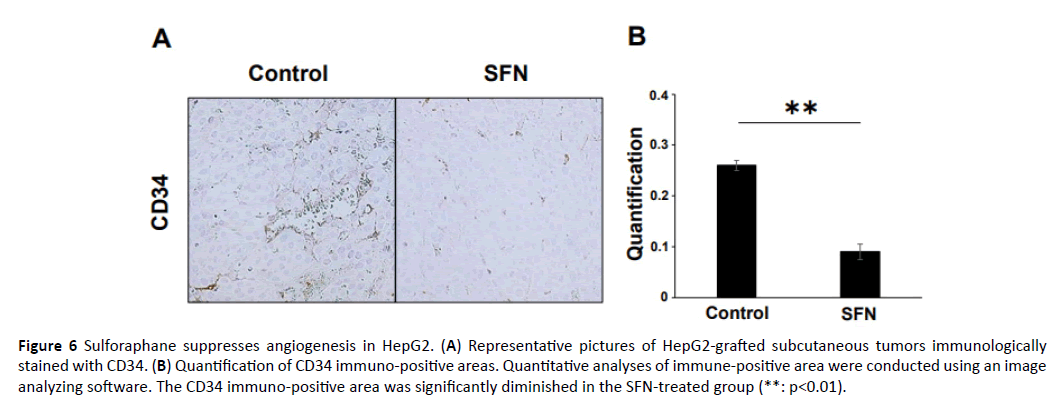
Figure 6: Sulforaphane suppresses angiogenesis in HepG2. (A) Representative pictures of HepG2-grafted subcutaneous tumors immunologically stained with CD34. (B) Quantification of CD34 immuno-positive areas. Quantitative analyses of immune-positive area were conducted using an image analyzing software. The CD34 immuno-positive area was significantly diminished in the SFN-treated group (**: p<0.01).
Thus, we successfully demonstrated that SFN exerted an inhibitory effect on the human liver cancer cell lines. Furthermore, we found that SFN inhibited the proliferation and angiogenesis of the human liver cancer cell lines, partially mediated by the Nrf2 signalling cascade.
Discussion and Conclusion
SFN, a dietary isothiocyanate that is present in broccoli and cauliflower, has been widely used for treating inflammatory diseases, and recent studies have demonstrated its inhibitory activities in tumor cell lines and animals models [11,12,25-27]. Moreover, SFN from broccoli sprouts has already been evaluated in a phase I clinical trial that demonstrated a good safety profile of SFN [28], a phase II clinical trial that aims to treat patients with recurrent prostate cancer is currently ongoing (ClinicalTrials.gov, NCT01228084). These reports indicate that SFN is a potential inhibitory agent for treating cancer. However, its inhibiting effect on liver cancer is still unclear. In the present study, we demonstrated that SFN considerably suppressed the growth of human liver cancer cells in vitro and in vivo.
Actually, several studies have been reported for the efficacy of SFN as an inhibitory agent against the HepG2 cell line [29-31]. However, these reports evaluated the effect of SFN in the “ex vivo” or “in vitro” study. Our study assessed the inhibitory effect of SFN in the “in vivo” study using the xenograft model which was simple and would reflect the clinical treatment.
Our data showed that SFN stimulated the Nrf2 signalling cascade in the liver cancer cell lines, stimulating the SFN-treated liver cancer cells to arrest their proliferation, accompanied by the inhibition of CCND1, CCNB1, CDK1 and CDK2 mRNA expression levels. Several reports indicated that sulforaphane arrested the cell cycle by decreasing retinoblastoma (Rb) phosphorylation in diverse cancers [32-34]. Moreover, SFN had a substantial in vivo suppressive effect on tumor growth in xenografted liver cancer. Similar to that in in vitro studies, the Nrf2 signalling cascade was activated in the tumors of mice treated with SFN. Using some immunohistochemical findings, we successfully showed that SFN effectively suppresses cell proliferation and angiogenesis in xenograft tumors. These results suggest that SFN exerts an inhibitory effect on cell proliferation and angiogenesis of human liver cancer. In this study, the IC50 value of sulforaphane on HepG2 cells was 22 μM (Figure 1). Biotechnical sheet of SFN shows that the IC50 value of this substance on HepG2 cells is to be 24.85 μM, and these concentrations are similar to each other. Furthermore, the IC50 value of sulforaphane on Huh 7 cells were 20 μM. We also found a paper in which similar results were shown concerning of the IC50 of SFN on Huh7 cells [35].
