Keywords
Benzothiazole; Anti-microbial; E. coli; Staphylococcus aureus
Introduction
Human kind has been subject to infection by microorganism since before the dawn of recorded history. One presumes that mankind has been searching for suitable therapy for nearly as long. Although one can find indications in old medical writing of folkloric use of plant and animal preparations, soybean curd, moldy bread and cheese, counter infection with other microbes, the slow development of public health measures, and an understanding of the desirability of personal cleanliness, these factors were erratically and inefficiently applied and often failed. Until after the discovery of bacteria 300 years ago, and subsequent understanding of their role in infection about 150 years ago, there was no hope for rational therapy [1].
The modern anti-infective era opened with the discovery of the sulfonamides in France and Germany in 1936 as an offshoot of Paul Ehrlich’s earlier achievements in treating infections with organometallics and his theories of vital staining. Initially the term ‘chemotherapeutic agent’ was restricted to synthetic compounds, but now since many antibiotics and their analogues have been synthesized, this criterion has become irrelevant; both synthetically and microbiologically produced drugs need to be included together. However, it would be more meaningful to use the term Antimicrobial Agent (AMA) to designate synthetic as well as naturally obtained drugs that attenuate microorganisms [2].
Basis of antimicrobial action
• Various antimicrobial agents act by interfering with Cell wall synthesis [3].
• Plasma membrane integrity.
• Nucleic acid synthesis.
• Ribosomal function.
• Folate synthesis.
Drug profile
Benzothiazole ring system consists of thiazole ring fused with benzene ring. Thiazole ring is a five?member ring containing one nitrogen and one sulfur atom in the ring system. Benzothiazoles are bicyclic ring system with multiple applications. In the 1950s, a number of 2?aminobenzothiazoles were intensively studied as central muscle relaxants. Since then medicinal chemists have not taken active interest in this chemical family. Biologist’s attention was drawn to this series when the pharmacological profile of Riluzole was discovered [4].
Benzothiazole ring is present in various marine or terrestrial natural compounds, which have useful biological properties. In last few years it was reported that benzothiazole, its bioisosters and derivatives had antimicrobial activity against Gram negative, Gram?positive bacteria and the yeast Candida albicans and antimicrobial activity especially against Enterobacter, Pseudomonas aeruginosa, E. coli, and Staphylococcus epidermidis. Other activities involves are antidiabetic, and bradykinin B2 receptor antagonist activity [5].
Benzothiazole is an aromatic heterocyclic compound with the chemical formula C7H5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature [6].
Chemical and physical properties
Several Benz?fused heterocyclic systems as Indole, Benzothiazole, Benzimidazole, Benzoxazole have been studied and found to be possessing various pharmacological activities such as antiviral, antibacterial, antimicrobial, and fungicidal activities, anti-allergic, anti-diabetic, anti-tumor, anti-inflammatory, anthelmintic, and anti HIV properties (Table 1).
| Description |
Yellow liquid with unpleasant odor |
| Boiling Point |
231%C |
| Melting Point |
2%C |
| Flash Point |
>110%C |
| Density |
1.2460 g/cm3 at 20%C |
| Solubility |
Slightly soluble in water; very soluble in ethanol, diethyl ether and carbon disulfide; soluble in acetone |
| Refractive Index |
1.6379 at 20%C |
| Vapor Pressure |
0.07 mm Hg at 20%C |
| Reactivity |
Reacts with aldehydes or ketones to generate a hydroxyl and carbonyl |
| Log P |
2.01 |
Table 1: Chemical and Physical Properties [7].
Survey of Literature reveals that no sufficient work regarding synthesis of imidazolinone derivatives of 2-aminobenzothiazole has been reported. They have been found effective in the treatment of cancer, tuberculosis and Amoebiasis.
Therefore the present research was designed to synthesize the new imidazolinone derivatives of benzothiazole and to evaluate its antimicrobial effect.
