Khan Mohemmed Faraz1, Verma Garima1, Akhtar Wasim1, Marella Akranth2, Alam Mohammad Mumtaz1, Akhter Mymoona1, Husain Asif1, Hasan Syed Misbahul3, Shaquiquzzaman Mohammad1* and Haider Syed Rashiduddin4*
1Department of Pharmaceutical Chemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, India
2Pharmalex India Pvt. Ltd., Sarita Vihar, India
3Faculty of Pharmacy, Integral University, India
4Department of Chemistry, Oriental College, Patna, India
*Corresponding Authors:
Shaquiquzzaman M
Department of Pharmaceutical Chemistry
School of Pharmaceutical Education
and Research, Jamia Hamdard, India
Tel: 9990663314
E-mail: shaqiq@gmail.com
Rashiduddin H
Department of Chemistry
Oriental College, Patna, Bihar, India
Tel: +91-9990663405
E-mail: aldol1234@gmail.com
Received Date: March 17, 2017 Accepted Date: April 28, 2017 Published Date: May 02, 2017
Keywords
Triazole; Synthesis; 1,2,3-Triazole
Introduction
Triazoles, five-membered heterocyclic compounds, with molecular formula C2H3N3, bearing three nitrogen atoms in the ring exist in two isomeric forms namely 1,2,3-triazoles and 1,2,4-triazoles [1,2] (Figure 1). Due to higher aromatic stabilization of 1,2,3-triazoles, they are resistant to oxidation, reduction, and hydrolysis in both acidic ad basic conditions. Their active participation in hydrogen bond formation, dipole-dipole and pi stacking interactions enhance their binding ability with different biological targets [3].

Figure 1: Isomers of triazole (a) 1,2,3-triazole (b) 1,2,3-triazole.
Over the past decade, scientists across the globe have shown substantial interest in the synthesis of 1,2,3-triazole units [4]. This moiety can be found in a number of pharmaceutical agents as shown in Figure 2. Tazobactam, a α-lactamase inhibitor, contains this moiety [5]. Cefatriazine, an orally active semisynthetic cephalosporin antibiotic [6] and rufinamide, an anticonvulsant agent bear 1,2,3-triazole [7]. This moiety finds several applications in the medical field such as antitubercular [8], antibacterial, antifungal [9], anticancer, antioxidant [10], antimalarial [11], antidiabetic [12] etc.
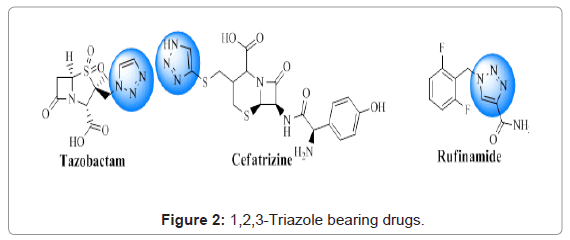
Figure 2: 1,2,3-Triazole bearing drugs.
Huisgen was the very first one to study the synthesis of 1,2,3-triazoles, naming it as Huisgen 1,3-dipolar cycloaddition. This reaction mechanism involves reaction of an alkyne with an azide to produce 1,4- and 1,5-disubstituted-triazole derivatives [13]. Further, in order to regioselectivity, different methodologies were developed. It was seen that copper catalyzed cycloaddition reaction led to the formation of 1,4-regioisomer whereas, 1,5-regiomer was formed in the presence of ruthenium. Products obtained from all these conditions have been shown in Figure 3 [14-16]. However, application of Cu-AAC is most commonly used employed in the synthesis as evident from the literature [17,18].
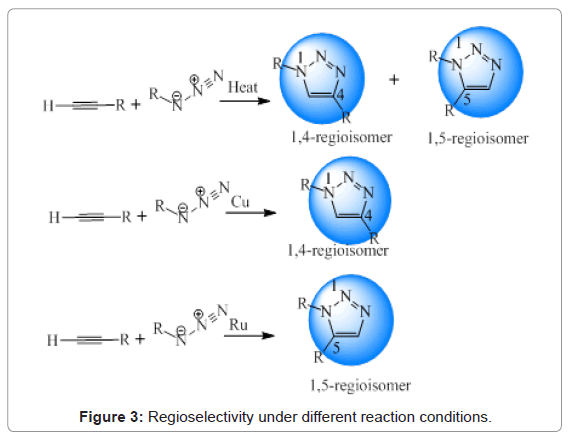
Figure 3: Regioselectivity under different reaction conditions.