Clinical study demonstrates a dose-dependent inhibitory effect for sulforaphane in cancer cells [36]. We selected the dose of SFN which was used in the previous reports. Even though it is difficult to predict whether micromolar concentrations of SFN are achievable in humans as pharmacokinetics of this agent, previous studies have estimated that a hundred grams of broccoli could yield up to 40 μmol of SFN [37,38]. A more recent pharmacokinetics study involving four human volunteers receiving single dose of 200 μmol of broccoli sprout indicated that isothiocyanates (ITCs) were absorbed rapidly and reached to the peak concentrations of 0.943-2.27 μmol in plasm, serum and erythrocytes 1 hour after ingestion of broccoli extract [39]. Of course, carefully designed pharmacokinetics studies of pure SFN are necessary to determine its ideal concentration.
In normal physiological conditions, Nrf2 is anchored in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), which also mediates proteasomal degradation of Nrf2. Oxidative and electrophilic stresses cause dissociation of Nrf2 from Keap1 and lead Nrf2 to translocate into the nucleus where it can bind to the Antioxidant Response Element (ARE), a cis-acting element on the promoter of multiple cytoprotective genes. Binding to ARE results in transactivation of these ARE-bearing genes [40,41]. SFN is known as the Nrf2 agonist [42,43]. Nrf2 activation results in that Nrf2 moves the nucleus where it can bind to the Antioxidant Response Element (ARE). So, the total amount of Nrf2 is not increased by the stimulation of SFN. Our study showed that the expression of Nrf2 was not different between groups (data not shown). That is the reason why we demonstrated the evidence of the Nrf2 activation by assessing the expression of corresponding downstream genes.
It is known that SFN activates Nrf2 and acts in part on the Keap1/Nrf2 pathway to regulate several cytoprotective genes [44]. It has been widely accepted that activating Nrf2 protects the cell homeostasis from reactive oxygen species (ROS) and the metabolites of carcinogens. In contrast, Nrf2 signalling can reportedly be compromised by cancer cells as the adaptive mechanism that alleviates ROS and electrophilic burdens within the tumor microenvironment, enabling cancer cell survival [45]. Moreover, the tumorigenic potential of Nrf2 was reported in the Diethyl Nitrosamine (DEN)-induced murine HCC model using Nrf2 KO mice [46]. These reports may conflict the current findings. However, Nrf2, the master regulator of cellular redox status, has also been reported to have a biphasic response in other studies as a potential risk factor in its application for cancer treatment. Although several future studies are necessary to confirm the mechanism of Nrf2 in malignant neoplasm, we speculate that Nrf2 could exert a suppressive effect on cell proliferation during early-stage liver cancer, while Nrf2 may have a progressive effect during the advanced stage. In the previous study, SFN inhibited thyroid cancer cell growth and invasiveness through repressing phosphorylation of Akt, enhancing p21 expression by the activation of Erk and p38 signalling cascades, and promoting mitochondrial-mediated apoptosis via reactive oxygen species (ROS)-dependent pathway [14]. Probably, SFN exerts an inhibitory effect on cell proliferation of human liver cancer by inducing apoptosis. Moreover, our findings suggest that SFN exerts an inhibitory effect on cell angiogenesis of human liver cancer, partially mediated by the Nrf2 cascade.
Nrf2 activators are considered effective for managing disorders associated with the accumulation of oxidative stress, such as cardiovascular diseases [47], diabetic complications [48], neurodegenerative disorders [49], and several cancers [50-53]. In fact, we did not perform in vivo and in vitro evaluations for the ROS degree of human hepatic cancer cells in this study. Future studies should attempt to clarify the association between ROS and the antitumor effect of SFN on liver cancer.
In sum, we showed that SFN suppressed the proliferation of liver cancer cells and induced cell cycle arrest. Thereafter, we demonstrated the inhibitory effect of SFN on liver cancer tumor angiogenesis and tumor growth during its initial stage. Thus, SFN could hold a high clinical potential for preventing liver cancer in the future.