Materials and Methods
These are the chemicals with their quantity required in the synthesis of the benzothiazole listed as follows (Table 2):
| Chemicals |
Quantity |
| 2-Aminobenzothiazole |
100 mg |
| Ethylchloroformate |
10 ml |
| Pyridine |
300 ml |
| Ethanol |
3 l |
| Glycine |
100 g |
| Benzoyl chloride |
150 ml |
| Sodium hydroxide pellets |
50 g |
| Hydrochloric acid |
50 ml |
| Acetic anhydride |
30 ml |
| Sodium acetate |
30 ml |
| Benzaldehyde |
4 ml |
| 4-methoxy benzaldehyde |
4 ml |
| 4-chloro benzaldehyde |
4 ml |
| 3-chloro benzaldehyde |
4 ml |
| 4-fluoro benzaldehyde |
4 ml |
| 2-methoxy benzaldehyde |
4 ml |
| Distilled water |
4 l |
| Absolute alcohol |
200 ml |
| Carbon tetrachloride |
300 ml |
| Methanol |
300 ml |
| Hexane |
300 ml |
| Chloroform |
200 ml |
| Ethyl acetate |
400 ml |
| Acetone |
100 ml |
| Silica gel G |
500 g |
| Sodium Chloride |
500 g |
| Calcium Chloride |
100 g |
| Nutrient Broth |
500 ml |
| Nutrient Agar Medium |
500 ml |
| Barium Chloride |
2 g |
| Sulphuric Acid |
10 ml |
| Tween 80 |
0.5 ml |
| Saborand Dextrose Medium |
500 ml |
| Saborand Dextrose Agar Medium |
500 ml |
Table 2: List of chemicals required.
Test strains
1. Gram +ve=Bacillus subtilis, Staphylococcus aureus.
2. Gram –ve=E. coli, Pseudomonas aeruginosa.
Composition of media
Nutrient Broth is used for the preparing stock cultures of bacteria (Table 3). Nutrient agar was prepared for the growth of bacteria in petri-dish which is to be inhibited by drugs (Table 4).
| Ingredients |
Quantity |
| Peptic digest of animal tissue |
10 g |
| Meat Extract |
10 g |
| NaCl |
5 g |
| Distilled water |
up to 1000 ml |
| pH |
7.5 ± 0.2 |
Table 3: Composition of nutrient broth.
| Ingredients |
Quantity |
| Peptone |
10 g |
| Beef Extract |
10 g |
| NaCl |
5 g |
| Agar |
20 g |
| Distilled water |
up to 1000 ml |
Table 4: Composition of Nutrient Agar Medium: (2%, pH ± 6.8, 0.2).
Synthesis of benzothiazole derivatives
The synthesis of benzothiazole derivatives was done by the following method:
a) Synthesis of Ethylbenzo [d] thiazol-2-ylcarbamate (S1) by the reaction of 2-aminobenzothiazole and ethyl-chloroformate in pyridine.
b) Synthesis of intermediate 4- (benzo [d] thiazole-2-yl) semicarbazide by refluxing (S1) with hydrazine hydrate.
c) Synthesis of 4-(substituted benzylidene)-2-phenyloxazol-5- one (X1-X6) as second intermediate by reacting benzoyl glycine with substituted benzaldehydes in the presence of acetic anhydride and sodium acetate (Literature Method).
d) Finally, the substituted benzylidene-2-phenyloxazolone (X1- X6) was reacted with 4-(benzo [d] thiazole-2-yl) semicarbazide (S 2) to yield different imidazolinone derivatives of benzothiazole.
Pharmacological activity
Antimicrobial activity: An antimicrobial is a substance that kills or inhibits the growth of microbes such as bacteria, fungi, or viruses. It is a general term that refers to a group of drugs that includes antibiotics, antifungals, antiprotozoals and antivirals and used to treat a microbial infection. Antimicrobial drugs either kills microbes (microbicidal) of prevent the growth of microbes (mocrobistatic).
In vitro tests were used as screening procedure for new agents and for testing the susceptibility of individual isolates from infections to determine which of the available drugs might be useful therapeutically [7-12].
