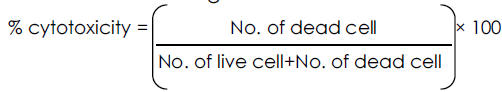Keywords
Chalcones, anticancer, antibacterial, antioxidant.
Introduction
The chalcones are α, β-Unsaturated ketones containing the reactive keto ethylene group -COCH= CH-, the presence of α,β-Unsaturated carbonyl system in chalcone makes it biologically active. Some substituted chalcones and their derivatives have been reported to exhibit a wide variety of biological properties such as anthelmintic [1], anti-microbial [2], antimycobacterial [4], antifungal [5], anticancer [6-9], anti-oxidant [10], and antiinflammatory [11] activity etc.
In the present work attention has been focused on the synthesis of chalcones from various aldehyde moieties and its derivatives. The structure of various synthesized compound was assigned on the basis of UV, IR, 1H-NMR, 13C-NMR, and Mass spectral data. The synthesized compounds were further screened for anticancer, antibacterial and antioxidant activity.
Material and Method
? Melting points of the synthesized compound was determined using Thiele’s melting point apparatus and was found uncorrected.
? Purity of the compounds was checked by thin layer chromatography using silicagelG in solvent system n-hexane-ethyl acetate (3:1) and the sport were located under iodine vapour and UV light.
? The UV spectra of the synthesized compounds were recorded on UV–Visible spectrophotometer (model Shimadzu 8700) using alcohol and the values of wave length (λ max) were reported in nm.
? IR spectra of all compounds were recorded on FTIR spectrometer (model Shimadzu 8700) in the range of 400 -4000 using KBr.
? 1H NMR spectra were recorded on Amx - 400 MHz NMR spectrometer using CDCl3 and chemical shifts (δ) are reported in parts per million downfield using Tetramethylsilane (TMS).
? 13C NMR (400 MHz) spectra were recorded in deuterated CDCl3 in Amx-400 liquid state NMR spectrometer .Chemical shifts (δ) are reported in parts per million.
? Mass spectra were recorded on Mass spectrophotometer (model Shimadzu) by MS
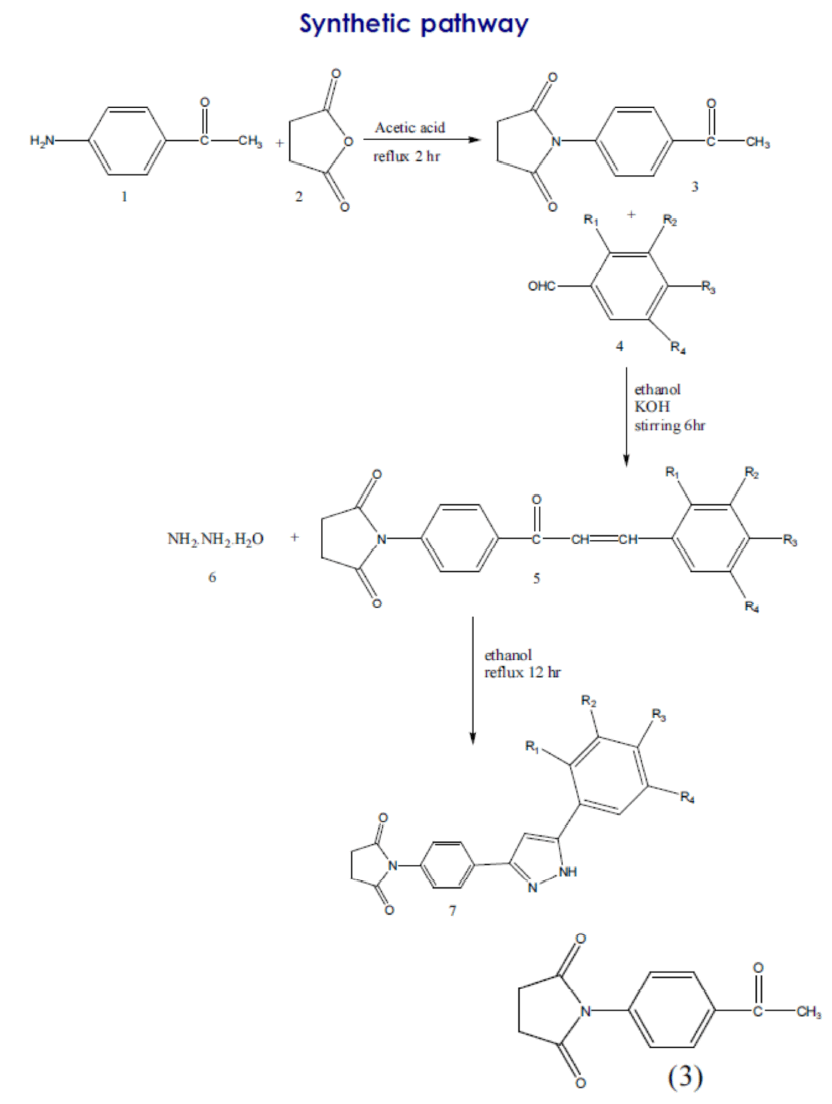
Procedure for the preparation of 1-(4-Acetylphenyl)- pyrrolidine-2, 5-dione
