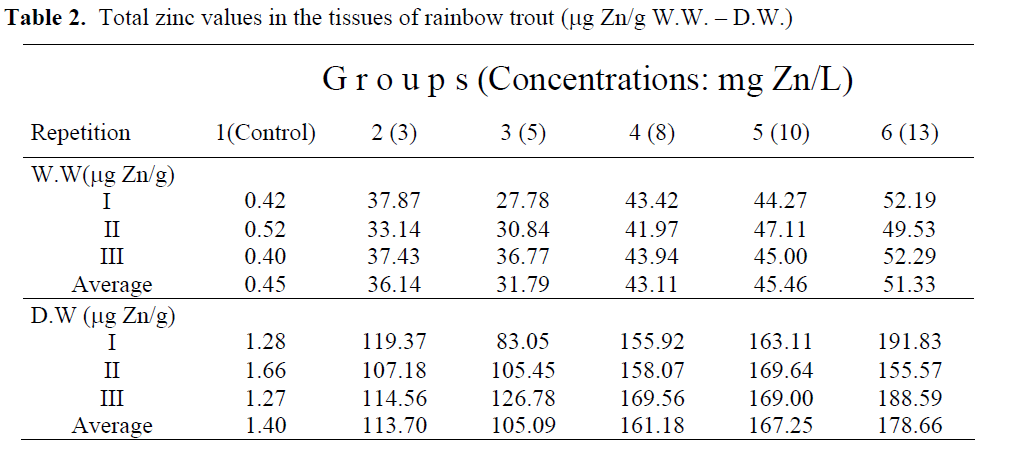Keywords
Zinc, copper, mortality, rainbow trout, heavy metal accumulation
Introduction
Water sources constitute the major parts of the biosphere. With the thought of the cleaning capacity of this huge water column’s itself; industrial wastes, household wastes and other polluting substances are released into this environment by nearly all countries. By the time being, their accumulation may have devastating effects on living organisms biologically (Kocata?, 1986).
Fish and other marine organisms are one of the main seafood sources. They are affected by increasing water pollution in consequence of technological improvement. Rivers and seas are excessively contaminated with heavy metals, hydrocarbons, pesticides and organic matters released from domestic, industrial, mining and agricultural effluents. The most important ingredients of these pollutants are the chemical substances that stay longer and are toxic in the water columns. Within them, heavy metals have positive effects on the vital activities of several organisms when they are in acceptable concentration limits, but if they reached above or below of the limits, they threaten human health through the impairment in the food chain by affecting biological activities of the living organisms in the ecosystem (Canl? et al., 1998).
Natural waters which are an environment for living organisms comprise a long food chain that characterizes the life itself. Phytoplanktons are eaten by zooplankton and zooplanktons are consumed by larger animals (Bat et al. 1999; Bat et al., 2000). Living organisms in every circlet of the food chain are able to absorb metals in the presence of heavy metals in the water (Uysal, 1978; Karahan, 1991; Öztürk, 1994; Öztürk et al., 1995; Sunlu and Egemen, 1997). In general, metals are taken by fish in several routes; (a) from water columns by the gills as dissolved ions, (b) from food accumulated in fish feed, (c) from other organisms living with fish and carrying metals in their body (Geldiay and Kocata?, 1988). The accumulation levels of the metals in living organisms are dependent to species, the size of individuals (Uysal et al., 1986), tissues and organs as well as the type of metal (Öztürk, 1994; Lloyd, 1992). Several authors reported that the toxic effects of zinc and copper vary depending on the species and age of fish, water temperature, pH, organic matters in water and salinity (Sprague and Ramsay, 1965; Gordon et al., 1974; Waiwood and Beamish, 1978; Hansen et al., 2002). Karakoç (1999) conducted an experiment using Tilapia nilotica by exposing to 0.1 mg Cu/l solution containing ‰5 and ‰20 NaCl in water for 30 days and found averages of 152.19 μg/g and 61.30 μg/g W.W. copper in the muscle tissues of fish, respectively. Results clearly showed that the accumulation value of copper in tissues vary depending on the NaCl content of the water.
In the study conducted by McGeer and et al. (2000); rainbow trout was exposed to 0,075 mgl/l copper solution for 20 days. The total hardiness and pH of the medium was 140 mg/L CaCO3 and 8 respectively. They found that mean value of copper in total fish tissues of control group was of 1.87 ±0.09 μg /g Cu for W.W and the accumulation average was of 2.46 ±0.27μg Cu/g (D.W.) in the muscle of fish.