There are two official methods for determining antimicrobial activity:
1. Paper-disk-plate technique (cylinder plate method).
2. Tube-dilution technique (broth micro dilution technique).
Paper-disk-plate technique (Cylinder plate method): Sensitivity testing was done to determine the range of microorganisms being susceptible to the compound under specified conditions. It was done by disk diffusion method. This method is suitable for the organisms that grow well overnight such as most of the common aerobes and facultative anaerobes and rapidly growing fungi. This method is solely depends upon the diffusion of the drug substance from a disk to an extent such than the observed growth of the microorganism is prevented totally in a zone just around the disk impregnated in a solution of drug substances. A zone of inhibition (a clear area) around the disk indicates that the organism was inhibited by the drug, which diffused into the agar from the disk.
Preparation of nutrient broth: Peptone (5 g) beef extract (5 g) and sodium chloride (2.5 g) (All of biological grades) were weighed and dissolved in 400 ml of distilled water in a 500 ml volumetric flask and warmed. This nutrient broth was sterilized in an autoclave at 15 lb/inch2 pressure (121°C) for 30 min.
Preparation of nutrient agar
Peptone (5 g) beef extract (5 g) and sodium chloride (2.5 g) (All of biological grades) were weighed and dissolved in 400 ml of distilled water in a 500 ml volumetric flask and warmed. 10 g agar was dissolved in 50 ml of warm distilled water. The two solutions were mixed and the volume in volumetric flask was made up to 500 ml with warm distilled water. This nutrient agar media was sterilized in an autoclave at 15 lb/ inch2 pressure (121°C) for 30 min.
Standard drug and test compounds
Preparation of solution of synthesized compound: Accurately weighed 25 mg and 50 mg of each synthesized compound were transferred to different 50 ml Beakers. These compounds were then dissolved in appropriate DMSO to form clear solution and volumes in each flask were made up to 10 ml with sterile distilled water. These suspension (each having conc. 50 and 100 μg/ml) were used as drug derivative suspensions.
Six mm disc of whatman paper were prepared, sterilized and dipped in the drug solutions so that the drug absorbs itself on the disc.
Preparation of standard drug (Ciprofloxacin): Disc of standard drug 10 μg/ml was used as standard for comparison. McFarland standards were used as a reference to adjust the turbidity of bacterial suspensions so that the number of bacteria will be within a given range. McFarland standards were mixed with specified amounts of barium chloride and sulfuric acid together. Mixing the two compounds forms a barium sulfate precipitate, which causes turbidity in the solution. A 0.5 McFarland standard was prepared by mixing 0.05 mL of 1.175% barium chloride dihydrate (BaCl2•2H2O), with 9.95 mL of 1% sulfuric acid (H2SO4) (Table 5). Mc Farland Standard 0.5 was used for the activity.
| McFarland Standard No. |
0.5 |
1 |
2 |
3 |
4 |
| 1.0% Barium chloride (ml) |
0.05 |
0.1 |
0.2 |
0.3 |
0.4 |
| 1.0% Sulfuric acid (ml) |
9.95 |
9.9 |
9.8 |
9.7 |
9.6 |
| Approx. cell density (1 ÃÂâ€â€Â 108 CFU/mL) |
1.5 |
3 |
6 |
9 |
12 |
|  % Transmittance* |
74.3 |
55.6 |
35.6 |
26.4 |
21.5 |
| Absorbance* |
0.132 |
0.257 |
0.451 |
0.582 |
0.669 |
Table 5: McFarland Standards.
Following steps were followed for determination of antibacterial activity of synthesized compounds
Step 1: 1 loop full strains of S. aureus, B. subtilis, E. coli and P. aeruginosa were transferred to 100 ml of sterilized Nutrient Broth under aseptic conditions. This was kept under incubation at 37°C for 24 hours.
Step 2: The seeded broth was used after 24 hrs. 1 ml of the seeded broth was diluted to prepare different dilutions of bacteria and a pinch of Tween 80 (8 drops of tween 80% in 100 ml of normal saline) was added.