4-Aminoacetophenone (0.01M), succinic anhydride (0.01M) and acetic acid (40 ml) was added in round bottom flask, refluxed for 2 hr and kept overnight, filtered and recrystalized from ethanol. The elution was done with nhexane: ethyl acetate (4: 1) crystallized from ethanol as white colour, yield 52 %, m.p 129- 131°C, Rf- 0.6 (structure-3)
Procedure for the preparation of 1-(4(3- Substituted phenyl) acryloyl)phenyl} pyrrolidine- 2, 5-dione
A mixture of 1-(4-acetyl-phenyl)-pyrrolidine-2, 5- dione (0.01M) and aryl aldehyde (0.01M) was stirred in ethanol (40 ml) and an aqueous solution of KOH (40%, 15 ml) was added to it. The stirring was continued for 6 hr and the mixture was kept overnight at room temperature and it was then poured into crushed ice and acidified with HCl. The solid separated was filtered and recrystallized from ethanol. (Table-1)
Procedure for the preparation of 1-{4[5- substitutedphenyl)-1H-pyrazol-3yl] phenyl} Pyrrolidine-2, 5 Dione.
A mixture of chalcone (0.01M), hydrazine hydrates (0.01M) and ethanol 25ml was refluxed for 12 hr .The mixture was concentrated by distilling out of the solvent under reduced pressure and poured into ice water. The precipitate obtained was filtered, washed and recrystalized with ethanol.
| Compound Code |
R1 |
R2 |
R3 |
R4 |
Molecular formula |
Molecular weight |
M.P°C |
% of yield |
| 1a |
OCH3 |
|
OCH3 |
|
C21H19NO5 |
365 |
147-148 |
62 |
| 1b |
|
OCH3 |
OCH3 |
OCH3 |
C22H21NO6 |
395 |
161-163 |
91.4 |
| 1c |
|
NO2 |
|
|
C19H14N2O5 |
350 |
158-160 |
78.2 |
| 1d |
Cl |
|
|
|
C19H14O3NCl |
339 |
137-138 |
95 |
| 1e |
OH |
|
|
OCH3 |
C20H17NO5 |
351 |
142-144 |
92 |
| 1f |
|
|
NO2 |
|
C19H14N2O5 |
350 |
132-134 |
92 |
| 1g |
|
|
OH |
|
C19H15NO4 |
321 |
162-164 |
91 |
| 1h |
|
|
OC2H5 |
|
C21H19NO4 |
349 |
132-134 |
85 |
| 1i |
NO2 |
|
|
|
C19H14N2O5 |
350 |
96-98 |
75 |
| 1j |
Cl |
|
Cl |
|
C19H13O3NCl2 |
350 |
118-120 |
85 |
| 1k |
N(C2H5)2 |
|
|
|
C19H16N2O3 |
320 |
95-98 |
79 |
Table 1: Physiochemical parameters of 1-(4(3-Substituted phenyl) acryloyl)phenyl} pyrrolidine-2, 5 dione
