Key words
|
| |
| Anthranilic acid derivatives, antiinflammatory, pyrazolin-3"-yl |
| |
INTRODUCTION
|
| |
| Anthranilic acid is a constituent of many of the bioactive compounds that exhibits a range of biological activities. Specifically, the nucleus of anthranilic acid is the biochemical precursor to the amino acid and its derivatives as well as constituents of several alkaloids. The biological activities of this basic moiety have been well reported. Some of them like mefenamic acid and meclofenamates, both Nphenyl anthranilic acid derivatives, have been used as anti-inflammatory agents. Looking at the importance of anthranilic acid in biological system it was thought worthwhile to design and synthesize derivatives of anthranilic acid and screen them for their potential biological activities. Derivatives of anthranilic acid (1- 9) were synthesized successfully with good yield and their molecular structures were confirmed by the FTIR, 1H-NMR and elemental analysis data. The FTIR spectra of newly synthesized compounds showed the presence of characteristic absorption bands in the region 3250-3350, 3000-3100,1670- 1730,1430-1600 and 1100-1200 cm-1 which can be NH Stretching, Ar-H stretching, C=O stretching, C=N stretching and C-N stretching, respectively. |
| |
EXPERIMENTAL
|
| |
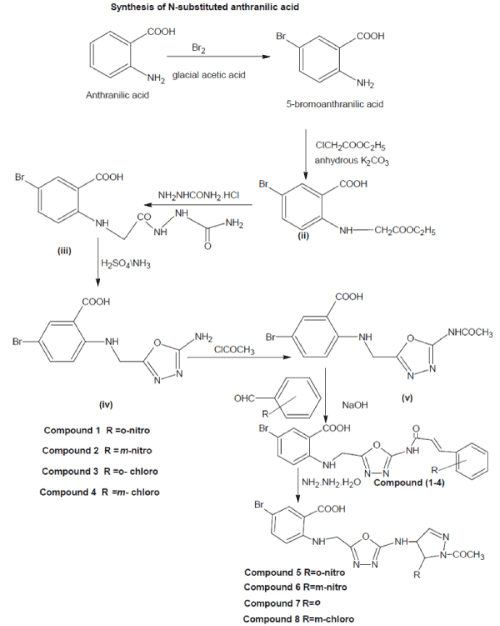
| The melting points of newly synthesized anthranilic acid derivatives were checked in capillary tubes were uncorrected. The reactions were monitored by TLC using chloroform: methanol (8:2) solvent system. The purity of compounds was checked by TLC using iodine vapour as detecting agent. The IR spectra were recorded in KBr on a FTIR Paragon 500 (Perkin Elmer) (ν max in cm-1) and 1H NMR spectra were recorded in DMSO-d6 on Bruker-300 FT instrument (chemical shift [δ] in ppm). Tetra-methyl silane (TMS) was used as internal standard. N- substituted anthranilic acid derivatives were synthesized by reacting anthranilic acid with bromine, after bromination it had given 5-bromoanthranilic acid (i) 5-bromoanthranilic acid with ethylchloroacetate had given compound 5-bromo-N-(ethylanthranilic anthranilic acids} (ii) which was further reacted with semicarbazide hydrochloride to yield the compound 5-Bromo-N-(semicarbazido carbonyl methyl) anthranilic acid, (iii) which on dehydration with H2SO4, afforded compound 5-Bromo-N-(2’-amino- 1’,3’,4’-oxadiazol-5,’ylmethyl) anthranilic acid was made. (IV) Compound made at step (IV) when reacted with acetyl chloride, gives compound 5-Bromo-N-(2’- amino acetyl-1’,3’,4’-oxadiazol-5,’ylmethyl) anthranilic acid, (V) which, on condensation with proper aromatic aldehyde yields into compound (1- 4). Compounds (5-8) were synthesized by the condensation of compounds (1-4) with the hydrazine hydrate in the presence of few drops of glacial acetic acid. |
| |
|
Synthesis of anthranilic acid derivatives (1-8) General procedure for synthesis
|
| |