Methodology for Synthesis
Synthesis using metals
Copper catalyzed 1,3-dipolar cycloaddition: Copper caltalyzed 1,3-dipolar cycloaddition reaction between an alkyne and azide (Cu- AAC) is the most commonly employed method for the synthesis of 1,2,3-triazoles [19,20]. General conditions for cycloaddition include the presence of Cu(I) or Cu(II) salts along with a reducing agent in some organic solvent or a mixture of water and tert-butyl alcohol at room temperature [21,22]. Zheng and Shi reported a Cu-catalyzed route taking N-tosylhydrazones and azides as the substrates [23]. Chen and co-workers have also demonstrated 1,2,3-triazoles synthesis from N-tosylhydrazones and anilines [24]. An overview of these routes is provided in Figure 4.
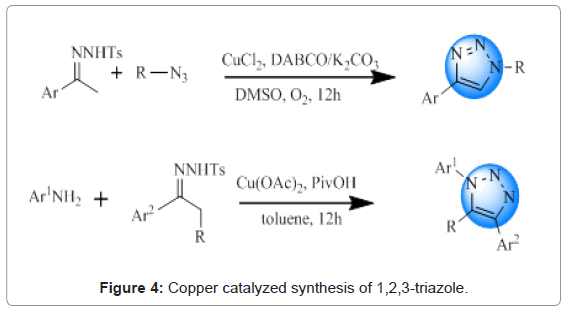
Figure 4: Copper catalyzed synthesis of 1,2,3-triazole.
Ruthenium catalyzed reactions: Ruthenium catalysts are most widely employed for the preparation of 1,5-disubstituted triazoles from azide and alkyne. However, this method suffers from the drawback that this is not efficient in case of sterically demanding substrates, leading to the formation of by-products. Ferrini et al. have reported rutheniumcatalyzed synthesis of 5-amino-1,2,3-triazoles [25]. Ruthenium(II) carboxylate complexes were used for efficient cross-dehydrogenative alkenylations of N-aryl-1,2,3-triazoles [26]. These routes have been shown in Figure 5.
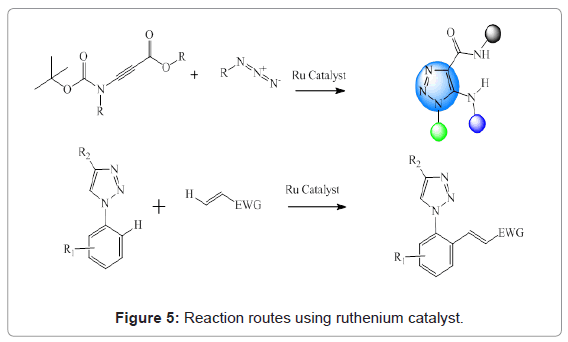
Figure 5: Reaction routes using ruthenium catalyst.
Lithium catalyzed reactions: Meza-Avina et al. reported the reaction between acetylides and sulfonyl azides for the formation of selective 1,5-substituted sulfonyl triazoles. This sort of reaction provided regioisomeric product in comparison to the conventional copper-catalysed azide-alkyne cycloaddition [27]. Overview of this reaction is given in Figures 6 and 7.
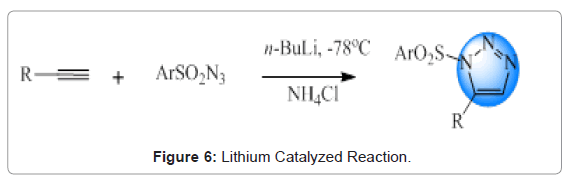
Figure 6: Lithium Catalyzed Reaction.