| Compound Code |
R1 |
R2 |
R3 |
R4 |
Molecular formula |
Molecular weight |
M.P°C |
% of yield |
| 2a |
OCH3 |
|
OCH3 |
|
C21H19NO4 |
377 |
110-112 |
77.7 |
| 2b |
|
OCH3 |
OCH3 |
OCH3 |
C22H21N3O5 |
407 |
110-112 |
21.1 |
| 2c |
|
NO2 |
|
|
C19H14N4O4 |
362 |
110-112 |
81.2 |
| 2d |
Cl |
|
|
|
C19H14N3O2Cl |
361 |
115-120 |
32.3 |
| 2e |
OH |
|
|
OCH3 |
C20H17N3O4 |
363 |
118-120 |
33.3 |
| 2f |
|
|
OC2H5 |
|
C21H19N3O3 |
361 |
45-48 |
27 |
Table 2: Physiochemical parameters of Procedure of 1-{4[5-substitutedphenyl)-1H-pyrazol-3yl] phenyl} Pyrrolidine-2, 5 Dione.
| Compound code |
λmax
(nm) |
Mass m/e |
IR (KBr)
Vmax cm-1 |
1H NMR δ ppm |
13C NMR |
| 1a |
365 |
|
3002( Ar C-H)
2927(Ali C-H)
1602(C=C)
1307(OCH3) |
δ 7.5-8.13 4H, (m, 2H of ArH + 2H of CH=CH) ;
δ 6.40-6.7 3H (m, ArH of
dimethoxy benzene) ; δ 3.84-3.88 6H
(s,2xOCH3) ; δ 1.8 4H (s,
2xCH2 of succinimide) |
|
| 1b |
329 |
|
3100 (Ar C-H)
2935(Ali C-H)
1589(C=C)
1280 (OCH3 )
1178 (C-N) |
δ 7.94 1H (s, CH=CH) ; δ
7.95 1H (s, CO CH=CH)
;δ 7.2-7.82 4H (m ,ArH) ;δ 6.60-6.85 2H (m, ArH of
trimethoxy benzene) ;δ 3.8-3.9 9H (s, 3xOCH3); δ
1.8 4H (s, 2xCH2 of succinimide) |
|
| 1d |
317 |
395(M+2) |
3059 (Ar C-H)
2923 (AliC-H)
1649 (C=O)
1608 (C=C)
1178 (C-N) |
δ 8.17 1H (s, CH=CH); δ
8.09 1H (s, CO CH=CH); δ 6.62-7.95 8H (m, ArH); δ 1.21-1.90 4H (s, 2xCH2 of succinimide). |
188.36 (C=Oenone), 151.56
(C=O,succinimide), 139.36 (Ci),
135.66, 131.61, 134.15,125.36 [(Ca),
(Cc) and (Ce), (Cd), (Cb) and (Cf) respectively of C6H4- succinimide].128. 13(Ch), 131.6,
131.07, 130.6, 128.74, 127.35 [(Ca’),
(Cb’), (Cc’), (Cd’ and Ce’) Cf’
respectively of o-Chloro C6H3] |
Table 3: Spectral characteristics of 1-(4(3-Substituted phenyl) acryloyl)phenyl} pyrrolidine-2,5-dione.
| Compound code |
λmax (nm) |
IR (KBr) Vmax cm-1 |
| 2a |
314 |
3358(N-H)
1596(C=N) |
| 2b |
327 |
3361(N-H)
1600(C=N) |
| 2c |
327 |
3357(N-H)
1591(C=N) |
| 2d |
382 |
3342(N-H)
1612(C=N) |
| 2e |
522 |
3213(N-H)
1599(C=N) |
Table 4: Spectral characteristics of 1-{4[5-substitutedphenyl)-1H- pyrazol3yl] phenyl} Pyrrolidine-2, 5-Dione.