Rainbow trout is commonly cultured in either fresh water or sea water in the recent years and its production and consumption rates are gradually increasing. Therefore, it was chosen as the research material for this study due to the economic importance.
Materials and Methods
Rainbow trout used in the experiments were brought to the research area (Aquaculture Division of the Faculty) and acclimatised at natural condition for 30 days. Before the experiments started, zinc and copper concentration and some physico-chemical peculiarities of the water were analyzed. Temperature (°C), dissolved O2, pH and ammonia were measured daily, and the average values of those were 14.62 ±0.41°C, 7.49 ±0.15 mg/L O2, 7.48 ±0.12 and 0.013 ±0.002 mg/L NH3–N, respectively (total hardness of 249.56 mg/L CaCO3). Average weight of fish were determined by collective weighting before placing them into the media and special attention was paid to put similar weighing fish at each experimental groups. Additionally, the length and weight of fish were measured one by one either at the end of the experiment or when any of them died. As heavy metals, zinc chloride (ZnCl2) and copper sulphate (CuSO4) were used. Two separate experiments were conducted which are experiment I and II including different concentrations of zinc and copper, respectively. Concentrations were; 3, 5, 8, 10, 13 mg/L for zinc and 0.01, 0.025, 0.050, 0.075, 0.1 mg/L for copper as well as control. Experiments were designed as 3 replicates in the tanks containing 100 L of water. Each experiment consists of 6 groups. Zinc and copper were not added into the control groups, it was also determined that tap water did not contain either of heavy metals. At both experiments, a total of 180 fish were used and randomly divided total 18 groups making 10 fish in each group.
Observations were carried out regularly during the experimental period. Any of fish death or an abnormality in swimming behaviour was immediately noted. Regular feeding and water changes were applied and after the 1 month duration, experiments were terminated. The death fish were counted and put into plastic bags after determining the length and weight of fish. All samples were kept at -21°C in a deep freeze and heavy metal concentrations in fish tissues were measured by Wet Burning Method (Benhard, 1976).
Accumulation zinc and copper in total body tissues of rainbow trout at different medium concentrations were determined by Atomic Absorption Spectrometric Methods at the end of 30-days experimental period. (Anonymous, 1976; Sar?kaya et al., 1987). Statistical analyse of the data was carried out with Minitab 13 statistical package programs and ANOVA was used to compare the accumulation levels for both heavy metals.
Results and Discussion
Fish in the control group showed calm swimming behaviour and swam closely to each other whereas fish exposed to metal solutions had signs of extreme stress, difficulty in respiration, reluctance to food and swam separately from each other. In control group fish did not have any deformation in any of the body tissues, but there were evident deformation in the metal exposed to fish groups.
Table1. showed that minimum and maximum average weights were of 2.99 ±0.23g (group 6) and 5.16 ±0.61g (group 1). Final minimum and maximum average length were 6.60 ±0.18 cm (group 6), and 7.42 ±0.31 cm (group 1) in the fish exposed to zinc. However, final minimum and maximum weights were 5.97 ±0.45 g (group 6), and 7.79 ±0.65 g (group 1), minimum and maximum length were 8.09 ±0.22 cm (group 6) and maximum length was 8.62 ±0.26 cm (group 1) in the fish exposed to copper. At the end of the experiment, it was concluded that fish in the control groups took the feed well and grew better comparing to the fish exposed to metal concentrations.
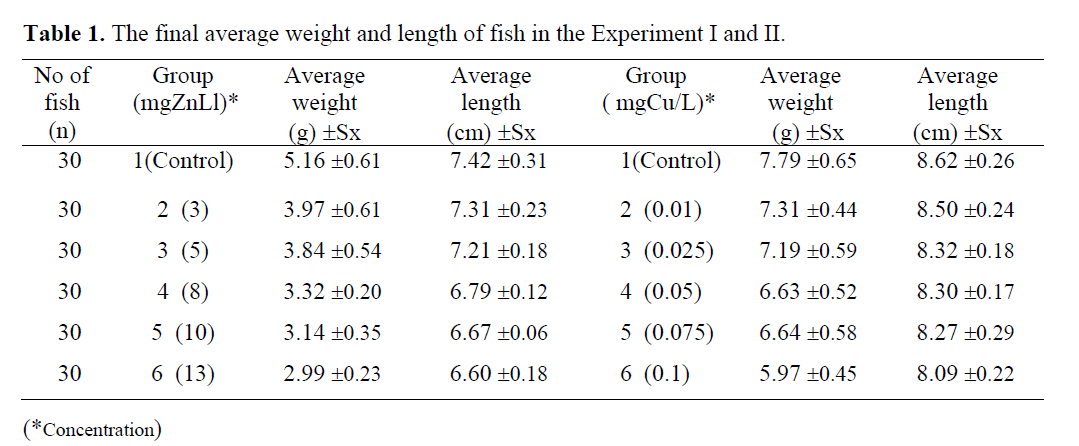
Table 1: The final average weight and length of fish in the Experiment I and II.