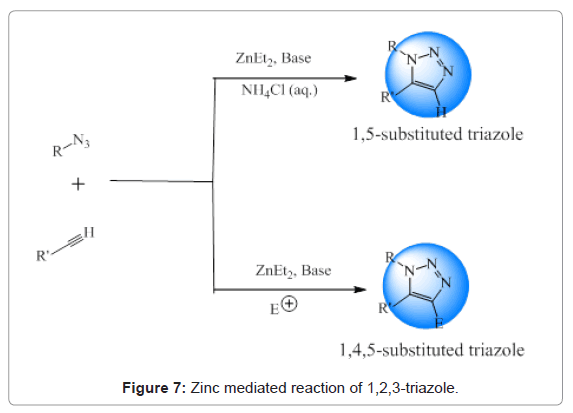
Figure 7: Zinc mediated reaction of 1,2,3-triazole.
Zinc mediated synthesis: Smith and Greaney performed zinc mediated ligation of azide-alkyne to form 1,5- and 1,4,5-substituted 1,2,3-triazoles. Reactions were carried out at room temperature [28].
Metal-less reactions
In an attempt to develop method for synthesis of 1,2,3-triazoles without using metals, they synthesized 1,5-disubstituted 1,2,3-triazoles using primary amines, enolizable ketones and 4-nitrophenyl azide [29]. Kwok et al. adopted a synthetic route for synthesis of 1,5-diarylsubstituted 1,2,3-triazoles from azides and terminal alkynes in DMSO in the presence of catalytic tetraalkylammonium hydroxide [30]. Bonacorso and co-workers reported synthesis of antiepileptic drug, rufinamide in the absence of any solvent, metal catalyst and reducing agent. Desired product was obtained in good yields [31]. Singh et al. developed a metal free route for development of 1,2,3-triazoles. [3+2] cycloaddition of aryl azides with activated cyclic C-H acids was brought about in the presence of DBU [32]. Jia et al. carried out 1,3-dipolar cycloaddition of commercially available aldehydes with azides and secondary amines in the absence of metal catalyst [33]. Overview of all routes is given in Figure 8.
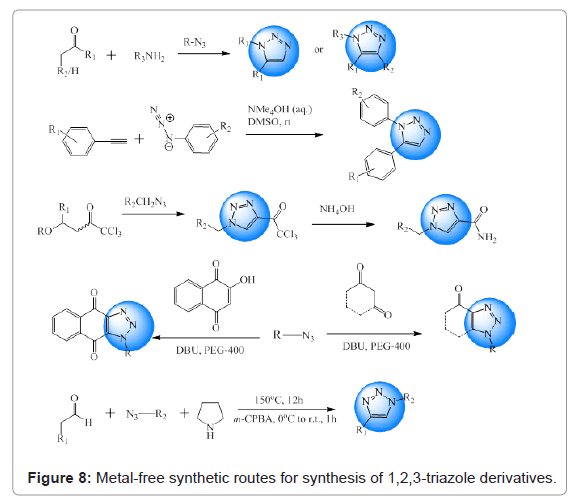
Figure 8: Metal-free synthetic routes for synthesis of 1,2,3-triazole derivatives.
Ultrasound-assisted synthesis
Silva et al. reported synthesis of twelve isatin derivatives in the presence of different alkynes and ultrasound irradiation. Better yields and less time consumption were advantages over the conventional methods [34]. Triazole derivatives were obtained via 1,3-dipolar cycloaddition reaction between 2-azido-N-(benzo[d]thiazol-2-yl) acetamide derivatives and different alkynes in the presence of ultrasound radiation. Reaction was carried out in solvent system comprising of t-BuOH/H2O (1:1 v/v) with CuSO4.5H2O as the catalyst [35]. 1,2,3-triazoles as PDE4 inhibitors were prepared by CuAAC method under ultrasound irradiation at room temperature [36]. CuAAC was employed to catalyze the reaction to obtain a series of 1,2,3-triazoles in benign solvents under ultrasound irradiation. Sonication served the benefits of reduced reaction time and better yields [37]. Zhang and coworkers reported an efficient synthesis of 1,2,3-triazole derivatives via 1,3-dipolar cycloaddition using copper acetate and sodium ascorbate as catalyst under ultrasonic radiation [38]. Overview of these reactions is given in Figure 9.