Biological activity
1. Anticancer studies [6-9]:
? Method: Trypan blue exclusion method(Dalton lymphoma ascities)
? Animal used: Tumor bearing mice
? Chemical used: Phosphate buffered saline
Method
? Cells were aspirated from the peritoneal cavity of tumor bearing mice.
? The cells were washed three times using PBS.
? The viability of the cells were checked using trypan blue (cell viability should be above 98%)
? The number of cells were counted using haemocytometer and after approximate dilution cell number adjusted to 1×10-7 cell/ml
? The experiment was setup by incubating different concentration of the drug with 1×106 cells
? Final volume of the assay mixture was made upto 1ml using PBS and incubated at 37°C for about 3 hours.
? 0.1ml of trypan blue was added after incubation and number of dead cell was counted using haemocytometer.
The number of stained and unstained cells was counted separately and percentage cell death was calculated using the formula:
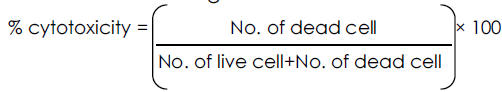
| Drug conc |
Percent cell Death (DLA) |
| µg/ml |
1a |
1b |
1d |
1h |
2a |
2b |
2e |
| 200 µg |
57% |
9% |
15% |
2% |
27% |
21% |
84% |
| 100 µg |
39% |
6% |
10% |
42% |
14% |
14% |
75% |
| 50 µg |
25% |
2% |
7% |
34% |
8% |
5% |
60% |
| 20 µg |
15% |
0 |
5% |
20% |
5% |
2% |
48% |
| 10 µg |
5% |
0 |
0 |
8% |
2% |
0 |
33% |
Table 5: Anticancer activity of chalcone by using DLA method
| Compound code |
Zone of inhibition (in mm) |
| Staphylococcus aureus |
Bacillus subtilis |
Streptococcus pneumonia |
Salmonella typhi |
| Ampicillin |
39 |
40 |
38 |
40 |
| 1a |
29 |
32 |
28 |
38 |
| 1b |
33 |
36 |
31 |
34 |
| 1c |
32 |
34 |
30 |
33 |
| 1d |
36 |
38 |
32 |
36 |
| 1h |
- |
18 |
13 |
- |
| 2a |
- |
15 |
13 |
- |
| 2b |
31 |
30 |
32 |
30 |
| 2c |
25 |
19 |
17 |
13 |
Table 6: in-vitro antibacterial activity of chalcones determined by agar diffusion method
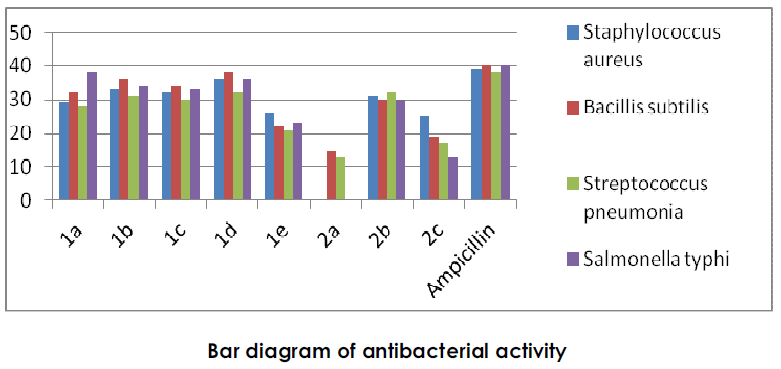
2. Antibacterial activity [2]:
The synthesised compound were screened for their antibacterial activity against two gram positive bacterial strains B.subtilis( NCIM 2697), S.aureus( NCIM 2079) and two gram negative bacterial strains S.pneumonia( NCIM 5082), S.typhi(NCIM 2263) by using cup plate method.The zone of inhibition was measured in mm, under similar condition the controlled experiment was carried out using antibiotics( Ampicillin) as a standard drug for comparison.