Total zinc concentrations are presented in Table 2. According to wet and dry weights of fish exposed to metal concentrations, the minimum zinc accumulation was 0.45 μg/g W.W, 1.40μg/g D.W. in whole fish tissues in the control group (group 1), whereas the maximum zinc accumulation was 51.33 μg/g W.W., 178. 66 μg/g D.W. in fish tissues in the group 6. Zinc accumulation in whole fish tissues has increased by increasing levels of zinc concentration. The wet and dry weight of rainbow trout tissues exposed to zinc solutions were also determined (Table 3, Figure 1, 2). Statistically significant differences were found both in wet and dry weights at different concentration levels of zinc (P<0.05). Control and highest zinc concentrations was quite different than the others for total WW although first two zinc concentrations had similar effect. Concentrations for 8, 10 and 13 mg Zn/L had also no differences statistically than others. Total DW in the highest three zinc concentrations took place in the same group whereas the lower two were in another group. Control was different than all groups.
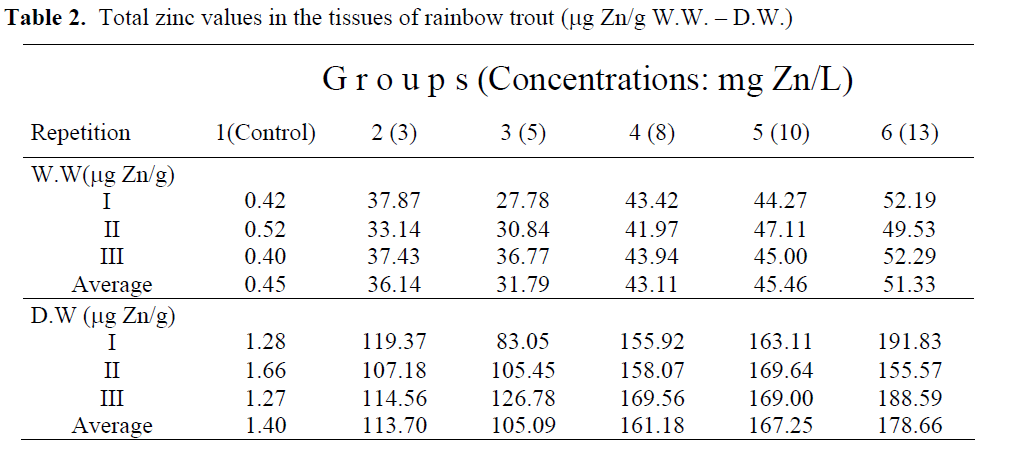
Table 2: Total zinc values in the tissues of rainbow trout (μg Zn/g W.W. – D.W.)
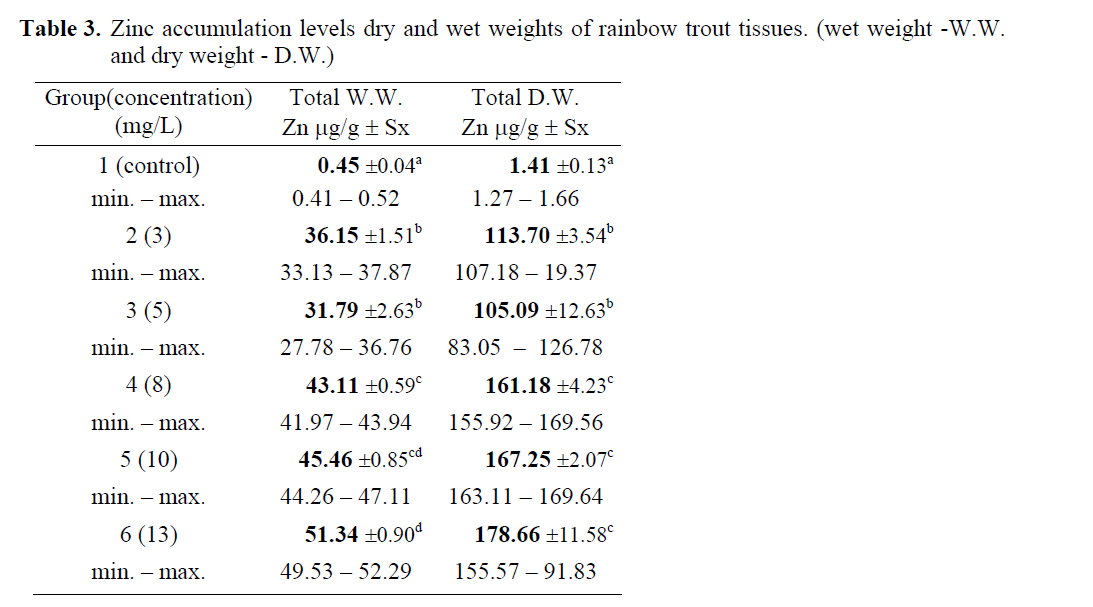
Table 3: Zinc accumulation levels dry and wet weights of rainbow trout tissues. (wet weight -W.W. and dry weight - D.W.)