Acknowledgments
The authors are grateful to the staff of the animal facility of Nara Medical University. This study was supported in part by a grantin- aid for research from Nara Medical University. The earlier version of this study was presented at the meeting of AASLD Liver Learning on Oct 2017.
Authors’ Contributions
Concept and design: SS, KM, HY. Performing experiments: SS, KM, MF, NS, YS, KS, KK, AM, YO, JY, HY. Writing of article: SS. Editing of article: KM. Data analysis: MF, TN, MK, HK, TA. All the authors have seen and approved the final version of this manuscript.
23799
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. (2015) Global cancer statistics. CA Cancer J Clin. 65: 87-108.
- Venook AP, Papandreou C, Furuse J, De Guevara LL (2010) The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 15: 5-13.
- International Agency for Research on Cancer (2012) GLOBOCAN: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 2: 1.
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34.
- Houghton CA, Fassett RG, Coombes JS (2013) Sulforaphane: Translational research from laboratory bench to clinic. Nutr Rev 71: 709-726.
- Yin TF, Wang M, Qing Y, Lin YM, Wu D (2016) Research progress on chemo-preventive effects of phytochemicals on colorectal cancer and their mechanisms. World J Gastroenterol 22: 7058-7068.
- Jeong WS, Kim IW, Hu R, Kong AN (2004) Modulatory properties of various natural chemo-preventive agents on the activation of NF-kappaB signaling pathway. Pharm Res 21: 661-670.
- Park HS, Han MH, Kim GY, Moon SK, Kim WJ, et al. (2014) Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem Toxicol. 64: 157-165.
- Leone A, Diorio G, Sexton W, Schell M, Alexandrow M, et al. (2017) Sulforaphane for the chemoprevention of bladder cancer: Molecular mechanism targeted approach. Oncotarget 8: 35412-35424.
- Estaquier J, Vallette F, Vayssiere JL, Mignotte B (2012) The mitochondrial pathways of apoptosis. Adv Exp Med Biol 942: 157-183.
- Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, et al. (2006) ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis 27: 437-445.
- Jackson SJ, Singletary KW (2004) Sulforaphane: A naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis 25: 219-227.
- Clulow JA, Storck EM, Lanyon-Hogg T, Kalesh KA, Jones LH, et al. (2017) Competition-based, quantitative chemical proteomics in breast cancer cells identifies new target profiles for sulforaphane. Chem Commun (Camb) 53: 5182-5185.
- Yang Q, Ji M, Guan H, Shi B, Hou P (2013) Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. J Clin Endocrinol Metab 98: E1909-1917.
- Wang L, Tian Z, Yang Q, Li H, Guan H, et al. (2015) Sulforaphane inhibits thyroid cancer cell growth and invasiveness through the reactive oxygen species-dependent pathway. Oncotarget 6: 25917-52931.
- Fimognari C, NÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂüsse M, Cesari R, Iori R, Cantelli-Forti G, et al. (2002) Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis 23: 581-586.
- Choi WY, Choi BT, Lee WH, Choi YH (2008) Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed Pharmacother 62: 637-644.
- Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, et al. (2014) Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol 49: 481-491.
- Aihara Y, Yoshiji H, Noguchi R, Kaji K, Namisaki T, et al. (2013) Direct renin inhibitor, aliskiren, attenuates the progression of non-alcoholic steatohepatitis in the rat model. Hepatol Res 43: 1241-1250.
- Singh AV, Xiao D, Lew KL, Dhir R, Singh SV (2004) Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 25: 83-90.
- Herz C, Hertrampf A, Zimmermann S, Stetter N, Wagner M, et al. (2014) The isothiocyanate erucin abrogates telomerase in hepatocellular carcinoma cells in vitro and in an orthotopic xenograft tumour model of HCC. J Cell Mol Med 18: 2393-2403.
- Abbaoui B, Riedl KM, Ralston RA, Thomas-Ahner JM, Schwartz SJ, et al. (2012) Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: Characterization, metabolism, and interconversion. Mol Nutr Food Res 56: 1675-1687.
- Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, et al. (2010) Selective aldosterone blocker, eplerenone, attenuates hepatocellular carcinoma growth and angiogenesis in mice. Hepatol Res 40: 540-549.
- Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249-257.
- Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, et al. (2006) Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis 27: 2038-2046.
