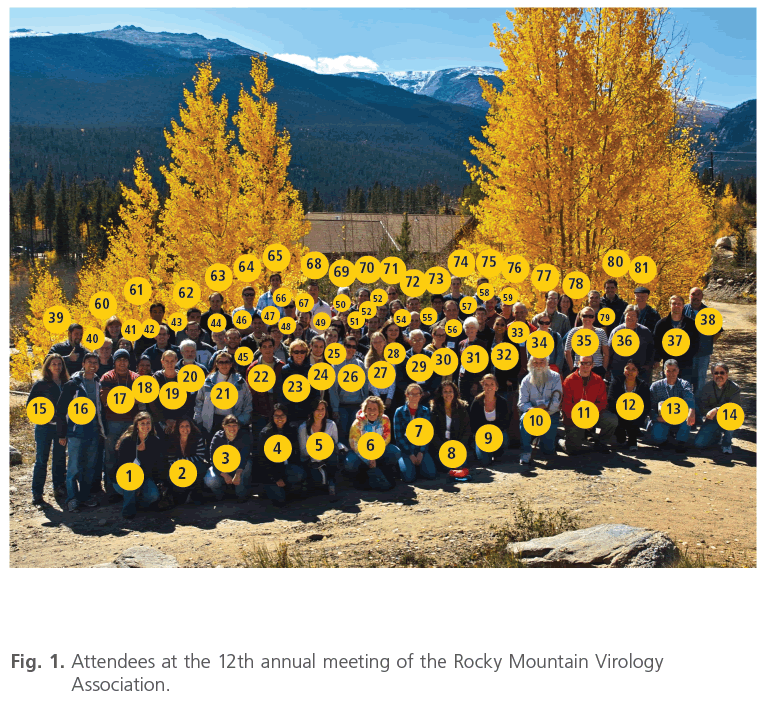
Figure 1: Attendees at the 12th annual meeting of the Rocky Mountain Virology Association.
1, Courtney Springer; 2, Grace Campagnola; 3, Amber Rico; 4, Nunya Chotiwan; 5, Ashlynne Goodloe; 6, Kassandra Willingham; 7, Danielle Adney; 8, Aimee Ortega; 9, Dana Hill; 10, Randall Cohrs; 11, Mark Zabel; 12, Maria Nagel; 13, Luis Rodriguez; 14, Joel Rovnak; 15, Sue VandeWoude; 16, Yonghua Zhuang; 17, Eamon Quick; 18, Rushika Perera; 19, Laura Griffin; 20, Ken Olson; 21, Irma Sanchez-Vargas; 22, Martin Lapel; 23, Penny Clarke; 24, Nelly Khmeleva; 25, Katherine Shives; 26, Daphne Cooper; 27, Claire Birkenheuer; 28, Natalia Voge; 29, Charlie Calisher; 30, Stephanie Moon; 31, Carol Blair ; 32, Phillida Charley; 33, Jeff Wilusz; 34, Heather Bender; 35, Christy Wyckoff; 36, Kathryn Holmes; 37, Justin Lee; 38, Tony Schountz; 39, Craig Miller; 40, Brian Geiss; 41, Sahaja Templin-Hladky; 42, Tem Morrison; 43, Trevor Blain; 44, Kendra Jones; 45, Nathan Grubaugh; 46, Louis Cicchini; 47, Forrest Kim; 48, Amy Stone; 49, Toni Schwarz; 50, Crystal Meyerett-Reid; 51, Abhishek Prasad; 52, Daniel Haden; 53, Eric Ross; 54, Carla Calvi; 55, Michael Barnhart; 56, Mark Burgoon; 57, David Hawman; 58, Aaron Phillips; 59, Olve Peersen; 60, Martha MacMillan; 61, Jifeng Bian; 62, A. Nonymous; 63, Ryan Troyer; 64, Brady Michel; 65, Ian Hoskins; 66, John Kriesel; 67, Jeff Christiansen; 68, Alexander Choe; 69, Sandra Quackenbush; 70, Richard Bessen; 71, Charles Scianna; 72, Igor Traktinskiy; 73, Jesús Peña ; 74, Jordan Steel; 75, Nik Mueller; 76, Jonathon Huntington; 77, Glenn Capodagli; 78, Caroline Kulesza; 79, Scott Pegan; 80, Henri Jupille; 81, Randall Holmes; not pictured, BreAnna Bonner; Ben Chan; Davin Henderson; Edward Hoover; Daniel Konet; Geoff Letchworth; Myrna Miller; Bruce Pulford; Kelsie Speiser; Glenn Telling.
Basic research is the foundation of evidence-based medicine whose translation into new, specific, effective therapeutic interventions is the linchpin of translational medicine. Traditionally virology has fostered basic research in microbiology, molecular biology, immunology, epidemiology, epigenetics and gene regulation, pathogenesis, genetics and evolution. Viruses quickly and efficiently disarm host intrinsic, innate and adaptive defenses and usurp cellular anabolic pathways resulting in virus production accompanied by cell death (permissive infection), virus production without cell death (chronic infection) or inhibition of virus replication with the possibility of future reactivation (latent infection). Recently an unprecedented class of infectious pathogens that invariably result in fatal neurogenerative diseases has been identified. These PRoteinaceous Infectious ONly diseaseS are caused by prions that induce structural transitions of host proteins into pathogenic aggregates. Recent evidence suggest prion strain variation and underlines the fact that the full spectrum of disease caused by this novel class of pathogens is not known.
The 12th annual meeting of the Rocky Mountain Virology Association (RMVA) witnessed expansion of the association to include significant presentation from experts in Prionology. The meeting was held Sept. 28-30, 2012 at the forestry extension campus of Colorado State University (Pingree Park) to coincide with the peak Aspen color and again we experienced a moose sighting. RMVA (www.RockyMountainVirologyClub. org) began in 2001 as a regional gathering of virologists to share and discuss scientific data and ideas, and to provide a venue for graduate students to present their findings in a relaxed but professional setting. Since its inception, RMVA has attracted virologist and now Prion experts from the Rocky Mountain area along with nationally and internationally recognized scientists, and has fostered interdisciplinary collaborations, assisted in grant development and has been the sounding board for multiple manuscripts. This year’s plenary presentation by Luis Rodriguez highlighted his collaborative studies with Jonathan Arzt, James Zhu, Manuel Borca, Elizabeth Rieder, Teresa De Los Santos and Marvin Grubman at the Plum Island Animal Disease Center U.S Department of Agriculture in Orient Point, NY. Following are selected abstracts presented by the contributing author (listed in alphabetically order and pictured in Fig. 1) beginning with the invited guest speaker.

Luis L. Rodriguez, Plum Island Animal Disease Center U.S Department of Agriculture .
presented Research Towards The Global Control And Eradication of Foot-And-Mouth Disease Virus. Foot and mouth disease (FMD) is one of the most economically and socially devastating diseases affecting animal agriculture throughout the world. Although FMD mortality is low in adults, mortality can be high in younger animals particularly when exposed to new or emerging highly virulent strains. Additionally, millions of animals (mostly non-infected) have been destroyed in sometimes unsuccessful efforts to rapidly control and eradicate the disease when incursions occur into FMD-free countries. The causing virus (FMDV) is a highly variable positive-sense RNA virus (Family Picornaviridae, genus Aphtovirus) occurring in seven serotypes; A, O, C, Asia 1, Sat 1, Sat and Sat 3 and multiple subtypes which require specifically matched vaccines. Although effective killed antigen vaccines protect against clinical disease, they induce short term immunity (<6 months) and fail to prevent primary infection resulting in persistently infected (carrier) animals. Using vaccination strategies Europe eradicated FMDV decades ago, although costly viral incursions have occurred. Other parts of the world invest billions of dollars in bi-annual vaccination campaigns to decrease the incidence of clinical disease and associated negative economic impact. Despite these control efforts, FMDV thrives in endemic regions usually located in poor countries having significant impact on millions of people whose livelihoods depend on livestock for income, food and draught. Our research efforts are focused on combining a deep understanding of viral pathogenesis and functional genomics integrating various disciplines including veterinary medicine, pathology, cell biology, bioinformatics and immunology toward the development of vaccines and other countermeasures rationally designed for FMD control and eradication. These vaccines need to be safe and inexpensive to produce, easy to deliver and capable of inducing broadly protective long-term immunity. When these critical components are available we will be closer to global control and eradication of FMDV

Michael D. Barnhart, Colorado State University
described the commandeering of the cellular HuR protein as a general property of Alphavirus infections that results in post-transcriptional changes in cellular gene expression. We have previously shown that cellular HuR protein binds to a U-rich region in the 3’UTR of Sindbis virus RNA, resulting in stabilization of viral transcripts and increased replication efficiency. While the presence of this U-rich region is generally conserved among Alphaviruses, a subset lacks a U-rich. The 3’ UTR of two Alphaviruses—Ross River virus and Chikungunya virus—that do not contain a U-rich region, were obtained from Dr. Tem Morrision and tested for HuR interactions by EMSA. HuR protein bound these 3’ UTRs with nanomolar affinities, similar to what was observed for the U-rich region of Sindbis virus. These observations demonstrate that the critical role for HuR-mediated viral RNA stabilization is likely a conserved property of most if not all members of the virus family. By analyzing deletion derivatives, we mapped the novel HuR binding sites in these two viruses to specific regions in the 3’ UTR. Finally, we examined the impact of Alphavirus-induced HuR relocalization to the cytoplasm and interaction with viral transcripts on cellular mRNA interactions and accumulation. HuR interactions with numerous cellular mRNAs were found to be drastically decreased during Sindbis virus infection. This decrease in HuR interaction was associated with a dramatic decrease in the stability of numerous cellular mRNAs as determined by mRNA half-life analysis. Collectively, these data indicate that in the process of using HuR protein to stabilize viral transcripts, the virus also effectively destabilizes numerous cellular mRNAs, many of which play a key role in innate antiviral immune responses and other fundamental cellular processes. These data suggest that viral-induced alterations in cellular mRNA stability may play a very important but underappreciated role in pathogenesis.