| 1) Solution containing 5-bromo-N-(2'-aminoacetyl- 1',3',4'-oxadiazol-5'-ylmethyl) Anthranilic acid (3.55 gm, 0.01mol) in 60.0 ml of ethanol with different aldehydes (0.01 mol) in the presence of 2.0% sodium hydroxide were refluxed for 10 h, while progress and completion of reaction was monitored by TLC. The reaction mixture was distilled off, cooled and poured in to ice-water and filtered. The product formed was filtered, dried in vacuum and recrystallized from ethanol-water to give desired compounds (1-4). |
| |
| 2) Solution of the compound, 5-bromo-2-{[5-{[(2E)- 3-(2-substituted phenyl) prop-2-enoyl]amino}-1,3,4,- oxadiazol-2-yl)methyl]amino} benzoic acid (0.991 gm, 0.002 mol) in absolute alcohol, 99% hydrazine hydrate (0.004 mol) was added drop by drop with constant stirring in the presence of few drops of glacial acetic acid. The reaction mixture was refluxed for 12 h, distilled off and cooled. The separated solid was filtered, washed with petroleum ether and recrystallized from ethanol-water to give compounds (5-8). Dileep Tiwari et al: Synthesis and pharmacological screening of N- substituted anthranilic acid derivatives Ful l Leng th Re s ea r ch Pape r Cov e |
| |
|
Compound 1
|
| |
|
5-Bromo-2-{[5-{[(2E)-3-(2-nitrophenyl) prop-2-enoyl]amino}-1,3,4,-oxadiazol-2-yl) methyl]amino}benzoic acid (1)
|
| |
| IR (KBr) n: 3516.4 (O-H stretching), 3216.5 (N-H stretching), 3020.1 (aromatic C-H stretching), 2926.5 (C-H sym stretching), 1710 (C=O stretching ), 1603 (ring C........ C stretching), 1652.9 (C=N stretching), 1215.8 (C-N stretching), 1144.5.(C-O-C stretching), 928.7 (N-N stretching), 521.1cm-1 (C-Br stretching). |
| |
| 1H NMR (DMSO) δ: 1.23 (s,2H, CH2), 3.34 (s, 1H, NH), 5.65 (d, 1H, COCH), 5.81 (d, 1H, CH-Ar), 7.42- 7.99 (m, 4H, aromatic), 8.28-8.64 (m, 3H, aromatic), 10.23 ppm (s, 1H, O-H). |
| |
|
SCHEME-1
|
| |
 |
| |
|
Compound 2:
|
| |
|
5-Bromo-2-{[5-{[(2E)-3-(3-nitrophenyl) prop-2-enoyl] amino}-1,3,4,-oxadiazol-2- yl) methyl] amino} benzoic acid
|
| |
| IR (KBr) n: 3400.6 (O-H stretching ), 3211.0 (N-H stretching), 3021.0 (aromatic C-H stretching), 2952.9 (C-H stretching), 1706.5 (C=O stretching), 1536.1 (ring C.......C stretching), 1615.8 (C=N stretching), 1215.7 (C-N stretching ), 1089.1 (C-O-C stretching), 931.2 (N-N stretching), 519.1 cm-1 (C-B stretching). |
| |
| 1H NMR (DMSO) δ: 2.80 (s, 2H, CH2), 4.20 (s, 1H, NH), 4.81 (d, 1H, COCH), 6.11 (d, 1H, CH-Ar), 7.55- 7.99 (m, 4H, aromatic), 8.28-8.64 (m, 3H, aromatic), 10.11 ppm (s, 1H, O-H). |
| |
|
Compound 3:
|
| |
|
5-Bromo-2-{[5-{[(2E)-3-(2-chlorophenyl) prop-2-enoyl] amino}-1,3,4,-oxadiazol-2-yl) methyl] amino} benzoic acid
|
| |
| IR (KBr) n: 3620.3 (O-H stretching), 3221.0 (N-H stretching), 3021.0 (aromatic C-H stretching), 2926.1 (C-H sym stretching), 1680.8 (C=O), 1580.5 (C=N stretching), 1541.3 (ring C.......C stretching), 1216.2 (C-N stretching), 1141.3 (C-O-C stretching), 1026.9 (N-N stretching), 580 cm-1 (C-Br stretching).1H NMR (DMSO)d: 2.64-2.91 (s, 2H, CH2), 3.24 (s, 1H, NH), 4.81 (d, 1H, COCH), 6.60 (d, 1H, CH-Ar), 7.60-7.99 (m, 4H, aromatic), 8.16-8.52 (m, 3H, aromatic), 10.18 ppm (s, 1H, O-H). |
| |
|
Compound 4:
|
| |
|
5-Bromo-2-{[5-{[(2E)-3-(3-chlorophenyl) prop-2-enoyl] amino}-1,3,4,-oxadiazol-2-yl) methyl] amino} benzoic acid
|
| |