- Bergantin E, Quarta C, Nanni C, Fanti S, Pession A, et al. (2014) Sulforaphane induces apoptosis in rhabdomyosarcoma and restores TRAIL-sensitivity in the aggressive alveolar subtype leading to tumor elimination in mice. Cancer Biol Ther 15: 1219-1225.
- Wang DX, Zou YJ, Zhuang XB, Chen SX, Lin Y, et al. (2017) Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3ÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâ¦ÃÂø/ÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâ¦ÃÂø-catenin signaling pathways. Acta Pharmacol Sin 38: 241-251.
- Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, et al. (2006) Safety, tolerance and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr Cancer 55: 53-62.
- Liu P, Wang W, Zhou Z, Smith AJO, Bowater RP, et al. (2018) Chemo-preventive activities of sulforaphane and its metabolites in human hepatoma HepG2 cells. Nutrients 9: 10.
- Liu P, Atkinson SJ, Akbareian SE, Zhou Z, Munsterberg A, et al. (2017) Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1a/VEGF signalling. Sci Rep 4: 7.
- Zou X, Qu Z, Fang Y, Shi X, Ji Y (2017) Endoplasmic reticulum stress mediates sulforaphane-induced apoptosis of HepG2 human hepatocellular carcinoma cells. Mol Med Rep 15: 331-338.
- Choi KM, Lee YS, Sin DM, Lee S, Lee MK, et al. (2012) Sulforaphane inhibits mitotic clonal expansion during adipogenesis through cell cycle arrest. Obesity (Silver Spring) 20: 1365-1371.
- Bryant CS, Kumar S, Chamala S, Shah J, Pal J, et al. (2010) Sulforaphane induces cell cycle arrest by protecting RB-E2F-1 complex in epithelial ovarian cancer cells. Mol Cancer 9: 47.
- Wang L, Liu D, Ahmed T, Chung FL, Conaway C, et al. (2004) Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int J Oncol 24: 187-192.
- Schrader C, Graeser AC, Huebbe P, Wagner AE, Rimbach G (2012) Allyl isothiocyanate as a potential inducer of paraoxonase-1 studies in cultured hepatocytes and in mice. IUBMB Life 64: 162-168.
- Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E (2006) Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis 27: 811-819.
- Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P (1998) Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev 7: 1091-1100.
- Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, et al. (2000) Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 38: 168-178.
- Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, et al. (2002) Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta 316: 43-53.
- Kobayashi M, Yamamoto M (2005) Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7: 385-394.
- Li W, Kong AN (2009) Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48: 91-104.
- Li J, Shen F, Guan C, Wang W, Sun X, et al. (2014) Activation of Nrf2 protects against triptolide-induced hepatotoxicity. PLoS One 2: 9.
- Kim HJ, Barajas B, Wang M, Andre E (2008) Nrf2 activation by sulforaphane restores the age-related decline of Th1 immunity: Role of dendritic cells. J Allergy Clin Immunol 121: 1255-1261.
- Chen YT, Shi D, Yang D, Yan B (2012) Antioxidant sulforaphane and sensitizer trinitrobenzene sulfonate induce carboxylesterase-1 through a novel element transactivated by nuclear factor-E2 related factor-2. Biochem Pharmacol 84: 864-871.
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, et al. (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475: 106-109.
- Ngo HKC, Kim DH, Cha YN, Na HK, Surh YJ (2017) Nrf2 mutagenic activation drives hepatocarcinogenesis. Cancer Res 77: 4797-4808.
- Howden R (2013) Nrf2 and cardiovascular defense. Oxid Med Cell Longev p: 104308.
- Xu Z, Wang S, Ji H, Zhang Z, Chen J, et al. (2016) Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci Rep 6: 30252.
- Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL (2015) NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest 125: 1433-1445.
- Surh YJ (2013) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3: 768-780.
- Jiang LL, Zhou SJ, Zhang XM, Chen HQ, Liu W (2016) Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed Pharmacother 78: 74-80.
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P, et al. (1994) Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA 91: 3147-3150.
- Chuang LT, Moqattash ST, Gretz HF, Nezhat F, Rahaman J, et al. (2007) Sulforaphane induces growth arrest and apoptosis in human ovarian cancer cells. Acta Obstet Gynecol Scand 86: 1263-1268.