Jifeng Bian, Colorado State University presented Novel cell cultural approaches for deciphering the pathogenesis of chronic wasting disease. Chronic wasting disease (CWD) is an emerging prion disease of deer, elk and moose. While we have generated transgenic mouse models of CWD (Browning, S. R., Mason, G. L., Seward, T., Green, M., Eliason, G. A., Mathiason, C., Miller, M. W., Williams, E. S., Hoover, E., and Telling, G. C. (2004) Journal of virology 78, 13345-13350; Angers, R. C., Seward, T. S., Napier, D., Green, M., Hoover, E., Spraker, T., O’Rourke, K., Balachandran, A., and Telling, G. C. (2009) Emerging Infectious Diseases 15, 696-703) the constraints of long incubation times and costs remain limitations. We found that RK13 rabbit kidney epithelial cells engineered to express mouse prion protein (PrP) can be readily infected with mouse prions. While RK13 cells expressing deer or elk PrP had a limited capacity to sustain CWD prion infection, we discovered that co-expression of the HIV-1 GAG precursor protein increased levels of PrPSc, and resulted in chronically infected cell clones, referred to as Elk21+ and Deer5E9+. Inoculation of CWD-susceptible transgenic mice with prions from these cells resulted in disease that was indistinguishable from naturally occurring CWD. We used Elk21+ cells to evaluate compounds that inhibit CWD prion propagation. As a first approach, we used compounds that had proven effective against experimentally adapted scrapie prions. We cured Elk21+ and Deer5E9+ cells of prion infectivity using dextran sulfate 500, or isolated spontaneously cured cells by single cell cloning, referred to as Elk21- and Deer5E9-S1 cells. In each case, reinfection with CWD prions was possible, and allowed us to develop cell culture systems to quantify CWD prion infectivity. Using this cervid prion cell assay (CPCA), we estimated titers of 106.1 to 106.7 /gram brain material from deer or elk with CWD (Bian, J., Napier, D., Khaychuck, V., Angers, R., Graham, C., and Telling, G. (2010) Journal of virology 84, 8322- 8326). Recent modifications have increased CPCA sensitivity by 100-fold. We also found that the single amino acid variation at residue 226 between deer and elk PrP, dramatically affected cell susceptibility to deer and elk prion infection. We also evaluated the efficacy of quinacrine, which has also been shown to have inhibitory activity for mouse prions. While we confirmed inhibition of mouse prion propagation in RK13 cells, quinacrine paradoxically increased PrPSc levels in Elk21+ cells. Inoculation of transgenic mice expressing deer or elk PrP with quinacrine-treated Elk21+ cells prolonged prion incubation times, and produced different neuropathology and PrPSc distribution patterns in the brains of diseased mice. Our results show that the effects of quinacrine depend on the strain of prion agent, and moreover suggest that, in the case of CWD, quinacrine treatment results in the selection of a novel prion strain.

Claire Birkenheuer, Colorado State University, Fort Collins described the implication of the interaction between RV-cyclin and CDK8. Walleye dermal sarcoma virus (WDSV) is a complex retrovirus, which causes seasonal tumors in walleye fish. The stages of the tumor life cycle are associated with differential expression of the three accessory proteins encoded by the virus. Retroviral cyclin (RV-cyclin) is one of these accessory proteins, and its expression correlates with tumor development. RV-cyclin contains a cyclin box domain, which comprises the majority of the protein and interacts with cyclin dependent kinase 8 (CDK8). CDK8 positively affects transcription of the serum response genes, FOS, EGR1 and JUN. In vitro kinase assays using immune precipitated CDK8 show that RV-cyclin increases CDK8 phosphorylation of the carboxy terminal domain of RNA Pol II. Due to this interaction, and the nature of RV-cyclin’s localization, we hypothesize RV-cyclin plays a role in altering transcription of regulatory genes to aid in the development of a tumor. Transient transfections and serum starvation/stimulation experiments were performed to determine if the RV-cyclin alters expression of these genes in mammalian cell lines. HeLa cells at 10 hours post transfection with RV-cyclin showed activation of the serum response genes up to 70 fold compared to control. In HCT116 cells that stably express RV-cyclin, serum response gene expression was enhanced up to 2 fold compared to control upon serum restoration. Additionally mutant RV-cyclin which cannot bind CDK8 did not enhance serum response gene expression under serum stimulation conditions. Chromatin immunoprecipitation experiments demonstrated that the presence of RV-cyclin correlates with an increased CDK8 occupancy along the EGR1 gene locus. Additionally, walleye egr1 transcripts were shown to be up-regulated in developing fall tumors when compared to regressing spring tumors. These data suggest that RV-cyclin is able to increase transcript levels of the serum response genes though its interaction with CDK8. As well as demonstrating that RV-cyclin’s influence on mammalian gene expression correlates with observations taken from developing walleye dermal sarcoma.

Charles H. Calisher, Colorado State University presented a humorous take on learning, what is important, and what is ridiculous.
Benjamin K. Chan, University of Utah School of Medicine presented an unbiased determination of viral etiology in encephalitis using deep sequencing. Diagnosis of infectious diseases currently requires prior knowledge of pathogen(s) to be tested for. This prior knowledge is usually guided by practical clinical knowledge that directs testing to a few suspected candidates. Specific identification of the pathogen then entails techniques such as culture and/or PCR. Selection of incorrect candidates for testing, as could be the case in novel, emerging, zoonotic, or escape mutants of known pathogens can result in failed testing and potentially, inappropriate treatment strategies. Our approach allows for unbiased testing of a sample for all microbial pathogens in a single test.

Alexander Choe, University of Colorado School of Medicine described the development of a novel GeXP multiplex RT-PCR assay for analysis of the SVV transcriptome. Varicella zoster virus (VZV) is an exclusively human virus that causes varicella (chickenpox) on primary infection then establishes latency in ganglia along the neuraxis. With a decline in VZV-specific cell-mediated immunity (elderly and immunocompromised subjects), VZV reactivates and causes multiple neurological diseases including herpes zoster (shingles), postherpetic neuralgia, stroke, spinal cord disease, and vision loss. Understanding VZV latency and reactivation is essential in developing potential therapies to prevent attendant neurological disease. Since there is no animal model to study VZV, we used the Simian varicella zoster virus (SVV) model since primary infection, latency and reactivation are similar to that seen with VZV. Since analysis of the SVV transcriptome is challenging when virus is present in low abundance; we developed a novel GeXP multiple RT-PCR assay that allow allows analysis of all 69 unique SVV open reading frames (ORFs) in only four reaction tubes. We optimized and validated each pair of SVV ORF-specific primers within each reaction and demonstrated that in SVV- infected tissue culture cells from African Green monkeys and Rhesus Macaques, all SVV transcripts are detected. In addition, we also pooled multiple ganglia from an African Green monkey during primary infection with SVV and showed that the majority of SVV transcripts are expressed. Only transcripts corresponding to SVV ORFs 14 and 35 were not detected and may reflect lower abundance of these transcripts. Thus, our SVV GeXP multiplex RT-PCR assay will serve as a useful tool for characterizing the SVV transcriptome during latency and reactivation

Nunya Chotiwan, Centers for Disease Control and Prevention (Fort Collins, CO) and Colorado State University described Molecular Determinants of Dengue Virus-2 E Protein Critical for Antibody-Dependent Enhancement of Infection. Antibody-dependent enhancement (ADE) of infection has been thought to be a major factor in development of life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) in patients undergoing a secondary infection with a different serotype of dengue virus (DENV). The ADE is caused by cross-reactivity of non-neutralizing (or subneutralizing) Abs to the newly-infecting DENV serotype. These cross-reactive Abs form virus-Ab complexes and enhance virus infection by binding to the Fc receptors (FcR) on DENV-susceptible cells. The early events in regular (non-ADE) DENV infection have been previously studied, but the pathway of ADE infection through the FcR is still largely unclear. In this study, we successfully established assays to measure ADE infection of DENV type 2 (DENV-2) with a subneutralizing cross-reactive MAb. We analyzed ADE infection by several DENV-2 envelope (E) protein mutants and identified critical domains of the viral E protein in ADE infection of FcR-bearing cells. Interestingly, the functional domains essential for ADE infection are also essential for non-antibody-enhanced infection. Our results confirmed that antibody at subneutralizing levels can promote ADE infection of DENV in FcR-bearing cells, but binding of the virus-Ab complex with FcR alone is not sufficient for ADE infection. It is likely that other specific cell receptors are required for successful internalization of virus-Ab complexes, and the virus-Ab complex enters the FcR- bearing cells through a similar endosomal pathway as the regular DENV infection. The FcR mainly plays an auxiliary role in concentrating the virus-Ab complex to the cell surface, resulting in enhancement of DENV infection.

