Keywords
Quinazoline, Azaisatin, Antibacterial, Antifungal.
Introduction
Quinazoline [1] is a versatile lead molecule for the design of potential bioactive agents because its derivative has been reported to possess broad spectrum biological activities. Quinazolinones are the oxidized form of quinazolines and as such are part of the quinoline alkaloids. Several quinazolinone alkaloids are known to elicit a wide variety of biological response including antibacterial [2], analgesic and anti-inflammatory [3], antifungal [4] anticonvulsant [5], anticancer [6], and antihypertensive [7] activities.
It is evident from Literature that azaisatin [8] are also biologically active and found to have various pharmacological activities. So, keeping this in view the present work is to synthesize the title compounds to obtain derivatives of azaisatin.
Materials and Methods
Melting points were taken in open capillary tubes on Sigma-Aldrich melting point apparatus and are uncorrected. All the synthesized compounds were purified by thin layer chromatogram on silica gel G using toluene: ethyl acetate (7:3) and visualized with UV light. IR spectra were recorded on PERKINELMER BX Series FTIR spectrometer using KBr pellets. 1HNMR spectra were recorded in a CDCl3 as a solvent and tetra methyl silane (TMS) as an interval standard. MASS Spectra of the compounds were recorded on a Agilent Mass spectroscopy 1100 series using ESI technique.
The physical constants of different synthesized azaisatins derivatives containing 2- phenylquinazolin4-(3H)-ones are shown in Table No.1 The spectral data of different synthesized azaisatins derivatives containing 2- phenylquinazolin4-(3H)-ones are shown in Table No.2
| Compound |
R |
Molecular weight (amu) |
Molecular Formula |
M.P |
%yield |
Recrystallization solvent |
| IV a |
– CH3 |
381 |
C22H15N5O2 |
216°C-218° C |
72 |
Absolute Ethanol |
| IV b |
– C2H5 |
395 |
C23H17N5O2 |
204°C-206° C |
75 |
Absolute Ethanol |
| IV c |
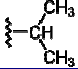 |
409 |
C24H19N5O2 |
213°C-215°C |
74 |
Absolute Ethanol |
| IV d |
– C6H13 |
451 |
C27H25N5O2 |
208°C-210°C |
73 |
Absolute Ethanol |
Table 1: Physical Constants Of Different Azaisatins Derivatives Containing 2-Phenylquinazolin-4-(3h)-Ones
| COMPOUND |
IR(KBr)cm-1 |
1H NMR(400 MHz, CDCl3) |
MASS SPECTRUM
m/z |
| Synthesis of 3-Amino-2- phenylquinazolin-4(3H)-one (III) |
3568.18,3309.03(d,NH,stretch),
1662.94 (C=N, stretch), 1718.24(C = O, stretch) |
d [ppm]: 8.306 (d, 1H, Ar-H),
7.800 (m,4H,Ar-H), 7.520 (m,
4H,Ar-H), 5.015 (s, 2H,NH2 |
The molecular ion was observed at 238 [M + H] +
260 [M +Na] +
and 497 [2M
+Na] + |
| 3-(1, 2-Dihydro-1-ethyl-2- oxopyrrolo [2, 3-b] pyridin-3- ylideneamino)-2- phenylquinazolin-4(3H)-one. [IV b] |
1718.17 (C = O, stretch), 1699.75 (C=O, stretch), 1654.24 (C=N,
stretch |
d [ppm]: 8.334 (d, 1H, Ar-H),
8.095 (d, 1H,Ar-H), 7.104 (t,
1H,Ar-H), 7.844 (d,3H,Ar-H),
7.670 (m, 3H,Ar-H), 7.542 (m,3H,Ar-H), 3.987 (q, 2H, CH2),
1.393 ( t, 3H,CH3) |
The molecular ion was observed at 396 [M + H] +
and 418 [M
+Na] + |