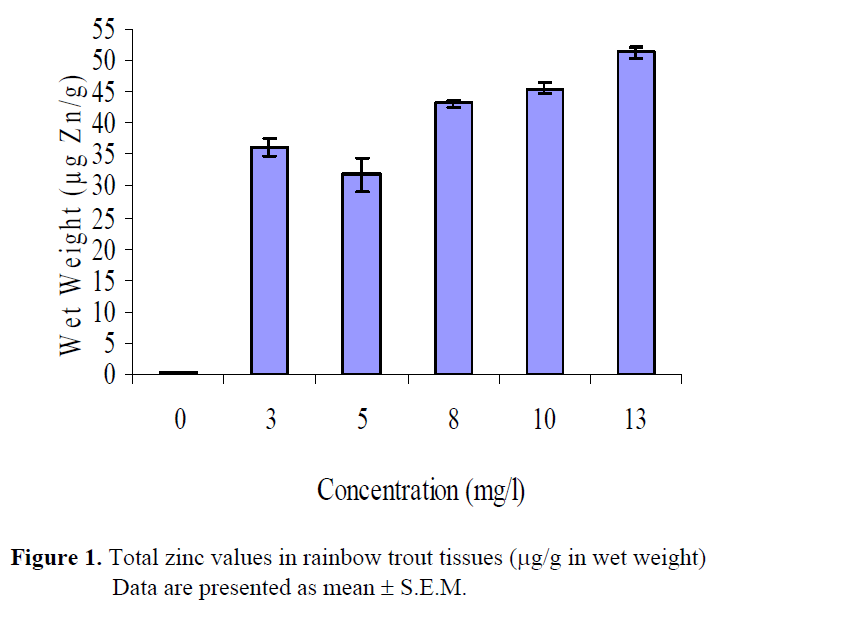
Figure 1: Total zinc values in rainbow trout tissues (μg/g in wet weight) Data are presented as mean ± S.E.M.
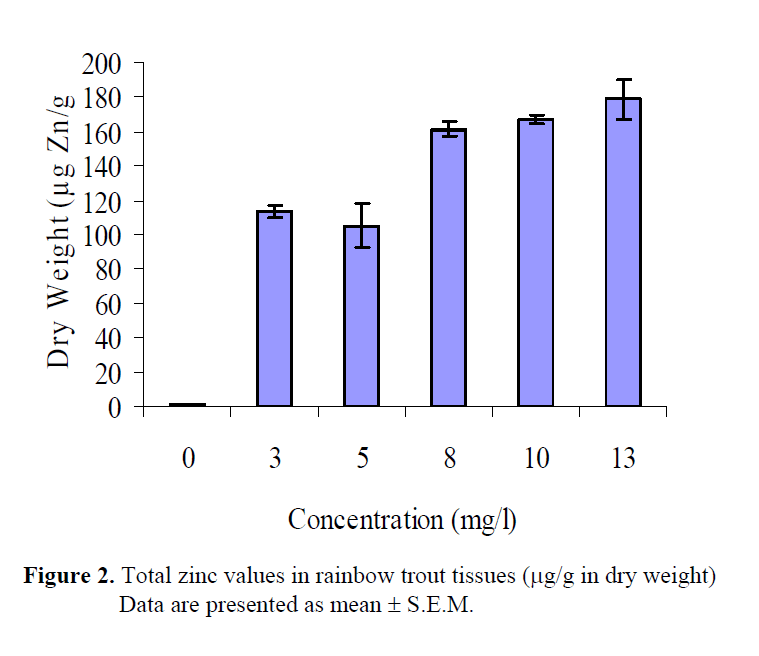
Figure 2: Total zinc values in rainbow trout tissues (μg/g in dry weight) Data are presented as mean ± S.E.M.