Penny Clarke, University of Colorado School of Medicine described the use of a novel ex vivo brain slice culture (BSC) model to confirm the role of death receptor (DR) apoptotic signaling in West Nile virus (WNV) pathogenesis. West Nile Virus infection is a significant cause of neurologic disease with no effective treatment or vaccine available. In this study, microarray and Ingenuity Pathway analyses identified genes associated with DR signaling as being differentially up-regulated in the brains of mice infected with WNV. The differential expression of DR-associated genes was confirmed by RTPCR and western blotting. In addition, activation of the DR-associated initiator caspase, caspase 8, was demonstrated in the brains of WNV-infected mice. Experiments in ex vivo BSC demonstrated that WNV-induced apoptosis (caspase 3 activation) and injury (lactate dehydrogenase release) was reduced in the presence of an inhibitor of caspase 8 indicating a role for DR apoptotic signaling in WNV pathogenesis.

Randall J. Cohrs, University of Colorado School of Medicine presented a review of herpes simplex virus type 1 (HSV-1) latency, current unpublished data on varicella zoster virus (VZV) genes transcribed in human trigeminal ganglia, and proposed a model to unify the seemingly different patterns of these human neurotropic alphaherpesvirus gene transcription during reactivation. In mouse trigeminal ganglia latently infected with HSV-1 only a single virus gene is transcribed. This LAT, latency associated transcript, functions at the RNA level through contained miRNA to inhibit neuronal apoptosis. In human trigeminal ganglia removed before 9 hrs following death only a single VZV gene is transcribed. This VZV gene encodes the immediate-early 63 protein may function at the RNA or protein level and may inhibit virus induced apoptosis. As time between death and analysis of the trigeminal ganglia increases, more HSV-1 and VZV gene are transcribed. However, instead of the ordered cascade of virus gene transcription seen in productively infected cells in tissue culture, virus gene transcription during reactivation (increased time between death and analysis) is disordered with general deregulation of transcriptional repression. The proposal presented to explain this disordered and general transcriptional deregulation involved a hypothesized structure in the latent virus genome that includes preformed mediator and RNA polII complexes stalled at cohesion junctions located at insulator binding sites. Experiments were proposed to identify these presumed complexes in latently infected human trigeminal ganglia which, if found, would advance the herpesvirus field by providing a molecular mechanism of virus gene regulation during latency and the early stages of virus reactivation.

Daphne A. Cooper, University of Colorado School of Medicine described Antiviral Endoribonuclease RNase L Targets on Specific Regions of Viral RNA. Ribonuclease L (RNase L) is an antiviral endoribonuclease known to cleave host and viral RNAs primarily at single-stranded UA and UU dinucleotides. The paucity of single-stranded UA and UU dinucleotides in GC-rich hepatitis C virus (HCV) genomes is consistent with the selective pressure of RNase L (Washenberger et al., 2007). RNase L activity has three antiviral mechanisms: 1) it cleaves viral RNAs of infected cells, inhibiting viral gene expression and replication; 2) it promotes apoptotic death of virus-infected cells; and 3) it produces RNA fragments that function as RIG-I ligands, leading to IFN and IFN-stimulated gene expression. One particular fragment of HCV RNA produced by RNase L is a potent RIG-I ligand (Malathi et al., 2010). While RNase L and the RIG-I ligands that it produces are predicted to influence the outcomes of HCV infections and interferonbased antiviral therapies, there have been no methods to easily measure the magnitudes of RNase L activity in patient samples and very few RIG-I ligands produced by RNase L have been carefully defined. Consequently, we developed novel cDNA synthesis and Illumina cDNA sequencing methods to easily identify RNase L cleavage sites in host and viral RNAs. These methods rely on the ability of A. thaliana tRNA ligase to recognize RNA fragments with terminal 2’, 3’-cyclic phosphates (Schutz et al., 2010), a biochemical feature of RNA fragments produced by endoribonucleases like RNase L. Using these methods, we confirmed that HCV RNA is cleaved by purified RNase L almost exclusively at single-stranded UU, UA, and UG dinucleotides. In addition, we observed abundant cleavage in the regions of HCV RNA encoding the hyper-variable regions of the envelope glycoproteins. Furthermore, we analyzed RNA fragments from poliovirus-infected HeLa cells expressing either wildtype or dominant-negative RNase L to define RNase L-dependent and independent cleavage sites in host and viral RNAs. We anticipate using our 2’, 3’-cyclic phosphate cDNA synthesis and Illumina sequencing methods on RNA fragments purified from HCV-infected liver biopsies in order to characterize the magnitudes of RNase L activity in HCV-infected patients.

Brian J. Geiss, Colorado State University described the Identification of a Novel Antiviral Inhibitor of the Flavivirus Guanylyltransferase Enzyme. Mosquito-borne flavivirus infection causes serious morbidity and mortality worldwide annually, but there are currently no effective antiviral chemotherapeutics available for human use. Therefore, it is critical that new therapeutics to virus-specific targets be developed. To identify new therapeutic structures that may be used as broadly active flavivirus inhibitors, we have performed a screen of 235,456 commercially available compounds for small molecule inhibitors of the dengue virus NS5 RNA capping enzyme at the NERCE National Screening Laboratory (NRSB). We have identified a family of compounds, the 2-thioxothiazolidin-4-ones, that show potent biochemical inhibition of GTP binding and guanylyltransferase function of the capping enzyme. During the course of structure-activity relationship analysis, a molecule within this family (BG323) was found to possess significant antiviral activity in a dengue virus subgenomic replicon assay. Further testing of BG323 demonstrated that this molecule can reduce the replication of infectious West Nile and yellow fever viruses in multi-step growth curve assays, reduces viral RNA released from infected cells, and shows little toxicity in cell culture. BG323 represents the first inhibitor that specifically targets the GTP-binding / guanylyltransferase activity of the flavivirus RNA capping enzyme and demonstrates the value of the flavivirus capping enzyme as a therapeutic drug development target. To improve biochemical affinity and reduce potential liabilities, available derivatives of BG323 are currently being tested and additional compounds are being synthesized to improve SAR and antiviral activity. Preliminary toxicity testing and pharmacokinetics analysis of BG323 in CD-1 mice indicate that BG-323 is non-toxic and detectable in mouse serum. These results suggest that BG-323 and derivitives may be useful as antivirals for the treatment of a number of different flaviviral diseases.

David Hawman, University of Colorado School of Medicine described how Host immunity impacts tissue-specific persistence of Chikungunya virus in a mouse model of disease. Chikungunya virus is an arthritogenic alphavirus spread by Aedes species mosquitoes that has caused explosive epidemics in Asia and Africa. Symptoms of the disease include a sudden onset of fever and intense, debilitating pain in the peripheral joints. There are no specific treatments and many patients report continuing musculoskeletal symptoms for months to years following the initial onset. Currently it is unclear if the prolonged symptoms of disease are due to persistent viral infection. To investigate mechanisms of chronic CHIKV disease, our lab uses a previously described mouse model in which the major disease signs, arthritis, myositis and tenosynovitis/tendonitis, are consistent with human disease. Using qRT-PCR, we detected CHIKV RNA in a variety of tissues in wild-type mice at early times post infection. By 14 dpi, CHIKV RNA was cleared from most tissues, however, we detected abundant CHIKV RNA in the ankles of mice out to 16 weeks post-inoculation (wpi) and in the spleen up to 6 wpi. The persistence of CHIKV RNA in ankle tissues was associated with histopathological evidence of arthritis, synovitis and tendonitis and persistence of CHIKV RNA in the spleen was associated with detection of CHIKV antigen. Rag-1- /- mice, which are deficient in mature B and T cells, had elevated levels of CHIKV RNA in ankle tissues and prolonged persistence in the quadriceps muscles suggesting that the adaptive immune response plays a critical role in clearance. Strikingly, CHIKV RNA was undetectable in the spleens of Rag-1-/- mice until 84 dpi, suggesting that a specific population of CHIKV target cells may be absent in the spleens of Rag-1-/- mice. Taken together, our findings suggest that chronic CHIKV musculoskeletal disease may be due to tissue-specific persistent CHIKV infection. Further, our findings in Rag-1-/- mice suggest that while the adaptive immune system is necessary for clearance from the muscle tissue, it fails to clear the virus from the ankle and spleen.