The dilutions obtained were studied by inoculating 0.2 ml of each dilution on solidified Nutrient Agar by spreading method under aseptic condition in laminar flow. It was incubated at 37°C for 24 hrs (Table 6).
| Seeded Broth |
Sterile water |
| 1 ml |
9 ml |
| 2 ml |
8 ml |
| 3 ml |
7 ml |
| 4 ml |
6 ml |
| 5 ml |
5 ml |
Table 6: Seeded broth and sterile water.
After 24 hrs No. of conspicuously present colonies on Petridis were studied. Seeded broth was diluted to contain between 105 to 106 m/o CFU per unit. This working stock was used for anti-bacterial studies.
Step 4: Inoculation of drug on the Petridis on which bacteria was spread laminar airflow bench was swapped with 70% alcohol and UV lamp was switched on. After 30 min, the UV lamp was switched off.
1. All the reagents, media, inoculum and glassware were placed in liminal airflow bench observing all aseptic conditions.
2. The sterilized solution was evenly spread in all pert dishes. It was allowed to stand for some time so that agar solidifies. After that 0.2 ml of bacteria’s stock solution was poured on the Petridis and was evenly spread with the help of L-shaped rod. The discs of the drug of different concentration were placed on the Petridis with the help of forceps at four sides of Petridis. All this was done in laminar flow. Another Petridis containing standard drug was also prepared.
3. The petri-dishes were then placed in an incubator for 24 hrs at 37°C.
4. Negative controlled plate- In this plate, only nutrient agar medium was poured i.e., it did not contain drug dilution and inoculum.
5. Positive controlled plate- In this plate, nutrient agar medium was poured and after its solidification, inoculum was spread over the surface. But this petri plate did not contain drug solution.
6. After 24 hrs the zone of inhibition of different drugs was measured by mm scale and it was compared with standard.
Results and Discussion
The result of antibacterial activity was reported in Table 7. From the antibacterial activity data, it was found that the synthesized compounds exhibited mild to good antibacterial activity against S. aureus, B. subtilis (gram-positive) and E. coli, Pseudomonas aeruginosa (gram-negative) at a concentration of 50, 100 μg/ml. The compound Z6 has shown the maximum zone of inhibition.
| S. No. |
Compound Code |
Zone of Inhibition (mm) |
| B. subtilis |
S. aureus |
E. coli (ATCC-11775) |
P. aeruginosa |
| (ATCC-441) |
(209p) |
(NCIM 2863) |
| 50 μg |
100 μg |
50 μg |
100 μg |
50 μg |
100 μg |
50 μg |
100 μg |
| 1 |
1A |
12 |
12 |
8 |
9 |
15 |
10 |
12 |
9 |
| 2 |
1B |
13 |
10 |
7 |
8 |
10 |
11 |
13 |
9 |
| 3 |
Z1 |
10 |
8 |
10 |
8 |
4 |
12 |
9 |
3 |
| 4 |
Z2 |
4 |
8 |
12 |
5 |
5 |
9 |
8 |
4 |
| 5 |
Z3 |
7 |
6 |
5 |
3 |
3 |
7 |
8 |
7 |
| 6 |
Z4 |
6 |
8 |
12 |
13 |
7 |
5 |
5 |
6 |
| 7 |
Z5 |
14 |
10 |
8 |
12 |
12 |
10 |
12 |
12 |
| 8 |
Z6 |
15 |
14 |
13 |
15 |
15 |
16 |
15 |
13 |
| 9 |
Ciprofloxacin |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
Table 7: Antibacterial activity data of synthesized compounds.
Table 7 summarizes the anti-bacterial potential of the synthesized product showed in Figures 1-6. They were significantly demonstrated mild to good antibacterial potential. It was evaluated against S. aureus, B. subtilis (gram-positive) and E. coli, Pseudomonas aeruginosa (gramnegative). It was found a good antibacterial agent at conc. 50 and 100 μg/ml. The compound Z6 has shown the maximum zone of inhibition. The mechanism behind its antibacterial potency is unknown but can be suggested due to its toxicity on the cell wall.