Similar results were obtained for the copper values in the tissues of fish exposed to different copper concentrations. Wet and dry weights values are presented in Table 4. The minimum and maximum copper accumulation mean values in total fish tissues were 0.44 μg/g for W.W., 1.45 μg/g for D.W. and 1.43 μg/g for W.W., 5.04 μg/g for D.W. in the control group (group 1) and in the group 6, respectively. An increase was found in the copper concentrations in fish tissues with an increasing concentration in the water (Table 4).
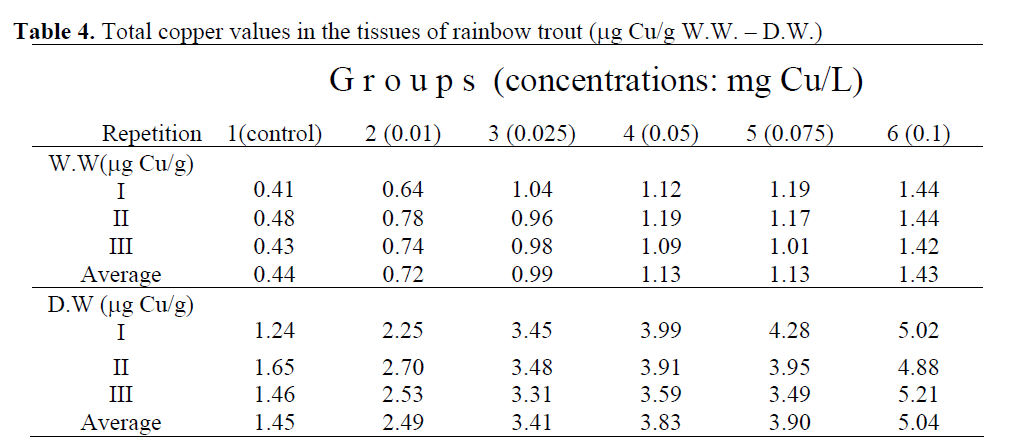
Table 4: Total copper values in the tissues of rainbow trout (μg Cu/g W.W. – D.W.)
According to the one-way analysis of variance, in the copper accumulation values in dry and wet weights of fish tissues exposed to different copper concentrations, statistically significant differences were determined (P<0.05), (Table 5; Figure 3, 4). Statistical groups were same both in WW and DW. Control was 2 mg/L and the highest copper concentration value was a group individually whereas the others were in the same statistical group.
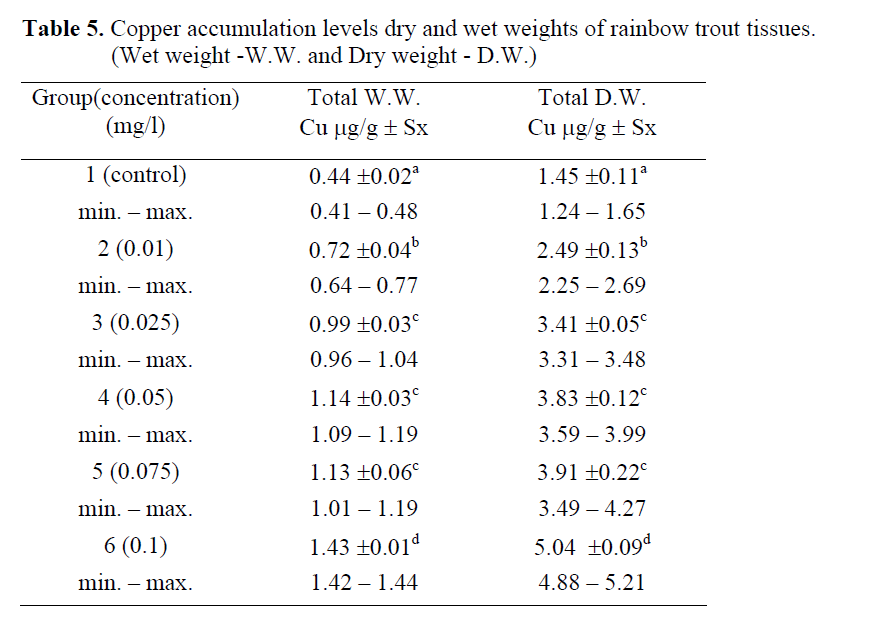
Table 5: Copper accumulation levels dry and wet weights of rainbow trout tissues. (Wet weight -W.W. and Dry weight - D.W.)