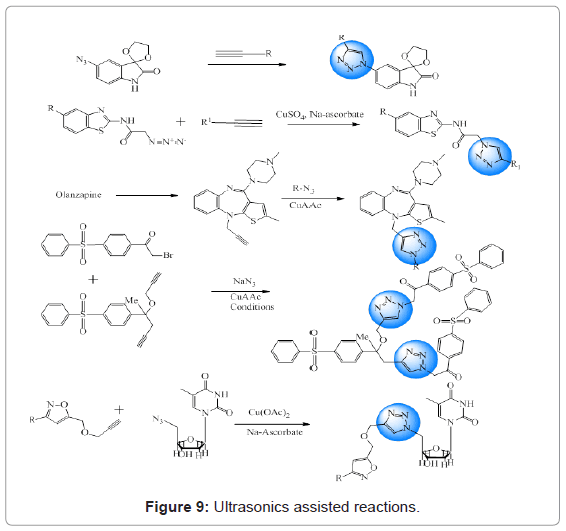
Figure 9: Ultrasonics assisted reactions.
Microwave assisted reactions
Microwave assisted synthesis of triazoles is well studied. Microwave irradiation allows efficient internal heat transfer, which reduces the reaction timing as well as elevates the reaction rate and yield [39]. Costa et al. reported microwave assisted synthesis of 1,2,3-trizole derivatives via 1,3-dipolar cycloaddition as anticancer agents. Souza and coworkers also synthesized novel triazoles based on a microwave-assisted multicomponent reaction [40]. Such reactions have been shown in Figure 10.
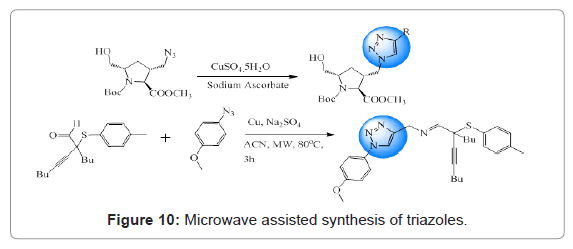
Figure 10: Microwave assisted synthesis of triazoles.
Conclusion
1,2,3-triazoles find their significant place in the field of drug discovery and development. This drives the interest of different scientists for development of novel methods for synthesis of 1,2,3-triazole derivatives. Different conventional methods, i.e., with metal or without metal employed in this way have been reported. A few alternative methods like ultrasonic or microwave irradiation have also been included.
19524
References
- Siddiqui N, Ahsan W, Alam MS, Ali R, Jain S, et al. (2011) Triazoles: as potential bioactive agents. Int J Pharm Sci Rev Res 8: 161-169.
- Shneine JK, Alaraji YH (2016) Chemistry of 1,2,4-traizole: a review article. Int J Sci Res 5: 1411-1423.
- Singhal N, Sharma PK, Dudhe R, Kumar N (2011) Recent advancements of traiazole derivatives and their biological significance. J Chem Pharm Res 3: 126-133.
- Totobenazara J, Burke AJ (2015) New click chemistry methods for 1,2,3-triazoles synthesis: recent advances and applications. Tetrahedron Lett 56: 2853-2859.
- Rodriguez CA, Agudelo M, Zuluga AF, Vesga O (2017) In vivo pharmacodynamics of piperacillin/tazobactam: implications for antimicrobial efficacy and resistance suppression with innovator and generic products. Int J Antimicrob Agents 49: 189-197.
- Choi HG, Jun HW, Kim DD, Sah H, Yoo BK, et al. (2004) Simultaneous determination of cefatrizine and clavulanic acid in dog plasma by HPLC. J Pharm Biomed Anal 35: 221-231.