Figure 1: Structure of Benzothiazole.
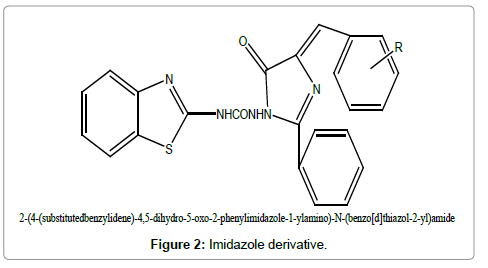
Figure 2: Imidazole derivative.

Figure 3: Synthesis of benzothiazole derivatives.
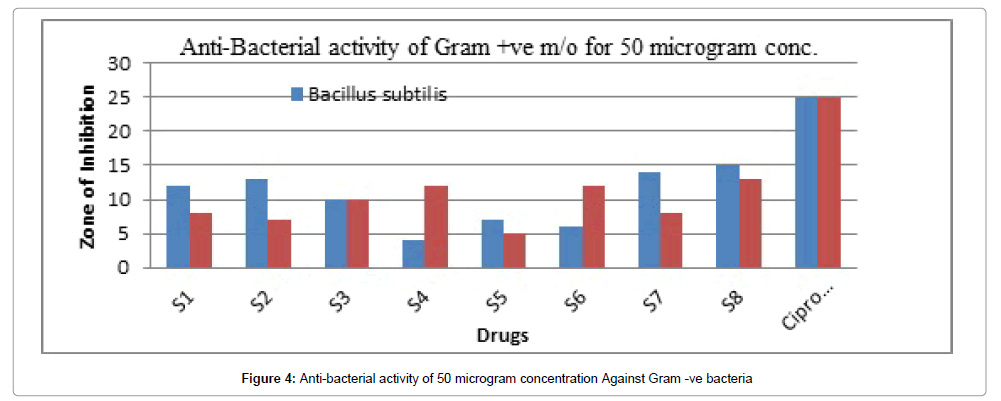
Figure 4: Anti-bacterial activity of 50 microgram concentration Against Gram -ve bacteria
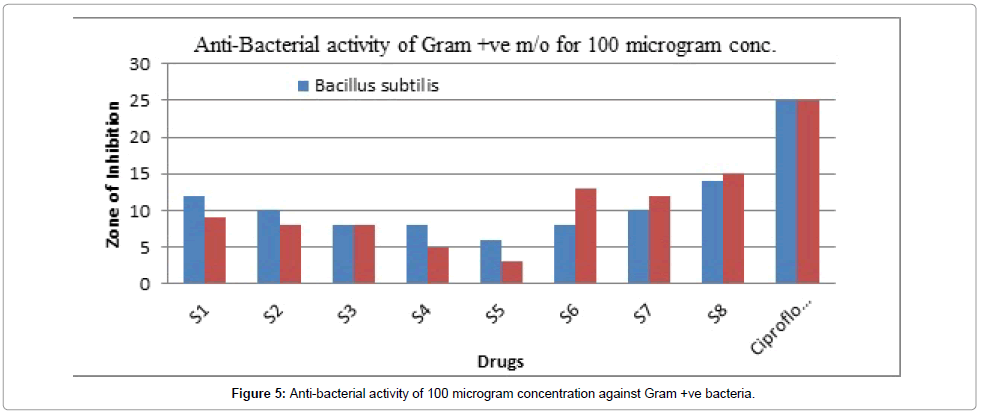
Figure 5: Anti-bacterial activity of 100 microgram concentration against Gram +ve bacteria.