Kathryn Holmes, University of Colorado School of Medicine presented information about bats as reservoirs for many viruses that are pathogenic for humans, including SARS coronavirus, Hendravirus, Ebolavirus, etc. Viruses related to SARS-CoV have been detected in bats of several species in Asia, Europe and Africa. To determine whether SARS-CoV-related viruses are also harbored in New World bats, members of the Colorado Bat Virus Consortium looked for coronavirus RNA by RT-PCR in fecal specimens from 17 species of bats in Colorado. We detected RNA of coronaviruses in Colorado bats of several species, including ones that interact commonly with humans. However we only detected alphacoronaviruses and not betacoronaviruses closely related to SARS-CoV. In Saudi Arabia and Qatar two humans recently developed severe respiratory disease and kidney failure that is associated with a novel betacoronavirus phylogenetically most related to two Asian bat betacoronaviruses, and in the same subgroup as SARSCoV. World health organization, CDC and other agencies and experts are currently investigating whether these two cases are the result of zoonotic transmission (perhaps from bats?) or are due to a previously undetected human coronaviruses. Rapid completion and publication of the full genome sequence of the virus and development of viral RNA detection assays and ELISAs to detect antibodies specific for the new coronavirus will aid in determining this. Koch’s postulates have not yet been fulfilled to show if the new virus actually caused the human disease. The tremendous diversity of coronaviruses in bats and the implication of bats in causing multiple spillovers into humans causing severe diseases justify continued surveillance of bats and other wildlife for coronaviruses, and other infectious agents, that might spill over to cause additional outbreaks of disease in humans, domestic animals or birds.

Randall K. Holmes, University of Colorado School of Medicine, presented studies performed in collaboration with Michael G. Jobling on the potential role of bacteriophages in production of type II enterotoxins (LT-IIs) and novel pertussis-toxin-like (PTL) toxins by isolates of enterotoxigenic Escherichia coli (ETEC). Our recent phylogenetic analysis of the LT-II loci from 50 diverse type II ETEC isolates showed that only 4 encode a previously characterized LT-IIa or LT-IIb enterotoxin (3 LT-IIa and 1 LT-IIb, respectively), whereas 46 encode highly related variants of a recently described LT-IIc enterotoxin that can be assigned to 6 phylogenetic subgroups (PLoS ONE 7(1):e29898). Additional analysis of representative isolates revealed that the LT-II operons are encoded by lambdoid type prophages (which may or may not be infectious) that also encode one of two novel pertussis-toxin-like (PTL) toxins heretofore unknown in ETEC. We have initiated studies to characterize the regulation and properties of these novel PTL toxins and their possible roles (either alone or in combination with LT-II enterotoxins) in pathogenesis of type II ETEC infections.

Ian Hoskins and Trevor Blain, University of Colorado described RNA polymerase II enrichment on regions of ORF 9 and 21 in wild-type VZV infected HFL cells. Varicella-zoster virus, a member of the alphaherpesviruses family, is the cause of chickenpox (upon primary infection), and shingles (upon reactivation from latency in neurons). Currently, the understanding of VZV gene regulation lags behind that of HSV-1, the prototypical alphaherpesvirus. To begin, we employed RT-PCR to record transcript abundances across genes of varying kinetic classes. Since it has been previously shown that these transcripts have similar degradation rates, differences in transcript abundance are a result of varying synthesis rates or transcriptional permissiveness. To probe a plausible cause behind the differential synthesis of ORFs in productive VZV, we used chromatin immunoprecipitation (ChIP) and subsequently, qPCR, to determine enrichment of RNA Pol II on ORFs 9 (early gene class), and 21 (late gene class). To better understand the epigenetic mechanisms in control of VZV transcription, ChIP assays targeting other regulatory proteins, such as mediator, cohesin, CTCF (an insulator), and modified histones, must be completed.

Henri J. Jupille, University of Colorado School of Medicine described a tyrosine to histidine mutation at position 18 of the Ross River virus E2 glycoprotein that modulates viral fitness in disparate hosts. Mosquito-borne alphaviruses such as Ross River virus (RRV) and chikungunya virus (CHIKV) cause debilitating and often chronic rheumatic disease in humans. These viruses are responsible for largescale epidemics that have resulted in millions of human disease cases. Despite these outbreaks, the pathogenesis of these viruses are poorly understood. Using chimeric viruses, we previously reported that substitution of the PE2 coding region of the T48 strain of RRV (RRV-T48) with that from the attenuated DC5692 strain, which differ by 7 amino acids, resulted in an attenuated disease phenotype in a mouse model of alphavirus induced musculoskeletal inflammatory disease. Introduction of one of these amino acid changes, a tyrosine (Y) to histidine (H) change at position 18 of the E2 glycoprotein (E2 Y18H), into the RRV-T48 genetic background was sufficient to generate a virus which caused dramatically less severe musculoskeletal disease in mice. The attenuated phenotype of RRV-T48 E2 Y18H was associated with reduced viral loads in musculoskeletal tissues, reduced serum viremia, and less efficient viral spread. Consistent with these findings, the E2 Y18H mutant replicated less well compared to RRV-T48 in murine muscle cells in vitro. In contrast, the E2 Y18H mutant replicated more efficiently than RRV-T48 in C6/36 mosquito cells. Competition studies confirmed that the E2 Y18H mutant virus had a fitness advantage in mosquito cells and a fitness disadvantage in mammalian cells. Interestingly, all sequenced RRV strains encode either a Y or H at E2 position 18, an observation which holds true across all viruses within the Semliki Forest virus antigenic complex. Taken together, these findings suggest that a tyrosine to histidine switch at E2 position 18 functions as a critical regulator of RRV fitness in vertebrate and invertebrate cells.

Forest Kim, University of Colorado School of Medicine presented matrix metalloproteases upregulation in VZV Vasculopathy. VZV is an exclusively, human alphaherpesvirus that causes chicken pox (shingles) on primary infection, after which it establishes latency in the cranial nerve, dorsal root and autonomic ganglia. With a decline in VZV-specific cell-mediated immunity (such as in elderly and immunocompromised individuals), VZV reactivates and typically causes zoster. Another serious complication of VZV reactivation is VZV vasculopathy or stroke caused by VZV infection of cerebral arteries. VZV infection of cerebral arteries causes thickening of the intima, which can contribute to ischemic stroke, and also loss of smooth muscle cells with weakening of the vessel wall, which can contribute to aneurysm formation and subsequent hemorrhagic stroke. The mechanism(s) by which VZV infection causes weakening of the vessel wall is unclear but matrix metalloproteases (MMPs) are known to degrade extracellular matrix and have been shown to be important in hemorrhagic conversion of ischemic stroke. Hence, we hypothesized that VZV infection of cerebral arteries causes upregulation of MMPs resulting in weakening of the vessel wall, aneurysm formation and hemorrhagic stroke. Herein, we infected human brain vascular adventitial fibroblasts with VZV and mock control. At 6 day post-infection (d.p.i.) cells were harvested, RNA extracted and analyzed by real-time PCR for MMP-2, MMP-3 and MMP-9 transcripts - which have been shown to be important in vascular remodeling. In addition, conditioned supernatant from VZV and mock-infected cells at 6 d.p.i. were analyzed by the MesoScale Discovery multiplex ELISA. At 6 dpi, MMP-3 and MMP-9 transcripts in VZV-infected cells were upregulated by 33.8 (+/- 9.5) fold and by 2.7 +/- 1.1 fold compared to mock-infected cells. MMP-2 was downregulated by 0.35 +/- 0.04 fold. Similarly, MMP-3 and MMP-9 protein was upregulated by 3.9 and 2.9 fold compared to mock-infected cells. Thus, upregulation of MMP-3 and MMP-9 during virus infection of vascular cells could potentially degrade the extracellular matrix, cause vessel wall weakening, aneurysm and hemorrhagic stroke. Studies on the effect of VZV on other MMP subtypes and quantification of activated MMPs are ongoing.
Dan Konet, Roche Applied Science described how the Universal ProbeLibrary (UPL) combines the flexibility, availability and convenience of SYBR Green I assays with the specificity of hydrolysis probe assays. The unique combination of online available assay design software and only 165 pre-validated, real-time PCR probes allows the user to quantify virtually any transcript in the transcriptomes of a large number of organisms.