| 3-(1, 2-Dihydro-1-isopropyl-2- oxopyrrolo [2, 3-b] pyridin-3- ylideneamino)-2- phenylquinazolin-4(3H)-one. [IV c] |
1718.20 (C = O, stretch), 1677.36 (C=O, stretch), 1654.24 (C=N,
stretch |
d [ppm]: 8.305 (d, 1H, Ar-H),
8.095 (d, 1H,Ar-H), 7.078 (t,
1H,Ar-H), 7.843 (d,3H,Ar-H),
7.669 (m, 3H,Ar-H), 7.540 (m,3H,Ar-H), 4.839 (m, 1H, CH),
1.615 ( d, 6H,CH3) |
The molecular ion was observed at 410 [M + H] +
and 432 [M
+Na] + |
Table 2: Spectral Data
Scheme
General Procedure
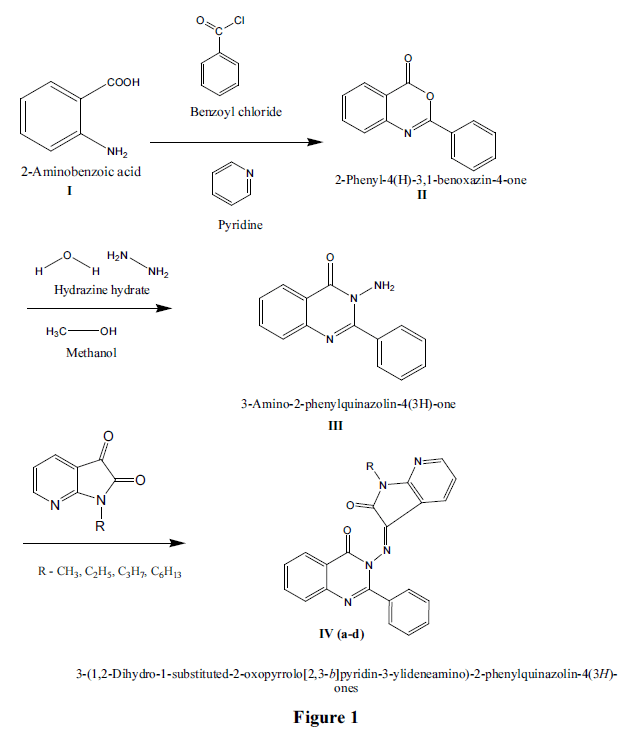
A) Synthesis of 2-Phenyl-(4H)-3, 1-benzoxazin-4- one [9] (II)
To a stirred solution of 2-aminobenzoic acid (I) (0.01 mole) in pyridine (6ml), benzoyl chloride (0.01 mole) was added drop wise maintaining the temperature near 8 0C for about 1.5 hours. Reaction mixture was stirred for another 3 hours at room temperature, while stirring a solid product separated out. Whole reaction mixture was neutralized with 5% NaHCO3 solution. A pale yellow deposited product was filtered, washed with cold water and dried. The solid product was recrystallized from ethanol.
Yield: 86%, m.p: 114 – 116 ° C
B) Synthesis of 3-Amino-2-phenylquinazolin- 4(3H)-one [9] (III)
In 100ml of round bottomed flask, a solution of hydrazine hydrate (0.10 mole) and 2-phenyl- 4Hbenzo[d] [3, 1] oxazin-4-one (II, 0.05 mole) in methanol were taken and refluxed for 3-6 hours and the reaction was monitored by TLC. The solution was cooled, poured into cold water and the product was filtered, washed with cold water and dried. The solid product was recrystallized from ethanol.
Yield: 82%, m.p: 168-172°C
C) Synthesis of 3-(1, 2-Dihydro-1-substituted-2- oxopyrrolo [2, 3-b] pyridin-3-ylideneamino)-2- phenylquinazolin-4(3H)-ones [10] (IV) a-d
A mixture of 3-Phenyl-2-methylquinazolin-4(3H)- one (VII, 0.001 mole) and substituted azaisatin (0.01 mole) in 10ml of glacial acetic acid were refluxed for 10-15minutes at 140 watt in Catalyst Systems Scientific microwave System and the reaction was monitored by TLC for completion. The resultant solution was poured into cold water. The product was filtered, washed with cold water and dried. The solid product was recrystallized from absolute ethanol.
Results
Antibacterial Activity
Antibacterial activity of compounds IV a-d was performed by disc diffusion method using nutrient agar medium against gram positive bacteria viz., Bacillus subtilis, Staphylococcus aureus, Streptococcus pneumonia and gram negative bacteria viz., E.coli, Proteus vulgaris, Klebsiella pneumonia, Pseudomonas aeruginosa at 10μg/ml concentration. Ciprofloxacin was used as standard for comparison. The results are given in Table No.3
| Compounds |
10µg/ml |
B.subtilis |
S.aureus |
S. pneumonia |
E.coli |
P.vulgaris |
K.pneumonia |
P. aeruginosa |
| IV a |
10 |
15 |
16 |
10 |
15 |
14 |
15 |
10 |
| IV b |
10 |
17 |
14 |
16 |
15 |
18 |
20 |
16 |
| IV c |
10 |
18 |
15 |
18 |
18 |
20 |
17 |
16 |
| IVd |
10 |
19 |
20 |
17 |
19 |
19 |
20 |
18 |
| Ciprofloxacin |
10 |
29 |
28 |
22 |
28 |
24 |
28 |
26 |
Table 3: Antibacterial Activities of synthesized compounds [Zone of inhibition zone in mm at 10µg/disc].