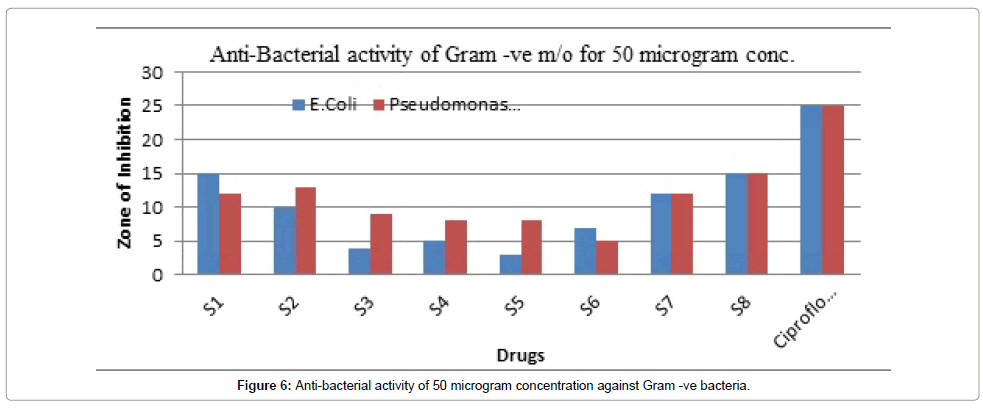
Figure 6: Anti-bacterial activity of 50 microgram concentration against Gram -ve bacteria.
It demonstrated a dose dependent response that makes it so valuable and important that can be utilized in the future.
The Ciprofloxacin was used as standard group from which the effect was compared.
Antifungal activity
From the antifungal activity data, it is found that the synthesized compounds exhibited mild to moderate antifungal activity against. A. niger and C. albicans at a concentration of 50 m 100 μg/ml. The compound Z2, Z3 and Z4 showed maximal anti-fungal activity (Table 8).
| S. No. |
Compounds Code |
Zone of Inhibition (mm) |
| A. niger |
C. albicans (ATCC 10231) |
| 50 μg |
100 μg |
50 μg |
100 μg |
| 1 |
1A |
9 |
13 |
9 |
10 |
| 2 |
1B |
12 |
11 |
7 |
8 |
| 3 |
Z1 |
10 |
3 |
3 |
9 |
| 4 |
Z2 |
10 |
9 |
12 |
9 |
| 5 |
Z3 |
12 |
11 |
11 |
12 |
| 6 |
Z4 |
9 |
12 |
14 |
15 |
| 7 |
Z5 |
12 |
9 |
12 |
9 |
| 8 |
Z6 |
6 |
8 |
9 |
5 |
| 9 |
Fluconazole |
25 |
25 |
25 |
25 |
Table 8: Antifungal activity data of the synthesized compounds.
Table 7 demonstrated the anti-fungal effect showed in Figures 7-9. All the synthesized compounds were evaluated for their antifungal activity against Aspergillus niger and Candida albicans (ATCC 10231) using Fluconazole as standard drug by paper disc method. We have adopted the same method as described in section 5.2.1 except the culture medium and incubation period. Sabouraud dextrose agar is used as culture medium and the petri dishes are incubated at 25°C for 48 hours. The standard and test compounds were treated at a concentration of 50, 100 μg/ml. Saborand dextrose medium has been made for preparing seeded broth of fungus. Fluconazole was kept as standard drug and from which the effect was compared.
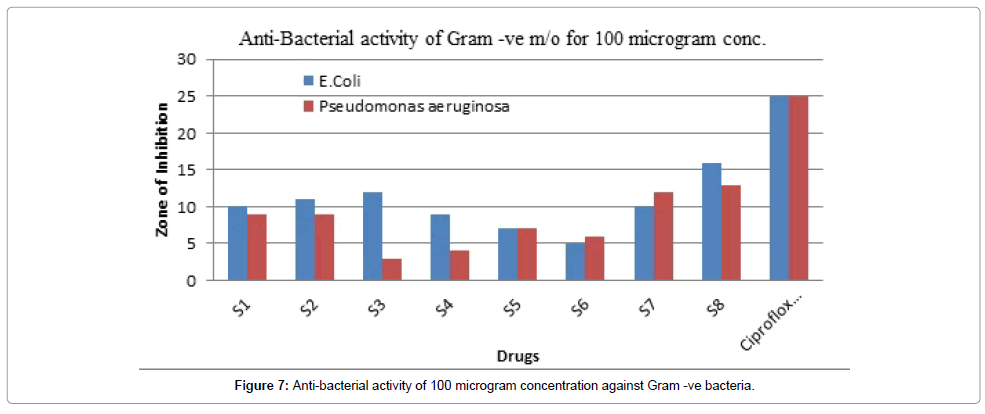
Figure 7: Anti-bacterial activity of 100 microgram concentration against Gram -ve bacteria.