- Kothare S, Kluger G, Sachdeo R, Williams B, Olhaye O, et al. (2017) Dosing considerations for rufinamide in patients with Lennox-Gastuat syndrome: phase III trial results and the real world clinical data. Seizure 47: 25-33.
- Menendez C, Gau S, Lherbet C, Rodriguez F, Inard C, et al. (2011) Synthesis and biological activities of triazole derivatives as inhibitors of InhA and antituberculosis agents. Eur J Med Chem 46: 5524-5531.
- Wang Q, Zhang J, Damu GLV, Wan K, Zhang HZ, et al. (2012) Synthesis and biological activities of thio-triazole derivatives as novel antibacterial and antifungal agents. Sci China Chem 55: 2134-2153.
- Nadeem H, Mohsin M, Afzaal H, Riaz S, Zahid A, et al. (2013) Synthesis and in vitro biological activities of 4,5-disubstituted 1,2,4-triazol-3-thols. Adv Microbiol 3: 366-375.
- Asif M (2014) A mini review on antimalarial activities of biologically active substituted triazole derivatives. Int J Adv Res Chem Sci 1: 22-28.
- Ferreira SB, Sodero ACR, Cardoso MFC, Lima ESL, Kaiser CR, et al. (2010) Synthesis, biological activity, and molecular modeling studies of 1H-1,2,3-triazole derivatives of carbohydrates as α-glucosidases inhibitors. J Med Chem 53: 2364-2375.
- Meldal M, Tornoe CW (2008) Cu-catalyzed azide-alkyne cycloaddition. Chem Rev 108: 2952-3015.
- Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67: 3057-3064.
- Rasmussen LK, Boren BC, Fokin VV (2007) Ruthenium-catalyzed cycloaddition of aryl azides and alkynes. Org Lett 9: 5337-5339.
- Zhang L, Chen X, Xue P, Sun HHY, Williams ID, et al. (2005) Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J Am Chem Soc 127: 15998-15999.
- Goyard D, Praly JP, Vidal S (2012) Synthesis of 5-halogenated 1,2,3-triazoles under stoichiometric Cu(I)-mediated azide-alkyne cycloaddition (CuAAC or Click Chemistry). Carb Res 362: 79-83.
- Li L, Zhang Z (2016) Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CUAAC) as a bioorthogonal reaction. Molecules 21: 1-22.
- Lipeeva AV, Pokrovsky MA, Baev DS, Shakirov MM, Bagryanskayan IY, et al. (2015) Synthesis of 1H-1,2,3-triazole linked aryl(arylamidomethyl)-dihydrofurocoumarin hybrids and analysis for their cytotoxicity. Eur J Med Chem 100: 119-128.
- Wang B, Ahmed MN, Zhang J, Chen W, Wang X, et al. (2013) Easy preparation of 1,4,5-trisubstituted 5-(2-alkoxy-1,2-dioxoethyl)-1,2,3-triazoles by chemoselective trapping of copper(I)-carbon bond with alkoxalyl chloride. Tetrahedron Lett 54: 6097-6100.
- Kaushik CP, Kumar K, Singh SK, Singh D, Saini S (2016) Synthesis and antimicrobial evaluation of 1,4-disubstitued 1,2,3-triazoles with aromatic ester functionality. Arabian J Chem 9: 865-871.
- Zao F, Chen Z, Xie K, Yang R, Jiang YB (2016) One pot synthesis of 1,4-disubstituted triazoles from nitrobenzenes. Chinese Chem Lett 27: 109-113.
- Zheng Z, Shi L (2016) An efficient regioselective copper-catalyzed approach to the synthesis of 1,2,3-triazoles from N-tosylhydrazones and azides. Tetrahedron Lett 57: 5132-5134.
- Chen Z, Yan Q, Liu Z, Xu Y, Zhang Y (2013) Copper-mediated synthesis of 1,2,3-triazoles from N-Tosylhydraznes and anilines. Angewandte Comm 52: 13324-13328.
