Keywords
Shabut, Arabibarbus grypus, fatty acid, cholesterol, fat-soluble vitamins.
Introduction
The lipids are the most important biochemical compounds of fish (Akp?nar, 1986). Fish store the lipids in various organs; particularly in muscles and liver. On the contrary, the mammals store in adipose tissue. A great amount of these lipids are transferred to the different parts of the body to be used for various physiological actions (Y?lmaz, 1995).
It is well known that lipids play an important role in human and animal nutrition by supplying both energy and essential fatty acids (FAs) necessary to satisfy the physiological needs of the organism. The n-3 and n-6 PUFAs are considered essential to the growth and development of children. They are precursors of composite hormones known as eicosanoids, involved in several metabolic processes of great importance for the human body, mainly related to cardiovascular activity (Eder, 1995; Inhamuns and Franco, 2008). There is a strong evidence that consumption of fish containing high levels of these fatty acids (FAs) is favourable for human health. Long-chain n-3 PUFAs cannot be synthesised by humans and must be obtained from the diet. Results of clinical and epidemiological research suggest that EPA and DHA, found only in fish and seafoods, have beneficial properties for the prevention of human coronary artery disease. Therefore fish lipids and their fatty acids have been a subject of investigation when considering human health.
The fat content and the fatty acid composition of the fish are not constant (Zlatanos and Laskaridis, 2007). The fatty acids composition of fish tissue can be affected by diet, size, age, reproductive cycle, salinity, temperature, season and geographical location (Henderson and Tocher, 1987; Zlatanos and Laskaridis, 2007; Inhamuns and Franco, 2008). It is generally recognised that PUFA composition may vary among species of fish. However, little attention has been paid to the composition of different species when selecting fish for the diet.
Fish is one of the main sources of vitamins (Cahu et al., 2004). In addition, some vitamins in fish such as the fat-soluble vitamins (A, D, E and K) also have therapeutic effects toward the prevention of particular diseases (Halver, 2002). Vitamin A required in vertebrates for the regeneration of light-sensitive rhodopsin in the retina (Lovell, 1998). Vitamin D is essential in maintaining homeostasis of calcium and inorganic phosphate which humans need for the normal development and maintenance of healthy teeth and bones. It also helps maintain proper blood levels of calcium and phosphorus (Halver, 2002). Vitamin E is an antioxidant also known as tocopherol. A major function of vitamin E is its role as a metabolic antioxidant, with a specific role in preventing oxidation of unsaturated phospholipids in cellular membranes, such as erythrocytes, and subcellular membranes such as mitochondria. Vitamin K is not listed among the essential vitamins. It is necessary for normal blood clotting in all animals, including fish. Therefore, fish is valuable source of essential fatty acids, vitamins and low levels on saturated fatty acids and cholesterol (Stancheva et al., 2010).
Barbus genus of Cyprinidae is widely distributed in eastern Asia, Eastern Europe, and Africa. It is commonly called barb or shabbout, also spelled shabboot or shabut and Shirbot. According to the records of FAO (Food and Agriculture Organization), Shabut, also known as A. grypus, is one of the most significant fish species listed in the fresh waters of Iraq and in the rivers along South and Southwest Iran, the Karoon river, and also in The Euphrates River and Tigris Rivers in Turkey (Zivotofskya and Amar, 2006; Dorostghoal et al., 2009).
A. grypus is mainly spread in Euphrate Basins and a vagile species that prefers rivers but is also found in estuaries. It is commercially fished and can reach nearly two meters and over 50 kg (Coad, 1996). Spawning generally occurs from May to mid June (Geldiay and Balik, 1988). The spawned eggs are scattered above aquatic plants and cling to the vegetation (Geldiay and Bal?k, 1988; Epler et al., 2001).
Keban Dam was built on Euphrates River in the eastern part of Turkey. Keban Dam Lake is the second largest dammed lake in Turkey (measured by surface area). About 28 fish species belonging to eight families living in the Euphrates River and its dam lakes have been recorded (Oymak et al., 2009). A. grypus is one of these species in Keban Dam Lake with more and more importance in economy because of human interest.
The freshwater fish constitute a great food potential for human. It is of great importance to know fat-soluble vitamins, cholesterol content and fatty acid compositions of the fish, which is economically important and willingly consumed. Also, new information will contribute to further projects.