3. Antioxidant activity [10]:
All the synthesized compounds were evaluated for their in-vitro free radical scavenging activity by DPPH (2, 2-diphenyl-1-picryl hydrazyl) reduction method using ascorbic acid as the standard.
Result and Discussion
? Structures of synthesized compounds was confirmed and characterized with the help of analytical data such as FTIR, mass spectroscopy, 1H NMR and C13 NMR.
? The synthesized compound 1a, 1h and 2d have shown good anti-cancer activity at concentration 100 μg/ml and 200 μg/ml. The compound 2a has shown moderate activity at concentration 200 μg/ml. The compound 1b, 1d and 2b did not exhibit prominent activity.
? Compound 1a, 1b, 1c, 1d and 2d exhibited good antibacterial activity at 100 μg/ ml. 1e, and 2c shows moderate activity while 1f, 1g, and 1i, exhibhited less antibacterial activity.
? 1a, 1b, 1c, 1d, 1e, 2a and 2d exhibited significant antioxidant activity with maximum inhibition at 40 μg/ml. 1g, 1j, 2b and 2c exhibited moderate antioxidant activity whereas 1h exhibited very low activity at 40 μg/ ml.
Acknowledgement
Authors are thankful to Prof. B.G. Shivananda, Principal, Al-Ameen College of Pharmacy, for providing all the facilities and timely help during the conduct of project in the college.
7285
References
- Das BC, Mariappan G, Saha S, Bhomik D, Chiranjib. Anthelmintic and anti-microbial activity of some novel chalcone derivatives. J Chem Pharm.Res.2010; 2(1):113-120
- Kamboj RC, Arora R, Sharma G, Kumar D, Sharma C, Joshi R, et al. Eco-friendly synthesis and antimicrobial activity of chalcones. Der Pharma Chemica.2010; 2(3):157-170
- Prasad YR, Kumar PR, Deepti CA, Venkatramana M. Synthesis and antimicrobial activity of some novel chalcones of 2-hydroxy-1- acetonapthone and 3-acetyl coumarin. E J Chem. 2006; 3(13):236-241.
- Trivedi AR,Dodiya DK,Ravat NR,Shah VH. Synthesis and biological evaluation of some new pyrimidines via a novel chalcones series. ARKIVOC. 2008; (xi):131-141
- Tiwari B, Pratapwar AS, Tapas AR, Butle SR, Vatkar BS. Synthesis and antimicrobial activity of some chalcone derivatives. Int J ChemTech Res.2010; 2(1):499-503.
- Shylesh BS, Nair SA, Subramoniam A. Induction of cell-specific apoptosis and protection from Dalton’s lymphoma challenge in mice by an active fraction from Emilia sonchifolia.Indian J Pharmacol.2005; 37(4):232-237
- Preethi KL, Kuttan G, Kuttan R. Antitumour activity of Ruta graveolens extract.Asian Pacific J Cancer Prev. 2006; 7:439-443
- Sashidhara KV, Kumar A, Sarkar J, Sinha S. synthesis and invitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg and Med Che Lett .2010; 20(24):7205-7211
- Radha R, Kaviman S, Ravichandra V. Antitumour activity of methanolic extract of Plumeria alba leaves against Dalton lymphoma ascites in mice. Int J Health Res.2008; 1(2): 79-85
- Ahmed MR, Sastry VG,Bano N, Ravichandra S, Raghavendra M. Synthesis and cytotoxic, antioxidant activities of new chalcone derivatives. J Chem 2011; 4(2): 289-294
- Tiwari B, Pratapwar AS, Tapas AR, Butle SR, Vatkar BS. Synthesis and antimicrobial activity of some chalcone derivatives. Int J ChemTech Res.2010; 2(1):499-503.