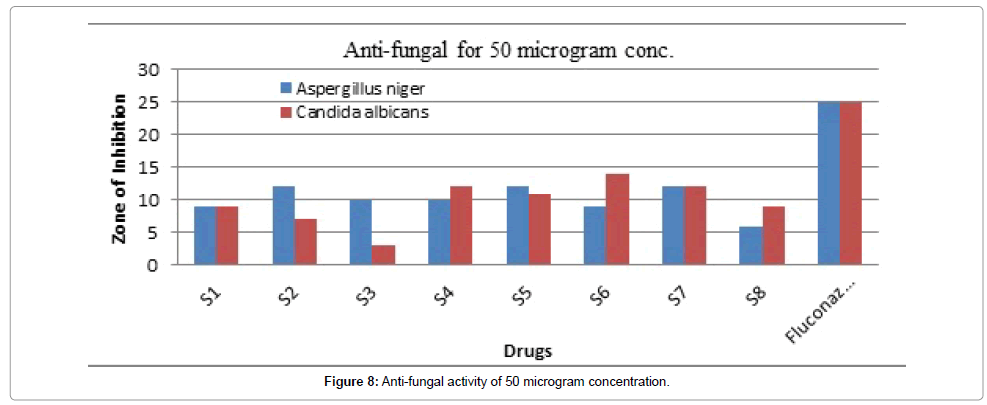
Figure 8: Anti-fungal activity of 50 microgram concentration.
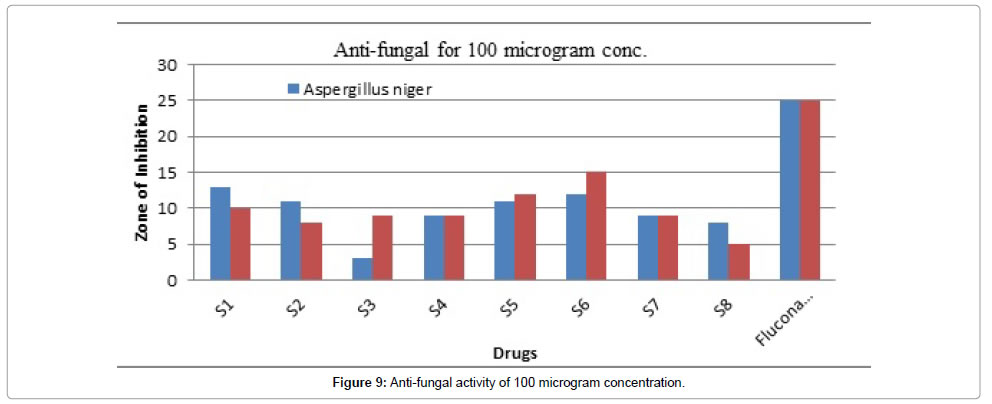
Figure 9: Anti-fungal activity of 100 microgram concentration.
Conclusion
According to objective of the research, the six new derivatives was prepared by refluxing various substitutes benzylidenes with 4(benzo[d] thiazol-2-yl)semicarbazide in the presence of pyridine for 6-8 hrs. Different benzylidenes have been prepared by the reaction of benzoyl glycine with substituted benzaldehydes in the presence of acetic anhydride and anhydrous sodium acetate. 4(benzo[d]thiazol-2-yl) semicarbazide has been prepared by reaction of 2-aminobenzothiazole with ethylchloroformate and the product was refluxed with hydrazine hydrate in the presence of ethanol. The identity of the compounds has been confirmed by M.P, TLC, IR and NMR studies.