Antifungal Activity
The compounds IV a-d were tested for their antifungal activity against four test organism Aspergillus niger, Aspergillus Flavus, Candida albicans, Fusarium oxysporium using potatodextrose- agar medium at 10μg/ml concentration. Inhibition zones were measured and the diameter was calculated in millimetre. The antifungal of these compounds was compared with standard drug Fluconazole and they have a promising activity. The results are given in Table No.4
| Compounds |
10µg/ml |
Aspergillus niger |
Aspergillus Flavus |
Candida albicans |
Fusarium oxysporium |
| IV a |
10 |
11 |
15 |
14 |
12 |
| IV b |
10 |
14 |
13 |
19 |
15 |
| IV c |
10 |
20 |
14 |
20 |
17 |
| IVd |
10 |
17 |
20 |
19 |
18 |
| Fluconazole |
10 |
27 |
26 |
29 |
24 |
Table 4: Antifungal activities of synthesized compounds [Zone of inhibition zone in mm at 10µg/disc]
Discussion
Antibacterial activity
Table No.3 shows the anti-bacterial activity data of 3-(1-substituted-1, 2-dihydro-2-oxopyrrolo-[2, 3- b] pyridin-3-ylideneamino)-2-phenyl-quinazolin- 4(3H)-ones.
Amongst them, compounds IV c, IV d have been found to be relatively more active against B.subtilis with a zone of inhibition of 18mm, and 19mm respectively, whereas compound IV d were effective against S.aureus with the zone of inhibition of 20mm respectively.
Compounds IV c, IV d were moderately effective against S. pneumonia with a zone of inhibition of 18mm and 17mm respectively, whereas compounds IV c, IV d were effective against E.coli with a zone of inhibition of 18mm, 19mm respectively.
Compounds IV b, IV c, IV d were more effective against P.vulgaris. with a zone of inhibition of 18mm, 20mm, 19mm respectively, whereas compounds IV b, IV d are effective against K.pneumonia with a zone of inhibition of 20mm and 20mm respectively. Compound IV d was effective against P. aeruginosa with a zone of inhibition of 18mm.
Antifungal activity
Table No.4 shows the antifungal activity data of 3-(1-substituted-1, 2-dihydro-2-oxopyrrolo-[2, 3-b] pyridin-3-ylideneamino)-2-phenyl-quinazolin- 4(3H)-ones.
Amongst them, compounds IV c has been found to be effective against Aspergillus Niger with the zone of inhibition of 20mm. Compounds IV d have been found to be effective against Aspergillus Flavus with the zone of inhibition of 20mm. Compounds IV b, IV c, IV d have been found to be more effective against Candida albicans with the zone of inhibition of 19mm, 20mm, 19mm respectively, whereas compounds IV d have been found to be effective against Fusarium oxysporium with the zone of inhibition of 18mm.
Conclusion
Antibacterial activity
All the synthesized compound were found to be very active at a concentration of (10μg/disc) against the gram-positive and gram-negative organisms.
The compounds with isopropyl and hexyl substitutions at position 1 in azaisatin moiety were found to have good antibacterial activity against all the organisms used.
Compared to other three compounds IV d has been found to be relatively more effective against bacterial strains.
Antifungal activity
All the synthesized compound were found to be very active at a concentration of (10μg/disc) against fungal strains used.
The compounds with ethyl, isopropyl and hexyl substitutions at position 1 in azaisatin moiety were found to have good antifungal activity against all the organisms used.
Compounds IV c and IV d are found to be more effective against fungal strains.
Acknowledgement
The authors are thankful to University College of Pharmaceutical Sciences, Kakatiya University, Warangal, and Andhra Pradesh for providing all the facilities and Heads of R and D institute, Vijayawada for providing the spectral data.
Conflict of Interest Statement
We declare that we have no conflict of interest.
5770
References
- Anjani K. Tiwari, Vinay Kumar Singh, ArunaBajpai, GauriShukla, Swetha Singh, Anil K. Mishra, European Journal of Medicinal Chemistry. 2007; 42: 1234-1238.
- K.Hemalatha, K.Girija, International Journal of Pharmacy and Pharmaceutical Sciences, 2011; Vol 3 (2): 103-106.
- Chatrasal Singh Rajput, Sanjeev Kumar and Ashok Kumar, International Journal of ChemTech Research.2010; Vol 2(3): 1653-1660.
- Hanan Georgey, Nagwa Abdel-Gawad and Safinaz Abbas, Molecules 2008;13: 2557-2569.
- Nagwa M. Abdel Gawad, Hanan H. Georgey, Riham M. Youssef, Nehad A. El-Sayed, European Journal of Medicinal Chemistry, 2010; 45 : 6058- 6067.
- R. K. Russell et.al, European Journal of Medicinal Chemistry, 1992; 27: 277-284.
- Muhammad Arif, and Khalid M. Khan. Pak. J. Pharm. Sci., 2008; Vo.21 (1): 36-39.
- A. Jafar. Ahamad, K.Vijaya Kumar; Der Pharma Chemica, 2010; 2 (5): 453-457.
- Praveen Kumar, BirendraShrivastava, Surendra N. Pandeya, James P. Stables; European Journal of Medicinal Chemistry 2011; 46: 1006-1018.