| IR (KBr) ν: 3418.9 (O-H stretching), 3225.0 (N-H stretching), 3021.0 (aromatic C-H stretching), 2927.7 (C-H Sym stretching), 1697.0 (C=O stretching), 1570.0 (ring C......C stretching), 1598.3 (C=N stretching), 1216.0 (C-N stretching ), 1192.6 (C-O-C stretching), 1016.0 (N-N stretching), 570.6 cm-1 (C-Br stretching). |
| |
| 1H NMR (DMSO) δ: 2.64-2.91 (s, 2H, CH2), 3.34 (s, 1H, NH)., 4.81(d, 1H, COCH), 6.60 (d, 1H, CH-Ar), 7.60-7.99 (m, 4H, aromatic), 8.28-8.53 (m, 3H, aromatic), 10.28 ppm (s, 1H, O-H). |
| |
|
Compound 5:
|
| |
|
5-Bromo-N-{2'-amino-[1''-acetyl-5''-(2-nitrophenyl)-2''-pyrazolin-3''-yl]-1',3',4'-oxadiazol-5'-ylmethyl] anthranilic acid
|
| |
| IR (KBr) ν: 3516.4 (O-H stretching), 3216.5 (N-H stretching), 3020.1 (C-H stretching), 2926.5 (C-H stretching, CH2), 2877.7 (C-H stretching, CH3), 1726.5 (C=O stretching), 1603.9 (ring C.....C stretching), 1652.9 (C=N stretching), 1521.2 (N=O stretching), 1454.1 (N=O stretching) 1215.8 (C-N stretching), 1144.5 (C-O-C stretching), 928.7 (N-N stretching), 521.1 cm-1 (C-Br stretching). |
| |
| 1H NMR (DMSO) δ: 1.48 (s, 3H, COCH3), 2.50 (d, 2H, CH2), 2.89-3.50 ppm (s, 1H, NH), 5.41 (s, 2H, NCH2), 6.46 (t, 1H, CH-Ar), 6.89-8.56 (m, 7H, aromatic), 10.08 (O-H) ppm (s, 1H, O-H). |
| |
|
Compound 6:
|
| |
|
5-Bromo-N-{2'-amino-[1''-acetyl-5''-(3-nitrophenyl)-2''-pyrazolin-3''-yl]-1',3',4'-oxadiazol-5'-ylmethyl] anthranilic acid
|
| |
| IR (KBr) n: 3516.4 (O-H stretching), 3216.5 (N-H stretching), 3020.1 (C-H stretching), 2926.5 (C-H stretching, CH2), 2877.7 (C-H stretching, CH3), 1726.5 (C=O stretching), 1603.9 (ring C.....C stretching), 1652.9 (C=N stretching), 1521.2 (N=O stretching), 1454.1 (N=O stretching) 1215.8 (C-N stretching), 1144.5 (C-O-C stretching), 928.7 (N-N stretching), 521.1 cm-1 (C-Br stretching). |
| |
| 1H NMR (DMSO) δ: 1.48 (s, 3H, COCH3), 2.50 (d, 2H, CH2), 2.89-3.50 ppm (s, 1H, NH), 5.41 (s, 2H, NCH2), 6.46 (t, 1H, CH-Ar), 6.89-8.56 (m, 7H, aromatic), 10.08 (O-H) ppm (s, 1H, O-H). |
| |
|
Compound 7:
|
| |
|
5-Bromo-N-{2'-amino-[1''-acetyl-5''-(3-chlorophenyl)-2''-pyrazolin-3''-yl]-1',3',4'-oxadiazol-5'-ylmethyl] anthranilic acid
|
| |
| IR (KBr) n: 3418.9(O-H stretching), 3225.0 (N-H stretching), 3021.0 (aromatic C-H stretching), 2927.7 (C-H stretching, CH2), 2857.4 (C-H stretching, CH3), 1697.0 (C=O stretching), 1570.0 (ring C.....C stretching), 1598.3 (C=N stretching), 1216.0 (C-N stretching), 1192.6 (C-O-C stretching), 1016.0 (N-N stretching ), 570.6 cm-1 (C-Br stretching). |
| |
| 1H NMR (DMSO) δ: 1.23 (s, 3H, COCH3), 2.27 (d, 2H, CH2), 3.34 (s, 2H, N-CH2), 4.65-4.81 (s, 2H, NCH2 ), 5.31 ppm (s, 1H, NH), 6.24 (t, 1H, CH-Ar), 7.42-8.64 (m, 7H, aromatic), 10.10 ppm (s,1H,O-H). |
| |
|
Compound 8:
|
| |
|
5-Bromo-N-{2'-amino-[1''-acetyl-5''-(3-chlorophenyl)-2''-pyrazolin-3''-yl]-1',3',4'-oxadiazol-5'-ylmethyl] anthranilic acid
|
| |
| IR (KBr) ν: 3418.9(O-H stretching), 3225.0 (N-H stretching), 3021.0 (aromatic C-H stretching), 2927.7 (C-H stretching, CH2), 2857.4 (C-H stretching, CH3), 1697.0 (C=O stretching), 1570.0 (ring C C stretching), 1598.3 (C=N stretching), 1216.0 (C-N stretching), 1192.6 (C-O-C stretching), 1016.0 (N-N stretching ), 570.6 cm-1 (C-Br stretching). |
| |