Martin Lapel, Metropolitan State University of Denver, presented studies on an animal model of Varicella zoster virus. Varicella zoster virus (VZV) causes chicken pox, becomes latent in ganglia and reactivates decades later to produce zoster in humans. Simian varicella virus (SVV) infection of primates causes similar disease and therefore serves as a good animal model to study VZV. SVV and VZV open reading frames (ORFs) share 25-75% homology. VZV ORF 66, a gene not required for replication inhibits production of class I major histocompatability complex (MHC) of infected cells in culture. En passant mutagenesis was used to construct two SVV mutants using a recombinant bacterial artificial chromosome (BAC) containing the complete SVV genome and green fluorescent protein (GFP). In the first mutant (SIV-gag WT), Simian immunodeficiency virus- gag (SIV-gag) driven by the EF-1A promoter was inserted at the 3’-end of wild-type SVV ORF 14. In the second mutant (SIV gag Δ66), in addition to the insertion of SIV gag, stop codons were introduced into SVV ORF 66. The mutant BACs were transfected into Vero (monkey kidney) cells to produce a cytopathic effect (CPE) that was GFP-positive. The SIV-gag Δ66 virus will be used to determine if ORF 66 expression is essential for immune evasion of SVV by blocking SIV-gag-specific T-cells. An understanding of the function of varicella ORF 66 may help in the development of novel vaccines in the future.

Martha MacMillan, Colorado State University described a Novel Antibody Therapy for Feline Immunodeficiency Virus. The cellular adhesion molecule LFA-1 is a heterodimer composed of CD11a and CD18 and has been reported to facilitate HIV infection by allowing adhesion of the viral envelope with target cell membranes. We sought to determine whether LFA-1 played a similar role in FIV infection. Initial experiments using a monoclonal antibody reactive to feline CD11a (TS1/22) demonstrated that an anti-feline CD11a antibody could interfere with virus infection in tissue culture. Inhibition was attained using virus prepared from either LFA-1 positive (104-C1) or LFA-1 negative (GFox) cells, suggesting that interference occurred via interaction at the target cell surface rather than on the virus particle. To determine if this activity could be translated to FIV infected animals, subsequent experiments were performed in vivo to determine whether treatment with TS1/22 could influence an ongoing virus infection in cats. Animals were treated with 4 mg/kg TS1/22 or a non-feline binding anti-CD11a specific monoclonal antibody by a single intraperitoneal inoculation. Viral load was assessed as viral RNA in blood and proviral load in lymphocytes. Total lymphocyte counts as well as CD4+ and CD8+ cells were analyzed over a 6-week period post treatment. TS1/22 treatment resulted in a marked transient increase in viral load as measured by plasma viral RNA. Proviral load did not change significantly over the course of the experiment. Interestingly, and in contrast to decreased peripheral CD4-T cell kinetics typically observed during increased FIV viremia, a concomitant rise in CD4+ and CD8+ lymphocytes, as well as a generalized lymphocytosis was noted in TS1/22 treated animals. Further, after 4 weeks, viral loads were transiently lower than those observed prior to antibody treatment. These results suggest LFA-1 is involved in FIV infection and can alter feline leukocyte parameters. The potential for any therapeutic application of anti-CD11a antibody awaits further experimentation.

Craig Miller, Colorado State University described the characterization of feline immunodeficiency virus excretion and tissue tropism in feline saliva and oral tissues. Feline immunodeficiency virus (FIV) is believed to be transmitted in saliva primarily by bite wounds, although mechanisms associated with salivary transmission have not been well studied. Human immunodeficiency virus (HIV) is also known to be present in the saliva of infected individuals and has been shown to be genetically, structurally, and biochemically similar to FIV. Studies involving HIV salivary pathogenesis have increased the prospect of alternative antiviral therapies and diagnostic methodologies in endemic areas. Therefore, further elucidation of lentiviral salivary excretion and transmission mechanisms may have significant implications in both medical and veterinary research. To characterize the excretion and tropism of FIV in saliva and oral tissues, and to hypothesize mechanisms associated with oral shedding and transmission of FIV, eighteen cats were intravenously inoculated with a well-characterized strain of FIV. Viral RNA and DNA present in saliva and oral mucosal and lymphoid tissues were quantified using RT-PCR analysis. Histologic evaluation of oral tissues was retrospectively performed by light microscope. We demonstrate that saliva contains significant amounts of both viral RNA and proviral DNA, and that viral and proviral loads in oral lymphoid tissues are significantly higher than oral mucosal tissues. Histology revealed moderate lymphoid hyper- plasia in lymphoid tissues, as well as mild to moderate, lymphoplasmacytic and mastocytic stomatitis and glossitis in mucosal tissues. Results suggest multi-organ involvement in viral shedding and infectivity that appears to predominate in oral lymphoid tissues.

Stephanie Moon, Colorado State University presented a talk entitled, “Dysregulation of host mRNA stability by a unique flavivirus non-coding RNA”. Arthropod-borne flaviviruses contain positivesense, single stranded RNA genomes with a single open reading frame and have been shown to generate a unique non-coding RNA (sfRNA) from the viral 3’ untranslated region (UTR). Like cellular mRNAs, viral RNAs are susceptible to degradation by the host mRNA decay machinery. Highly structured regions of the viral 3’ UTR cause the major host 5’-3’ exonuclease Xrn1 to stall on the RNA, leading to the formation of sfRNA. We have shown that formation of sfRNA also represses Xrn1 activity using in vitro mosquito and human cell extract systems and a cell culture model of Dengue virus and Kunjin virus infection. In association with the accumulation of sfRNA in virus-infected cells, we observed an increase in uncapped mRNA decay intermediates (normally susceptible to Xrn1-mediated decay) and increased stability of host mRNAs. Importantly, a mutant Kunjin virus unable to form sfRNA did not induce stabilization of host mRNA and expression of the Dengue virus sfRNA alone was associated with the stabilization of host transcripts in the absence of infection. Finally, a subset of cellular mRNAs was shown to be differentially expressed in an sfRNA-dependent fashion. The ability of sfRNA to inhibit the Xrn1 exonuclease- leading to changes in host mRNA turnover- could have a major impact on host gene expression and the response to viral infection.

Tem Morrison, University of Colorado School of Medicine described enhances clearance of an arthritogenic alphavirus by generic ablation of arginase 1 in macrophages and neutrophils. Chikungunya virus (CHIKV) and Ross River virus (RRV) cause a debilitating, and often chronic, musculoskeletal inflammatory disease in humans. We hypothesized that the severe damage to musculoskeletal tissues observed in RRV or CHIKV-infected humans and mice would promote a wound healing response characterized by M2-like macrophages. Utilizing preclinical mouse models, we found that RRV and CHIKV-induced musculoskeletal inflammatory lesions, and macrophages present in these lesions, have a unique gene expression pattern characterized by high expression of arginase 1 (Arg1) and Ym1/Ch3l3 in the absence of FIZZ1/Relmα that is consistent with an M2-like suppressive phenotype. Strikingly, mice specifically deleted for Arg1 in macrophages and neutrophils (LysMcre;Arg1F/F) had dramatically reduced viral loads at 14 and 21 days post-inoculation (dpi) but similar viral loads at 7 dpi, indicating that genetic deletion of Arg1 resulted in enhanced RRV clearance. The reduced levels of RRV RNA were associated with less severe muscle tissue pathology. Macrophages sorted from inflamed muscle tissues of RRV-infected WT mice at 10 dpi inhibited T cell proliferation ex vivo by a mechanism that was partially Arg1-dependent. Furthermore, depletion of CD8 T cells from LysMcre;Arg1F/F mice, but not Arg1-sufficient mice, significantly increased viral loads. These findings suggest that arthritogenic alphavirus infections drive a unique myeloid cell activation program in inflamed musculoskeletal tissues that inhibits CD8 T cell-mediated virus clearance and impedes disease resolution in an Arg1-dependent manner.
Maria Nagel, University of Colorado School of Medicine described purinergic signaling in VZV vasculopathy. Varicella zoster virus (VZV) is a neurotropic, alphaherpesvirus that reactivates from ganglia, infects adventitia of human cerebral arteries by transaxonal spread, and induces vessel wall changes that ultimately results in stroke (VZV vasculopathy). The mechanism(s) by which VZV successfully infects cerebrovascular adventitial fibroblasts and promotes vascular remodeling and stroke is unclear. However, recent studies indicate that purinergic signaling may play a significant role in both virus infection and vascular remodeling. Specifically, induced extracellular ATP has the potential to interact with up to fifteen P2 purinergic receptors, divided into P2X ion channel and P2Y G-protein coupled receptor subtypes, with each receptor modulating short and long term complex cellular processes, including NO production and vasodilation, vasoconstriction, platelet aggregation, and activation of mitogenic pathways. Thus, we hypothesized that VZV infection induces extracellular ATP release which then binds to specific purinergic receptor subtypes in cerebral vasculature to mediate VZV infection and promote vascular remodeling and stroke. Human brain vascular adventitial fibroblasts (BRAFs), which are the initial cells infected in VZV vasculopathy and key regulators of vascular tone and function, were infected with VZV and mock control. Extracellular ATP in the supernatant was measured. In addition, infected cells were treated with apyrase (which degrades extracellular ATP) and PPADs (general purinergic receptor antagonist), and VZV DNA and titers were measured at 1 and 3 days post-infection (d.p.i.). We found an increase in extracellular ATP in VZV-infected BRAFs compared to mock at 15 minutes post-infection (27.5 nM +/- 3.5 versus 18.5 nM +/- 0.5) and at 30 minutes post-infection (23.8 nM +/- 2.3 versus 16.0 +/- 2.3), similar in time and concentration to hypoxia and HIV-induced extracellular ATP release. In addition, blockade of purinergic signaling by apyrase and PPADs resulted in a reduction in VZV DNA at 1 d.p.i. (53% +/- 5% and 77% +/- 4%, respectively) and at 3 d.p.i. (27% +/- 3% and 38% +/- 7%, respectively) with concurrent 18% and 16% respective decreases in titers. These studies indicate that purinergic signaling contributes to VZV infection and future studies using specific receptor antagonists will elucidate which receptors are important for virus infection and vascular remodeling – providing a potential therapeutic drug target.