Several studies have investigated on A. grypus. Its growth, sexual maturity characteristics, reproduction biology, have been studied (Marammazi and Kahkesh, 2011; Olguno?lu and Olguno?lu 2011; Atar and Ates, 2010; Oymak et al., 2008; Maghami et al., 2008; Zivotofskya and Amar, 2006; Dorostghoal et al., 2009; Sahinöz et al., 2007; Kahkesh et al., 2011) but studies about fatty acid composition and fat soluable vitamins and cholesterol are limited. (Harlio?lu and Gölba?i 2013; Olguno?lu et al., 2011). In view of these facts, the objective of the present study was to determine fatty acid, cholesterol and fat soluble vitamin compositions of the Shabut from Keban Dam Lake, Elaz?g, Turkey, because it can be more valuable and attractive fish for human consumption.
Materials and Methods
Experimental animals
The level of fatty acids, cholesterol and fat-soluble vitamins were determined in 40 Shabut (A. grypus) specimens obtained from Keban Dam Lake, Elaz??, Turkey between December and March (2013). The water temperatures in Keban Dam Lake were between 6.5 and 8.6 °C during the time in which fish samples have been obtained. Mean total weight±standard error (SE) and mean total length±standard error (SE) of fish used in this study were 755.98±84.1 g and 409.3±4.04 mm respectively. Muscle samples (without skin) were taken from each fish for analysing of fatty acid components, fat-soluble vitamins (A, D, E and K) and cholesterol.
Extraction of lipids
Lipids of muscle samples were extracted with hexaneisopropanol (3:2 v/v) by the method of Hara and Radin (1978). Nearly 1 g tissue sample was homogenized with 10 ml hexaneisopropanol mixture. The homogenate was centrifuged at 5000 rpm for 5 min at 4 °C and parts of tissue remnants were precipitated. The supernatant part was used in the ADEK, cholesterol and fatty acid analysis.
Preparation of fatty acid methyl esters
Fatty acids in the lipid extracts were converted into methyl esters including 2% sulfuric acid (v/v) in methanol (Christie, 1992). The mixture was vortexed and then kept at 50 °C for 12 h. After it was being cooled to room temperature, 5 ml of 5% sodium chloride was added and then it was vortexed again. Fatty acid methyl esters were extracted with 2x5 ml hexane. Fatty acid methyl esters were treated with 5 ml 2% KHCO3 solution and then the hexane phase was evaporated by the nitrogen flow and then by dissolving in 0.5 ml fresh hexane (Christie, 1992), they were taken to auto sampler vials.
Gas chromatographic analysis of fatty acid methyl esters: Methyl esters were analyzed with the Shimadzu GC-17 Ver. 3 gas chromatography (Kyoto, Japan). For this analysis, 25 m of long Machery-Nagel (Germany) capillary colon with an inner diameter of 0.25 μm and a thickness of 25 micron film was used. During the analysis, the colon temperature was kept at 120-220°C, injection temperature was kept at 240 °C and the detector temperature was kept at 280 °C. The nitrogen carrier gas flow was 1 ml/min. The methyl esters of fatty acids were identified by comparison with authentic external standard mixtures analyzed under the same conditions. After this process, the necessary programming was made and the Class GC 10 software version 2.01 was used to process the data.
HPLC analysis of ADEK vitamins and Cholesterol
Five ml supernatant was taken to 25 ml tubes with caps and 5% KOH solution was added and immediately vortexed for 20s. The tubes were placed in a water bath at 85°C for 15 min. The tubes were then taken and cooled to room temperature and 5 ml of distilled water was added and mixed. Lipophilic molecules, that did not saponify, were extracted with 2x5 ml hexane. The hexane phase was evaporated with nitrogen flow. It was dissolved in 1 ml (50+50%, v v-1) acetonitrile/methanol mixture and then was taken to auto sampler vials and was analyzed. The analysis was made with the Shimadzu brand HPLC device. HPLC conditions were as follows: mobile phase 60:38:2 (v/v/v): acetonitrile/ methanol/water; The mobile phase flow rate was determined to be 1mL A UV detector was used for the analysis and as a column the Supelcosil LC 18 (15x4.6cm 5μm; Sigma USA) column was used. For vitamin E and cholesterol 202 nm, retinol, 326nm and for vitamin D and K, 265 nm was used (L’opez-Cervantes et al., 2006; Katsanidis and Addis, 1999).
Statistical analysis
The SPSS software (SPSS Inc, Chicago, IL, USA) was used for statistical analyses. Results for the groups are expressed as mean ± standard estimation (SEM). Differences between the group’s means were analyzed for significance using the ANOVA Duncan’s Multiple Range Test. Statistical significance was defined as P<0.05.