All the synthesized compounds have been found to exhibit moderate to good anti-microbial activity. 2-(4-(2-methoxybenzylidene)-4,5- dihydro-5-oxo-2-phenylimidazole-1-ylamino)-N-(benzo[d]thiazol-2- yl)amide showed maximum activity against gram negative and gram positive bacterias. All the compounds showed good anti-fungal activity. 2-(4-(3-chlorobenzylidene)-4, 5-dihydro-5-oxo-2-phenylimidazole-1- ylamino)-N-(benzo [d]thiazol-2-yl) amide showed significant result as an anti-fungal.
The present study shows that some of the benzothiazole derivatives can be further used as template for the designing of anti-microbial with some modification to obtain potent compounds. The underlying mechanism behind synthesized product’s anti-bacterial and antifungal potential is unknown but its potency makes it enough to search the mechanism of action for further use.
23479
References
- Russell AD, Chopra I (1996) Understanding Antibacterial Action and Resistance.
- Alang G, Kaur R, Singh A, Budhlakoti P, Singh A, et al. (2010) Synthesis, Characterization and Antifungal activity of Certain (E)-1-(1-(substitutedphenyl) ethylidene)-2-(6-methylbenzo [d] thiazol-2-yl) hydrazine analogues. International Journal of Pharmaceutical & Biological Archives 1: 56-61.
- Yadav SK, Malipatil SM (2011) Synthesis and biological evaluation of benzothiazole derivatives. International Journal of Drug Discovery and Herbal Research 1: 42-43.
- Prabhu PP, Shastryb CS, Pandea SS, Selvama TP (2011) Design, Synthesis, Characterization and biological evaluation of Benzothiazole-6-carboxylate derivatives. Research in Pharmacy 1: 6-12.
- Fungi: Mushrooms, Toadstools, Moulds, Yeasts and other Fungi (2010) 1st edn., Judy Wearing. Crabtree Publishing Company.
- (1998) Antifungal Azoles: A Comprehensive Survey of their Structures and Properties L. Zirngibl.
- Yadav PS, Devprakash D, Senthilkumar GP (2011) Different Methods of Synthesis and Diverse Biological Activities. International Journal of Pharmaceutical Sciences and Drug Research 3: 01-07.
- Summary of Data for Chemical Selection: Benzothiazole. Prepared by Technical Resources International, Inc. under contract No. NO2-CB-50511 (6/97; rev. 9/97).
- Bhosale VN, Vartale SP, Deshmukh VK, Kuberkar SV (2010) Novel synthesis and antibacterial activity of 3-amino-8-chloro-4-oxo-(2H)/Aryl/Heteryl-pyrazolo [3ÃÂÃÂÃÂâÂÂÃÂâââÂÂìÃÂ
áÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂâ,4ÃÂÃÂÃÂâÂÂÃÂâââÂÂìÃÂ
áÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂâ:4,5] pyrimido[2,1-b][1,3] benzothiazoles. J Chem Pharm Res 2: 51-58.
- Basavaraja KM, Somasekhar B, Shivakumar B (2010) Synthesis of 2-[(1-Phenyl) (Aryl) Azo] Methyleneimino-6-Chloro/ Fluoro Benzothiazoles and their Antibacterial Activity. International Journal of PharmTech Research 2: 1139-1143.
- Shendarkar GR, Labhsetwar LB, Butle SR, Karki SS, Sharma RH, et al. (2011) Synthesis and Antimicrobial Evaluation of Some Fused Iminopyrimido Benzothiazole Derivatives. International Journal of Research in Pharmaceutical and Biomedical Sciences 2: 1-15.
- Harish Kumar DR, Karvekar MD (2010) Synthesis of Benzofuran Derivatives and their Evaluation of Antimicrobial Activity. E-Journal of Chemistry 7: 636-640.