- Ferrini S, Chandanshive JZ, Lena S, Franchini MC, Giannini G, et al. (2015) Ruthenium-catalyzed synthesis of 5-Amino-1,2,3-triazole-4-carboxylates for triazole-based scaffolds: beyond the Dimorth rearrangement. J Org Chem 80: 2562-2572.
- Tirler C, Ackermann L (2015) Ruthenium(II)-catalyzed cross-dehydrogenative C-H alkenylations by triazole assistance. Tetrahedron 71: 4543-4551.
- Avina MEM, Patel MK, Lee CB, Dietz TJ, Croatt MP (2011) Selective formation of 1,5-substituted sulfonyl triazoles using acetylides and sulfonyl azides. Organic Lett 13: 2984-2987.
- Smith CD, Greaney MF (2013) Zinc mediated azide-alkyne ligation to 1,5- and 1,4,5-substitued 1,2,3-triazoles. Organic Lett 15: 4826-4829.
- Thomas J, Jana S, John J, Liekens S, Dehaen W (2016) A general metal-free route towards the synthesis of 1,2,3-triazoles from readily available primary amines and ketones. Chem Comm 52: 2885-2888.
- Kwok SW, Fotsing JR, Fraser RJ, Rodionov VO, Fokin VV (2010) Transition-metal free catalytic synthesis of 1,5-diaryl-1,2,3-riazoles. Org Lett 12: 4217-4219.
- Bonacorso HG, Moraes MC, Luz FM, Quintana PS, Zanatta N, et al. (2015) New solventless and metal-free synthesis of the antiepileptic drug, 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide (Rufinamide) and analogues. Tetrahedron Lett 56: 441-444.
- Singh H, Khanna G, Khurana JM (2016) DBU catalyzed metal free synthesis of fused 1,2,3-triazoles through [3+2] cycloaddition of aryl azides with activated cyclic C-H acids. Tetrahedron Lett 57: 3075-3080.
- Jia Q, Yang G, Chen L, Du Z, Wei J, et al. (2015) A facile one-pot metal-free synthesis of 1,4-disubstituted 1,2,3-triazoles. Eur J Org Chem 2015: 3435-3440.
- Silva BNM, Pinto AC, Silva FC, Ferreira VF, Silva BV (2016) Ultrasound-assisted synthesis of isatin-type 5’-(4-alkyl/aryl-1H-1,2,3-triazoles) via 1,3-dipolar cycloaddition reactions. J Braz Chem Soc 27: 2378-2382.
- Rezki N (2016) A green ultrasound synthesis, characterization and antibacterial evaluation of 1,4-disubstituted 1,2,3-triazoles tethering bioactive benzothiazole nucleus. Molecules 21: 1-13.
- Nallapati SB, Sreenivas BY, Bankala R, Parsa KVL, Sripelly S, et al. 1,2,3-triazoles derived from olanzapine: their synthesis via an ultrasound assisted CuAAC method and evaluation as inhibitors of PDE4B. RSC Adv 5: 94623-94628.
- Mady MF, Awad GEA, Jorgensen KB (2014) Ultrasound-assisted synthesis of novel 1,2,3-triazoles coupled diaryl sulfone moieties by the CuAAC reaction, and biological evaluation of them as antioxidant and antimicrobial agents. Eur J Med Chem 84: 433-443.
- Zhang D, Zhang Y, Li J, Zhao T, Gu Q, et al. (2017) Ultrasonic-assisted synthesis of 1,4-disubstituted 1,2,3-triazoles via various terminal acetylenes and azide and their quorum sensing inhibition. Ultrasonics Sonochem 36: 343-353.
- Costa JF, Mera XG, Caamano O, Brea JM, Loza MI (2015) Synthesis by microwave-assisted 1,3-dipoalr cycloaddition of 1,2,3-triazole 1’-homo-3’-isoazanucleosides and evaluation of their anticancer activity. Eur J Med Chem 98: 212-220.
- Souza FB, Pimenta DC, Stefani HA (2016) Microwave-assisted one-pot three-component synthesis of imine 1,2,3-triazoles. Tetrahedron Lett 57: 1592-1596.
