Figure 3: Total copper values in rainbow trout tissues (μg/g in wet weight). Data are presented as mean ± S.E.M.
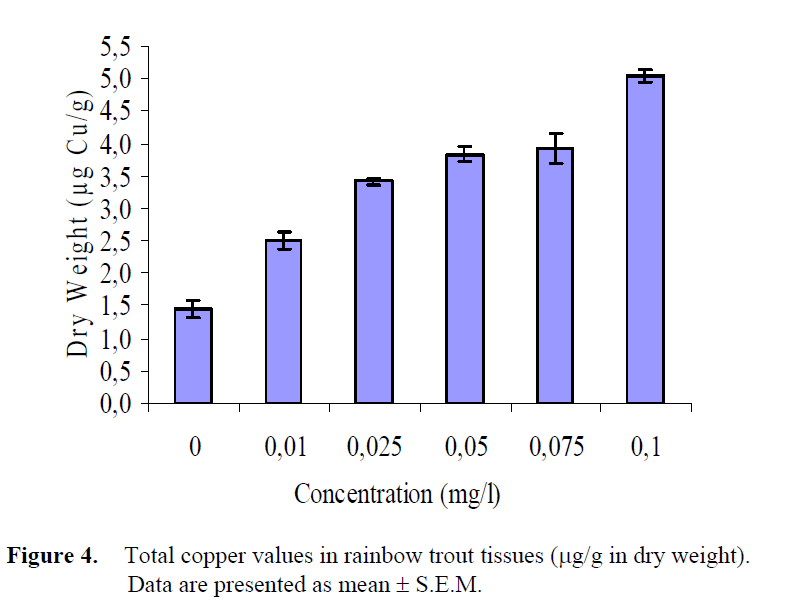
Figure 4: Total copper values in rainbow trout tissues (μg/g in dry weight). Data are presented as mean ± S.E.M.
Heavy metal accumulation and its toxic effect were reported by several authors in the tissues and organs of fish collected from different sources where metal pollution is present (Hejkal et al., 1983; Turner et al., 1986; Güven and Topcuo?lu, 1991; Göksu, 1993). Result of this study has indicated that accumulation of heavy metals in whole fish tissues increased by increasing levels of heavy metals used. These results are in agreement with the studies have shown that accumulation of metals in tissues of fish is dependent on exposure concentration and duration (McGeer and et al., 2000).
According to Lloyd (1992), copper and zinc have similar toxicity but copper has more toxic effects than zinc to fish and the toxicity varies depending on ionised form which is more toxic or connected with organic compounds position. Several authors also reported that the toxic effects of zinc and copper vary depending on the species and age of fish, water temperature, pH, organic matters in water and salinity (Sprague and Ramsay, 1965; Gordon et al., 1974; Waiwood and Beamish, 1978; Hansen et al., 2002, Karakoç, 1999). In the present study, extreme differences of zinc and copper values in rainbow trout tissues were due to differences of solution concentrations. Copper is more toxic than zinc (Bat et al., 1998) and the lethal dose limit for zinc is quite higher than that of copper as it is valid in this study.
Metal accumulation levels in fish tissues increase in connection with exposure period and concentration. However this effect changes according to the type of metal salts and species of organisms (Wong et al., 1977; Güven et al., 1999). Similarly, copper uptakes from water cause the accumulation of copper in the tissues and organs of fish (Yamamato et al., 1977; Merlini, 1980; Dixon and Hilton, 1985; Karg?n and Erdem, 1991).
Results of this research were not agreed with the results obtained by McGeer and et al. (2000). The differences of the average values of copper recorded in total body tissues between two studies may be due to the different experimental conditions (such as pH and total hardness), which are copper exposed period and initial copper levels of control groups. Our research was conducted at 249.56 mg/L CaCO3 total hardiness and 7.64 pH. While McGeer and et al. (2000) conducted their research in the medium with total hardiness at 140 mg/L CaCO3 and 8 pH. However, the copper exposed period was 30 days in our study that was ten days more comparing to the period in the study of McGeer and et al. (2000). Additionally, the initial copper level of the control groups in our study was nearly one fifth of their value. Nevertheless, in the comparison of daily accumulation levels with control, daily values are very close in both studies.