| 1H NMR (DMSO) δ: 1.23 (s, 3H, COCH3), 2.27 (d, 2H, CH2), 3.34 (s, 2H, N-CH2), 4.65-4.81 (s, 2H, NCH2), 5.31 ppm (s, 1H, NH), 6.24 (t, 1H, CH-Ar), 7.42-8.64 (m, 7H, aromatic), 10.10 ppm (s,1H,O-H). |
| |
|
Anti-inflammatory screening of synthesized anthranilic acid derivatives
|
| |
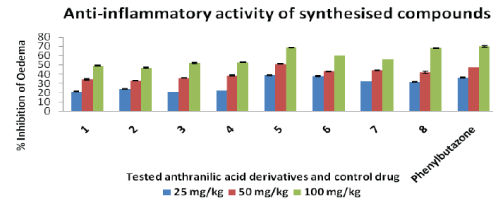
| Anti-inflammatory activity of the newly synthesized compounds was assayed by Winter et al., (1962) using carrageenan induced rat paw oedema method on adult rats (Wistar strain) of either sex weighing 200-300 mg. The rats were randomly divided into various groups, each consisted of a minimum of 6 animals The suspension of the newly synthesized compounds, uniformly dispersed in 2.0% Tween 80 were administered to test animals orally. The control groups received the same experimental handling as test groups in place of test compounds equivalent doses of vehicle alone were administered. Food and water were withdrawn during the test Phenylbutazone was used as the standard antiinflammatory drug. |
| |
|
Carrageenan-induced paw oedema method
|
| |
| Acute oedema were induced in the hind paw of rats by the injection of freshly prepared carragenan in distilled water (0.1 ml of freshly prepared, w/v carrageenan in distilled water). Oedema was determined immediately and 30, 60, 120 and 180 min after the injection using a mercury plethysmograph. Different doses of test compounds 3.5 mg/kg, 7.0 mg/kg and 14 mg/kg, respectively, and the control drug 7.0 mg/kg were administered 1 hr before the carragenan injection. The results are expressed as percentual inhibition of the oedema as compared to the control. The % inhibition of the inflammation after 3 h was calculated by using following formula: |
| |
| % Inhibition = 100 (a-x)/(b-y) |
| |
| Where ‘x’ and ‘a’ are the mean foot volume of the rats before and after the administration of carrageenan injection, respectively; in the rest of standard group ‘y’ and ‘b’ are the mean foot volumes of the rats before and after the administration of the carrageenan, respectively, in the control group. |
| |
 |
| |
RESULTS AND DISCUSSIONS
|
| |
| Anthranilic acid derivatives (1-8) were synthesized successfully with good purity and yield. TLC confirmed the purity of the title compounds. The structures of the newly synthesized compounds obtained have been confirmed on the basis of spectral (FTIR and 1H-NMR) data and elemental analysis. Of the synthesized compounds of the present series, compound 5 exhibited highest percentage inhibition of oedema and compound 6, 7 and 8 also shows the comparable inhibitory effect in comparison to the control drug Phenylbutazone. |
| |
CONCLUSION
|
| |
| The data obtained through screening of antiinflammatory activity of synthesized anthranilic acid derivatived revealed that addition of acidic functional five membered heterocyclic rings improved antiinflammatory effects. |
| |
Acknowledgements
|
| |
| Authors are grateful to the Mr. Sunil Kumar, Department of Chemistry, Delhi University (North Campus), India, for helping in FTIR & NMR spectral and analytical data. |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
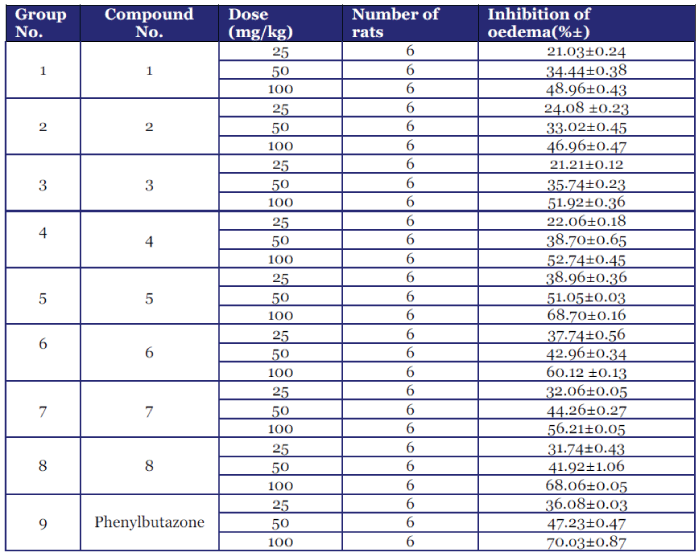 |
| Graph 1 |
|
| |