Scott Pegan, University of Denver, presented on viral ovarian tumor domain protease from nairoviruses. Specifically, he focused on the prospective roles of vOTUs in immune evasion originating from different viral families that seek to disrupt certain cellular processes through a conserved specificity for poly-Ub and ISG15. Furthermore, he presented data illustrating that vOTUs form different nairoviruses virus appear to have specificity towards certain mammalian hosts. A X-ray crystallographic structure of the vOTU from the Dugbe Virus, a nairovirus that is not lethal to humans, was solved in complex with Ub and compared to the structure of the vOTU-Ub complex from the Crimean-Congo Hemorrhagic Fever Virus, a nairovirus with a human mortality rate reaching as high as 80%.

Aaron Phillips, Colorado State University presented a talk describing what happens when Western equine encephalitis virus gets on your nerves. Eastern and western equine encephalitis viruses (EEEV and WEEV, respectively; Alphavirus; Togaviridae) are mosquito-borne viruses that can cause fatal encephalitis in humans. EEEV and WEEV can also infect animals by the olfactory route. Currently, there are no human antiviral therapies or vaccines for alphaviruses to mitigate disease in case of a large outbreak. We previously developed an outbred CD-1 mouse pathogenesis model based on the McMillan (McM) strain of WEEV, but McM, due to its high mortality within 4 days of infection provided a short window for applying therapeutic interventions. We have now adapted the model to infection with Montana 64 strain of WEEV because this virus has a slower disease progression (7-8 days) in mice and may be more reflective of the disease course in humans. We show here that cationic-liposome-nucleic acid complexes (CLNCs) elicit protective innate immune responses in mice 24h after subcutaneous challenge with Montana 64 WEEV. The addition of invertebrate or humanized recombinant glycoforms of the WEEV E1 and E2-E1 envelope glycoproteins (from the Jarvis lab) to these CLNCs actually lowered the therapeutic efficacy. However, CLNC’s mixed with E1 or E2-E1 provided significant protection from infection using a prime-boost vaccination strategy. Both human and insect glycoforms induced class switching to IgG and differences in antibody isotype were observed between the two E1 glycoforms. Immunization once with either human or insect glycoforms induced class switching to IgG2b specific to the human glycoform (presumably the carbohydrate groups), but not the insect glycoform. Both glycoforms resulted in 100% survival of mice challenged by subcutaneous route. The longevity of the protective response, cross-protection against other alphavirus species, and antigen dose dependence are currently being investigated. Additionally, we have adapted our mouse model for in vivo bioluminescent imaging of WEEV infection to quantify the antiviral efficacy of treatment strategies and develop a better understanding of WEEV neuroinvasion. Finally, we have generated preliminary data which shows that cytoplasmic polyadenylation and related synaptic translation control pathways represent viable antiviral targets for encephalitic alphavirus infection. We show increased survival in WEEV-infected mice treated with inhibitors of the cytoplasmic polyadenylation pathway.

Abhishek Prasad, Colorado State University described production of non-ping pong dependent PIWI RNA-like small RNAs in the mosquito midgut in response to West Nile virus infection. Small RNA regulatory pathways are an integral component of endogenous transcriptional regulation, as well as innate immunity. The role of small-interfering RNAs (siRNAs, 20-23 nts) in response to viral infection in invertebrates has been extensively studied and well-characterized. However, the extent to which other small RNA populations participate in antiviral defense is comparably less understood. Recent evidence has suggested that components of the PIWI class of nucleic acid binding proteins can also participate in antiviral defense in mosquitoes. The hypothesis for this observation is that the PIWI-interacting RNA (piRNA) pathway may act as a compensatory response to viral infection when the RNAi pathway is deficient or overburdened. Primary piRNAs show significant strand bias, and range from 24-30 nts in length. Recent studies with Chikungunya virus and Sindbis virus (Togaviridae) in Aedes aegypti and Ae. albopictus mosquitoes and cell lines have described the production of viral-derived piRNA-like small RNAs that exhibit “ping-pong” amplification. In this model, primary piRNA transcripts with a strong bias for a 5’ uridine terminus bind target transcripts and result in cleavage of the target 10 nucleotides upstream from the 5’ uridine residue. Due to this, secondary piRNAs produced in this manner exhibit an adenine residue in the 10 position. Here we characterize 24-30 nt piRNA-like small RNAs produced in response to West Nile virus (Flaviviridae) infection in Culex quinquefasciatus mosquitoes. Interestingly, while exhibiting a strong bias for sense-strand orientation, viral-derived piRNA-like RNAs did not exhibit signatures indicative of ping-pong amplification. Previous studies of dengue virus-infected Ae. aegypti support this observation, suggesting that the piRNA pathway may function differently in response to flaviviruses compared to alphaviruses.

Eamon Quick, University of Colorado School of Medicine presented a poster describing an ex vivo spinal cord slice culture (SCSC) model for studies of reovirus and West Nile virus (WNV) pathogenesis. Viral infections of the spinal cord are a significant cause of morbidity and mortality around the world. In this study, viral infection of ex vivo SCSCs caused increased cell death (MTT and LDH assays) and increased expression of genes associated with inflammatory responses compared to mock-infected controls. This suggests that the SCSC model can be used to assess local cellular responses to viral infections and to evaluate novel therapeutic agents for virus-induced spinal cord disease.

Irma Sanchez-Vargas, Colorado State University described vertical and venereal transmission of DENV2 in a Genetically Diverse Laboratory Strain of Ae. aegypti (GDLS) from Mexico. Dengue (DEN) is the most prevalent arthropod-borne viral disease in the world; DEN disease incidence has increased dramatically in the last 50 years. Ae. aegypti is the most important vector of DENV to human hosts and the vector remains persistently infected with DENV for life. Given the tremendous public health impact of dengue infections globally and the lack of commercially licensed vaccines or therapeutic dengue drugs, our best weapon for controlling DENV is vector control. Determination of secondary modes of DENV maintenance in mosquitoes and a better understanding of DENV transmission dynamics could help to target more vector control approaches to be used in integrated mosquito control programs.
Vertical and venereal transmission of DENV ensures presence of viral pathogens in mosquitoes independent of their feeding on viremic human blood carrying DENV and may play a crucial role in maintenance of the viruses especially during protracted dry seasons or inter-epidemic periods in an endemic setting. In addition vertical transmission has been reported in nature by the detection of DENV in the field-collected larvae and field caught adult male mosquitoes. The presence of the DENV in the male mosquitoes is suggestive that they can initiate a new cycle of infection by venereal transmission to the female mosquito during mating. Few studies have ever addressed the role of males in DENV transmission dynamics and only one study has focused on DENV venereal transmission in Ae. aegypti. In order to understand the factors that influence vertical and venereal transmission of in Ae. aegypti mosquito We conducted experiments to demonstrate that female mosquitoes orally infected with DENV are able to transmit the virus to male progeny by a vertical transmission mechanism. These infected male mosquitoes are able to transmit DENV to un-infected females during mating, which become infected and transmit the DENV horizontally and contribute to viral maintenance in nature. We observed that orally infected GDLS mosquitoes can vertically transmit DENV2 to their F1 progeny from the second gonotrophic cycle (E2). We found a vertical transmission rate of 19% in larval pools (4th instar), when females feed a second time with non-infectious meal 7 d later (E2-7d) and the vertical transmission rate was higher when females fed a second time at 10 d (E2-10d) or 21 d (E2-21d) after initial intake of a bloodmeal. However, there was no significant difference between E2-10d and E2-21d in vertical transmission rates. A significant difference was observed between the vertical transmission of E2-7d and E2-10d. The filial infection rate and the vertical transmission rates in virgin male progeny from individual infected female obtained were higher than those found in other studies. Likewise, no significant correlation was detected between virus titer from the female parent and proportion of progeny infected. In addition, we report, for the first time, that DENV2 vertically infected Ae. aegypti males are capable of transmitting the virus efficiently to females venereally during mating. Although, we had not observed difference in venereal infection of DENV between fed (non-infectious blood meal) or non-fed females prior to mating as previously reported in Ae. albopictus with DENV .We observed that virus dissemination rates were higher in venereal infected females that were fed before mating.