Conclusion
The accumulation level and toxicity of heavy metals to organisms vary according to the type of metal itself and the species of organisms. Even if within the same species accumulation level and toxicity of same heavy metal can vary as it was discussed above. Heavy metals can be transferred through the upper classes of the food chain once accumulated by an aquatic organizm. An increase in metal remnants in food chain present in an ecosystem reaches to thousands folds in birds and human fed on aquatic products. Fish is one of the main food sources, and as a part of aquatic life face to the toxic effects of metals. It is very important to determine the accumulation levels of heavy metals in fish that making high proportion of protein sources in the food chain for human health and sustainable ecological balance. Therefore, this study was conducted to determine the toxic effects of industrial wastes on the aquatic organisms and was supported by metal accumulation in the tissues and organs of some organisms collected from natural environment. As conclusion, results have indicated that accumulation of zinc and copper in the body tissues had increased with increasing metal concentrations in the medium.
Acknowledgement
I assure that whole activities in the present study were conducted in accordance with national and institutional guidelines for the protection of human subjects and animal welfare.
The authors would like to thank Ondokuz May?s University for its financial support for enabling to carry out the study.
1664
References
- Anonymous, (1976). S.M.E.W.W. (Standard Methods for the Examination of Water and Wastewater, Part 800 ). Bioassay methods for aquatic organisms. Amer. Water Works Ass.Water Pollutiation Fed., Washington DC., 683-872.
- Bat, L., Çulha, M., Akbulut, M., Gündogdu, A. and Sezgin, M. (1998). Toxicity of zinc and copper to the hermit crab Diogenes pugilator (Roux). Turkish Journal of Marine Sciences, 4, 39-48.
- Bat, L., Gündogdu, A., Öztürk, M. and Öztürk, M. (1999). Copper, zinc, lead and cadmium concentrations in the Mediterranean mussel Mytilus galloprovincialis. Lamarck 1819 from Sinop coast of the Black Sea. Turkish Journal of Zoology, 23, 321-326.
- Bat, L., Akbulut, M., Çulha, M.., Gündogdu, A, and Satilmis, H.H. (2000). Effect of temperature on the toxicity of zinc, copper and lead to the freshwater amphipod Gammarus pulex (L.). Turkish Journal of Zoology, 24(4): 409-415.
- Benhard, M., (1976). Manual of Methods in Aquatic Environment Research. FAO Fisheries Technical Paper FIRI / T 158 1- 123, Rome. Italy.
- Canli, M., Ay, Ö. and Kalay, M., (1998). Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissue of Cyprunus carpio, Barbus capito and Chondrotoma regium from the Seyhan river. Turkey. Turkish Journal of Zoology, 22: 149-157.
- Dixon, D.G. and Hilton, J.W., (1985). Effect of available dietary carbohydrate and water temperature on the chronic toxicity of waterborne copper to Rainbow trout (Salmo gairdneri ). Canadian J. Fisheries and Aquatic Sciences, 42: 1007- 1013.
- Geldiay, R. and Kocatas, A., (1988). Beginning to Marine Biology (In Turkish). Ege Ü. Fen Fakültesi Kitaplar Serisi No:31, Izmir, 459 s.
- Gordon, K. P., Rosemarie, C. R., and Robert, V. T. (1974). Effect of complecation on toxicity of copper to fishes. J. Fisheries Research Board of Canada, 31: 462-465.
- Göksu, H., (1993). Research on heavy metal accumulation in cod (Merlangius merlangus euxinus, Nordman, 1980) caught from Trabzon harbour and its vicinities. M. Sc. Thesis, K.T.Ü.,
- Fen Bilimleri Enstitüsü, Trabzon. Güven, K.C. and Topcuoglu, S., (1991). Pollution monitoring of the black sea by marine organism. The Black Sea Symposium, Proceedings of the ecological problems and economical prospects of the Black Sea, 16 – 18, 109 – 120,Istanbul.
- Güven, K., Özbay, C., Ünlü, E. and Satar, A., (1999). Acute lethal toxicity and accumulation of copper in Gammarus pulex (L.). Turkish Journal of Biology, 23: 513-521.
- Hansen, J.A., Lipton, J., Welsh, P.G., Morris, J., Cacela, D., and Suedkamp, M.J., (2002). Relationship between exposure duration, tissue residues, growth, and mortality in Rainbow trout Oncorhynchusmykiss) juveniles sub-chronically exposed to copper. Aquatic Toxicology, 58(3-4): 175 - 188.