Tony Schountz, University of Northern Colorado described his work involving Sin Nombre virus. Deer mice (Peromyscus maniculatus) are the principal reservoir hosts of Sin Nombre virus (SNV), the cause of the great majority of hantavirus cardiopulmonary syndrome (HCPS) cases in North America. SNV, like all hantaviruses with their reservoirs, causes persistent infection without pathology in deer mice and appear to elicit a regulatory T cell response. Deer mice are also susceptible to Andes virus (ANDV), which causes the great majority of HCPS cases in South America, but they clear infection by 56 days post infection without signs of disease. We examined lymph node cell responses of deer mice infected with ANDV to determine expression profiles upon in vitro recall challenge with viral antigen. Because the deer mouse genome is currently unannotated, we developed a bioinformatics pipeline to use known lab mouse (Mus musculus) cDNAs to predict genes within the deer mouse genome and design primers for quantitative PCR (https://dna.publichealth.uga.edu/BlastPrimer/BlastPrimer.php). Of 94 genes examined, 20 were elevated, the plurality of which were Th2-specific, whereas 12 were downregulated. Other genes represented Th1, regulatory T cells and follicular helper T cells, but not Th17 cells, and B cells, indicating that many cellular phenotypes participate in the host response to Andes virus.

Charles Scianna, University of Colorado School of Medicine, investigated Varicella Zoster Virus (VZV) gene expression in cell culture. Quantitative cDNA analysis VZV infected cells in tissue culture showed significantly more transcripts for VZV open reading frame (ORF) 9 than ORF 21. To explore the mechanism governing this difference in transcript abundance, it was initially found that the ORFs 9 and 21 transcripts decay at a similar rate. My hypothesis is that the difference in ORFs 9 and 21 mRNA abundance is dependent on the relative amount of RNA polymerase II bound to each gene. To test this hypothesis chromatin immunoprecipitation (ChIP) was used to determine the amount of RNA polII on similar regions of ORFs 9 and 21. The regions selected included the gene promoter, ORF and 3’ untranslated region. The preliminary results showed that for both ORFs 9 and 21, more RNA polII was present at the promoter than other regions of gene. Additionally, since both ORF 9 and 21 transcription is inhibited when the neurotropic alphaherpesvirus becomes latent, the seeding of heterochromatic histone markings, e.g. H3K9 tri-methylation, was also examined using ChIP. Initial results indicate that during steady state lytic virus infection, H3K9 tri-methylation is not present on either ORF.

J. Jordan Steel, Colorado State University described a Subgenomic Reporter RNA System for Detection of Wild-Type Sindbis Virus Infection in Live Mosquitoes. Alphaviruses are mosquito-borne pathogens that can cause severe human disease, several of which are considered potential biological weapons. Defining how alphaviruses infect their mosquito hosts and transmit to mammalian hosts is a critical area of research for understanding how infection may occur, but the tools for monitoring alphavirus infection in mosquitoes has relied largely on alphaviruses engineered to express virus encoded reporter proteins. These drastic modifications to the viral genome, sometimes increasing the genome size by over 10%, have large effects on viral replication and virulence in their hosts. To better visualize how wild type alphaviruses infect, disseminate, and transmit from mosquitoes, we are developing a transgenic mosquito system where reporter constructs encoded within the mosquito’s genome is activated only during infection.
We have developed a sindbis virus mini-genome style expression system that can produce an RNA species in mosquito cells that is recognized and replicated by trans-complemented viral non-structural proteins. This reporter RNA expression construct (which contains the Sindbis virus 5’ UTR, 3’UTR, eGFP fused to the first 143 amino acids of nsP1, and the viral subgenomic promoter 5’ to an mCherry reporter gene) can be transiently transfected into C6/36 mosquito cells and expresses eGFP but does not produce detectable mCherry. However, when transfected C6/36 cells are infected with Sindbis virus, mCherry fluorescence is detectable 24 hours-post infection. These results represent the first time a replicationcompetent RNA genome structure has been launched from DNA in mosquito cells, and opens the way for further work into mosquito DNA-launch systems.
Based on our success in vitro, we are in the process of developing transgenic Ae. aegypti mosquitoes that will constitutively express the reporter RNA. The Higgs White-Eye strain of Ae. aegypti were transduced with a Mariner Mos1 transposon containing a Sindbis reporter RNA expressing mCherry from the subgenomic promoter. We have established 7 transgenic families that we are currently analyzing for reporter RNA activity.

Amy Stone, University of Colorado School of Medicine described a study of human plasmacytoid dendritic cell’s (pDCs) Type I and Type III Interferon (IFN) response to a region of the Hepatitis C Virus genome called the pU/UC tract. Hepatitis C virus is a member of the Flaviviridea family. In the US, approximately 3.2 million people are infected with this small enveloped positive-strand RNA virus, making HCV the most common chronic bloodborne infection. Type III IFNs (IL-28A/B, IL-29) have been implicated in spontaneous and drug-induced clearance of HCV. pDCs are innate immune cells whose primary function is to detect viruses and respond with IFN production. Upon transfection with the pU/ UC tract, pDCs respond by producing Type I and III IFNs and upregulating Pattern Recognition Receptors. The conditioned media from a pDC cell line transfected with the pU/UC RNA was able to inhibit viral replication in the in vitro HCV replication model system (JFH-1/Huh7.5) through a JAK/STAT mediated pathway. Importantly, she showed that ex vivo pDCs from human subjects responded with Type I and III IFN production and the magnitude of that response varied by IL-28B genotype. Her study investigated the role of pU/UC tracts in induction of the IFN response.
Glenn C. Telling, Colorado State University described conformation-dependent prion protein epitopes. Whereas prion replication involves structural rearrangement of cellular prion protein (PrPC), the existence of conformational epitopes has been the subject of speculation and controversy, and PrP transformation is monitored by immunoblot detection of PrP27-30, a protease-resistant counterpart of pathogenic PrPSc. We now describe the involvement of specific amino acids in conformational determinants of novel monoclonal antibodies (mAbs) raised against randomly chimeric PrP. Epitope recognition of two mAbs depended on polymorphisms controlling disease susceptibility. Detection by one, referred to as PRC5, required alanine and asparagine at discontinuous mouse PrP residues 132 and 158, which acquire proximity when residues 126 to 218 form a structured globular domain. The discontinuous epitope of glycosylation-dependent mAb PRC7 also mapped within this domain at residues 154 and 185. In accordance with their conformational dependence, tertiary structure perturbations compromised recognition by PRC5, PRC7, as well as previously characterized mAbs whose epitopes also reside in the globular domain, whereas conformation independent epitopes proximal or distal to this region were refractory to such destabilizing treatments. We also show that PRC7 fails to recognize PrP that is glycosylated at asparagine 196, and that PrP glycosylated at asparagine 180 preferentially accumulates in the brains of animals infected with scrapie or CWD prions. Our studies also address the paradox of how conformational epitopes remain functional following denaturing treatments, and indicate that PrPC and PrP27-30 both renature to a common structure that reconstitutes the globular domain.