- Hejkal, T.W., Gerba, G.P., Henderson, S. and Freeze, M., (1983). Bacteriological, virological and chemical evaluation of a wastewater- aquaculture system. Water Research. 17: 1749 - 1755.
- Karahan, B., (1991). Study on the accumulation of copper (Cu) taken from fish feed in fish tissues and the effects on growing and breeding peculiarities of common carp (Cyprinus carpio L.). Ph.D. Thesis, Ankara Üniv., Fen Bilimleri Enstitüsü, Ankara, 127 pp. (In Turkish).
- Karakoç, M., (1999). Effects of salinity on the accumulation of copper in liver, gill and muscle tissues of Tilapia nilotica. Turkish Journal of Zoology, 23: 299-303.
- Karakoç, M. and Kargin, F., (1999). The accumulation of metal and metal mixture in the spleen, brain and muscle tissues of Tilapia nilotica. Turkish Journal of Zoology, 23: 719-724 (In Turkish).
- Kargin, F. and Erdem, C., 1991. Accumulation of copper in liver, spleen, stomach, intestine, gill and muscle of Cyprinus carpio. Turkish Journal of Zoology, 15: 306-314.
- Kocatas, A., (1986). Oseanology. Ege Üniv., Fen Fakültesi Kitaplar Serisi No: 142, Bornova, Izmir, 645 pp. (In Turkish).
- Lloyd, R., (1992). Pollution and Freshwater Fish. Fishing News Books Sci. Pul. Ltd., Londra, 161 pp.
- McGeer,, J.C., Szebedinszky, C., McDonald, D. G. and Wood, C. M., (2000). Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout 2: tissue specific metal accumulation. Aquatic Toxicology, 50(3): 245-256.
- Merlini, M., (1980). Some consideration on heavy metals in the marine hydrosphere and biosphere. Thalassia Jugoslavica, 16 (2-4): 367-376.
- Öztürk, M., (1994). Heavy metal levels in Patella coerulae (L.) and Enteromorpha linza (L.) J.agardh distributed through the bays and harbours in Sinop. Turkish Journal of Biology, 18: 195-211 (In Turkish).
- Öztürk, M., Bat, L. and Öztürk, M., (1995). The accumulation of some heavy metals in several organs and tissues of Cyprinus Carpio L., (1758) living in Altinkaya dam (Samsun). II. Ulusal Ekoloji ve Çevre Kongresi , 650-667, Ankara (In Turkish).
- Sarikaya, Y., Güler, Ç., Sarikahya, F., (1987). General Chemistry. Ege Üniv. Fen Fakültesi, Kim. Böl., Anorg. Kim. Anabilim Dali, Izmir, 831 pp. (In Turkish).
- Sprague, J.B. and Ramsay, B.A., (1965). Lethal levels of mixed copper-zinc solutions for juvenile salmon. J. Fisheries Research Board of Canada, 22(2): 425-432.
- Sunlu, U. and Egemen, Ö., (1997). Study on the determination of several heavy metals in lipsoz (Scorpaena porcus L.1758) distributed in Izmir bay. Med. Fish. Congress., Izmir, 9-11 Nisan (In Turkish).
- Turner, J.W.D., Sibbald, R.R. and Hemens,J., (1986). Chlorinated secondary domestic sewage effluent as a fertilizer for marine aquaculture. III. Assesment of bacterial and viral quality and accumulation of heavy metals and chlorinated pesticides incultured fish and prawns. Aquaculture, 53(2): 157 –168.
- Uysal, H., (1978). Accumulation and distribution of heavy metals in some marine organisms in the bay of and in Aegean coasts. IV Journees Etud. Poll., 213 – 217.
- Uysal, H., Tuncer, S. and Yaramaz, Ö., (1986). A comparative study on the heavy metals present in eatable organisms in the coasts of Eagean Sea. Çevre. 86 Sempozyum, 2-5 Haz., Izmir (In Turkish).
- Waiwood, K.G. and Beamish, F.W.H., (1978). The effect of copper and pH on the growth of Rainbow trout, Salmo gairdneri. J. Fish Biology, 13: 591-598.
- Wong, M.H., Luk, K.C. and Choi, K.Y., (1977). The effects of zinc and copper salts on Cyprinus carpio and Ctenopharyngodon idellus. Acta Anotom, 99: 450-454.
- Yamamato, Y., Ishii, T., Sato, M. and Ikeda, S., (1977). Studies on copper metabolism in fishes-II. The site of copper accumaliton in the tissues of carp. Bullinof the Japanese Socety Of Scienctific Fisheries. 43(11): 1327 – 1332.