Sahaja Templin-Hladky, Colorado State University, presented the quantification of feline immunodeficiency virus proviral load in FIVpco subtype A infected puma (Puma concolor) and bobcat (Lynx rufus). Feline immunodeficiency virus (FIV) is a lentivirus that naturally infects domestic and wild feline species. Individual feline species typically carry genetically-distinct species-specific FIV strains. One notable exception includes sympatric populations of puma (Puma concolor) and bobcat (Lynx rufus) in California and Florida, which are both infected with FIV-Puma concolor subtype A (FIVpcoA). In contrast, FIVpco subtype B is found only in pumas. We hypothesize that FIVpcoA may be a bobcat-adapted virus, which has more recently been transmitted to pumas. Host-adapted lentiviruses typically replicate to high viral load in their natural host species. Thus, if FIVpcoA is a bobcat-adapted virus, we expect FIVpcoA viral loads to be higher in bobcats than pumas. In order to test this hypothesis, we developed a quantitative polymerase chain reaction (qPCR) assay to compare FIVpcoA proviral loads in infected bobcats and pumas. This assay targets highly conserved sequences in the FIVpcoA envelope (env) gene and employs FIVpcoA env plasmid standards for proviral quantification and verification of assay efficiency. We compared proviral load for 6 pumas and 18 bobcats using DNA extracted from whole blood. We found that bobcats had significantly higher FIVpcoA proviral loads than pumas (p = 0.0006). Furthermore, we found that pumas infected with FIVpcoA had lower proviral loads than pumas infected with FIVpcoB (Blake et al., 2006). These preliminary data support the hypothesis that FIVpcoA evolved to replicate efficiently in bobcats but has lower fitness in pumas. In the future we plan to expand the number of bobcat and puma samples to further investigate this hypothesis.

Igor Traktinskiy, University of Colorado School of Medicine described how VZV infection of human brain vascular adventitial fibroblasts downregulates STAT mRNA expression and inhibits translocation pSTAT to the nucleus. Primary varicella zoster virus (VZV) infection causes varicella (chickenpox), after which virus becomes latent in ganglionic neurons. With advancing age, a declining cell-mediated immunity to VZV leads to virus reactivation and manifests as zoster (shingles). Zoster may be complicated by VZV vasculopathy (TIAs or stroke) caused by productive virus infection in arteries. Within the first year after zoster, there is a 30% increased risk of stroke. In VZV vasculopathy from 4 to 10 months after zoster, both viral antigen and abundant leukocyte subsets are found. To understand the mechanism(s) by which VZV persists despite a robust immune response, we focused on the Jak-STAT pathway that is commonly activated after viral infection. In uninfected cells, type I interferons (specifically IFNα) bind to interferon receptors, resulting in auto-phosphorylation of Jak proteins, which in turn phosphorylates one of the STAT proteins (pSTAT). pSTAT then complexes with interferon regulatory factor (IRF) 9, and the complex is translocated to the nucleus. After translocation, pSTAT upregulates transcription of multiple antiviral proteins (Mx1, RNAse L and protein kinase R). In SCID-hu xenograft models, VZV-infected skin cells have decreased amounts of IFNα, and pSTAT does not translocate to the nucleus, as in uninfected bystander cells. Herein, we showed that in VZV-infected primary human cerebrovascular adventitial fibroblasts, expression of STAT1? mRNA was downregulated by ~50% compared to mock-infected fibroblasts. In contrast, Jak mRNA was unaffected by VZV infection. Immunostaining revealed that pSTAT did not translocate to the nucleus in VZV-infected cells, but was seen in the nucleus of uninfected bystander cells as well in mock-infected cells. Overall, VZV infection of cerebral adventitial fibroblasts blocked translocation of pSTAT to the nucleus. Our findings provide a potential mechanism by which transcription of antiviral proteins are downregulated by VZV, which in turn leads to viral persistence, vascular remodeling and potential stroke.

Ryan Troyer, Colorado State University described Expression of APOBEC3 lentiviral restriction factors in felines. Feline immunodeficiency virus (FIV) is a naturally occurring T-cell tropic lentiviral disease of felids with many similarities to HIV/AIDS in humans. In contrast to infection with host-adapted FIV, experimental infection of domestic cats with a lentivirus native to the puma (PLV) results in productive yet avirulent infection which progresses to nearly undetectable viral load concurrent with extensive G-to-A hypermutation of PLV provirus, suggestive of APOBEC3-mediated viral restriction. Proteins encoded by feline APOBEC3 (A3) genes A3Z3 and A3Z2-Z3 can inhibit vif-deficient FIV in vitro. We developed realtime qPCR assays to examine expression of the feline A3 genes A3Z2, A3Z3 and A3Z2-Z3 to determine expression levels during host-adapted and non-adapted FIV infections. Regulation of A3 expression by cytokines, mitogens and FIV infection was characterized in cultured cells, and A3 expression in multiple different tissues and blood cells was determined in uninfected, FIV infected, PLV infected and FIV/PLV coinfected cats. In all feline cells and tissues studied, there was a striking difference in expression between the A3 genes which encode FIV inhibitors, with A3Z3 mRNA abundance exceeding that of A3Z2-Z3 by 300-fold or more. A3 genes were able to be regulated, with interferon-alpha treatment of cat T-cells resulting in upregulation of A3 expression, and treatment with the mitogen concanavalin A resulting in A3 downregulation. Basal levels of A3 expression in vivo varied between different cat tissues, with secondary lymphoid organs and PBMC having the highest expression levels. Acute FIV and PLV infection of cats resulted in no detectable change in A3 expression in PBMC, bone marrow or lymph nodes. However, FIV-infected cats had significantly elevated A3 expression in the thymus. Relative abundance of A3Z3 mRNA compared to A3Z2-Z3 suggests that A3Z3 may be the major anti-lentiviral APOBEC3 gene in felines. Feline A3 expression was regulated by cytokine and mitogen treatment, but FIV and PLV infected cat lymphoid tissues did not have altered A3 expression with the exception of the thymus, which had increased expression in FIV-infected cats.

Natalia Voge, Colorado State University presented the metabolomics-based diagnosis of dengue infections. Yearly, an estimated of 100 million cases of dengue fever (DF) and 500,000 cases of severe dengue occur (DHF/DSS), overwhelming public health capacity. The goal of our research is to identify and characterize candidate small molecule biomarkers of DF and DHF/DSS in acute phase serum and noninvasive saliva and urine clinical specimens using a liquid chromatography–tandem mass spectrometry based metabolomics approach. Metabolites are the intermediaries and products of metabolism, they can serve as valuable biomarkers for disease or indicators of toxicological exposure and reveal disease processes. We have identified some candidate biomarkers with this approach, we are currently conducting further validation test on the candidate biomarkers.

A. Christy Wyckoff, Colorado State University and the National Wildlife Research Center, United States Department of Agriculture presented information Estimating Prion Adsorption Capacity of Soil by BioAssay of Subtracted Infectivity from Complex Solutions (BASICS). Prions, the infectious agent of scrapie, chronic wasting disease and other transmissible spongiform encephalopathies, are misfolded proteins that are highly stable and resistant to degradation. Prions are known to associate with clay and other soil components, enhancing their persistence and surprisingly, the transmissibility. Currently, few detection and quantification methods exist for prions in soil, hindering an understanding of prion persistence and infectivity in the environment. Our objective was to quantify the prion adsorption capacity of whole soil (sandy loam) and purified montmorillonite clay by BioAssay of Subtracted Infectivity in Complex Solutions (BASICS). We incubated prion positive brain homogenate (RML) for 24 hours with 10% sandy loam soil or Mte. Soil-free supernatant was intracerebrally inoculated into susceptible mice. The number of days post inoculation to clinical disease was used to calculate the infectious titer of each treatment. BASICS indicated sandy loam soil bound and removed ³ 95% of infectivity. Mte treatments removed lethal doses (99.98%) of prions from inocula effectively preventing disease in the mice. Our data reveal significant prion-binding capacity of soil and the utility of BASICS to further investigate persistence and decomposition of prions in the environment. Additionally, since Mte successfully rescued the mice from prion disease, Mte might be used for remediation and decontamination protocols.

Yonghua Zhuang, University of Colorado School of Medicine described the role of Daxx in neuronal apoptosis occurring in reovirus-infected brains. Reovirus infection is a well-characterized experimental system for the study of viral pathogenesis and anti-viral immunity within the CNS. It has been reported that c-Jun N-terminal Kinase (JNK) and the Fas death receptor each play a role in neuronal apoptosis occurring in reovirus-infected brains. Death associated protein 6 (Daxx) is a cellular protein that mechanistically links Fas signaling to JNK signaling in several models of apoptosis. This study showed that Daxx is upregulated in the reovirus-infected brain. Daxx upregulation is limited to brain regions that undergo reovirus-induced apoptosis, and occurs specifically within the cytoplasm of infected neurons where the protein colocalizes with Fas. In vitro expression of a dominant-negative form of Daxx (DNDaxx) inhibited apoptosis of reovirus-infected cells, indicating that Daxx is an important regulator of reovirus-induced apoptosis. Overall, these data suggest that reoviral infection drives the expression of neuronal Daxx, which likely serves to lower apoptotic threshold by linking Fas and JNK signaling.
Acknowledgements
The 12th annual Rocky Mountain Virology Association meeting was supported in part by donations from Novus Biologicals, Roche Applied Science, PhDPosters, New Belgium Brewery, Pateros Creek Brewery, and our hosts at Pingree Park. Funding for this conference was made possible [in part] by 1 R13 AI 102540 - 01 from the National Institute of Allergy and Infectious Diseases. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
2484