Results and Discussion
Fatty acids composition
The fatty acid compositions (% of total fatty acids) in the muscle of Shabut are given in Table 1.
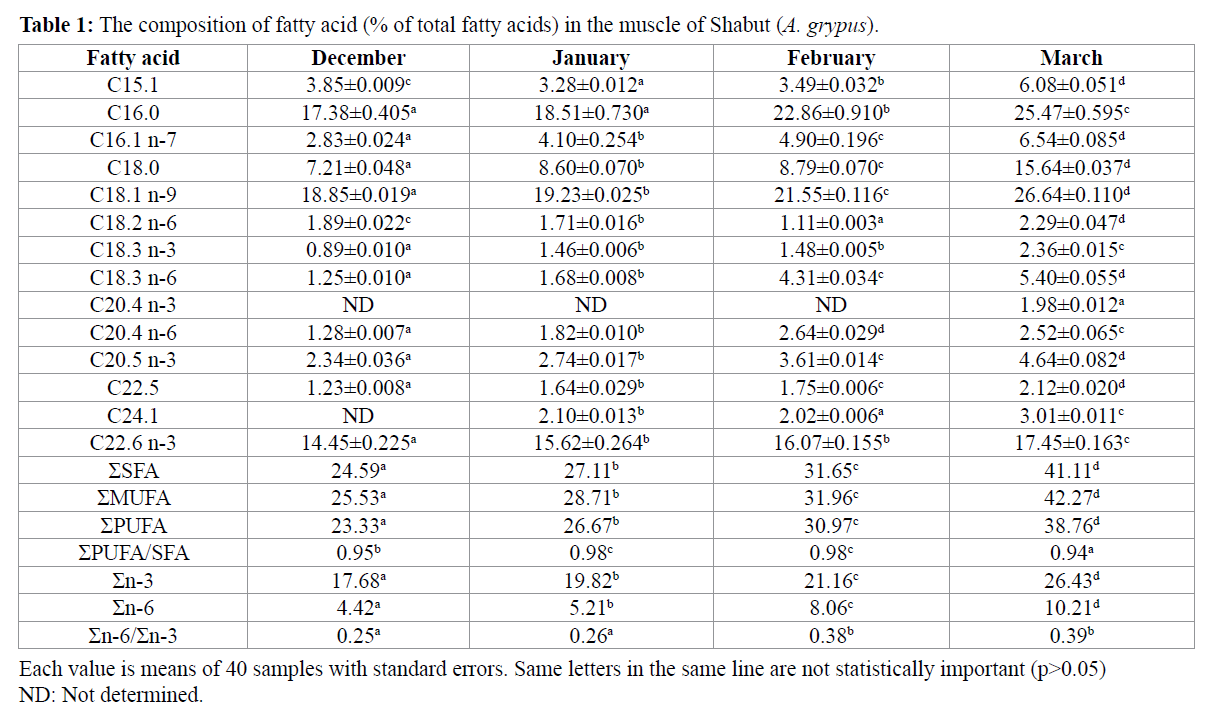
Table 1: The composition of fatty acid (% of total fatty acids) in the muscle of Shabut (A. grypus).
The fatty acids analysed were grouped as saturated fatty acid (SFA), mono unsaturated fatty acid (MUFA) and polyenoic fatty acids (PUFA). The results of present study showed that MUFA was the highest followed by SFA and PUFA in the muscle of all groups. The highest fatty acid levels found in Shabut throughout all months (December – March) were 16:0, 18:1, 22:6 n-3(DHA) and 20:5 n-3 (EPA).
As displayed in Table 1, Palmitic acid (C16:0) was the primary SFA in Shabut in all months followed by stearic acid (C18:0). Among the SFAs, palmitic (C16:0) and stearic acid (C 18:0) were the major SFAs in the muscle of Shabut in all months. Our results about fatty acid composition of Shabut showed similarity with the result of Olgunoglu et al. (2011).
In the present study it was also determined that oleic acid (C18:1 n-9) was the dominant MUFAs in Shabut. Through the study, oleic acid (C18:1 n-9), a monounsaturated fatty acid type (MUFA), was observed as the predominant primary fatty acid throughout four months followed by palmitoleic acid (C16:1 n-7) and c?s-10-pentadeceno?c ac?d ( C15:1). This finding seems differences with other study (Olgunoglu et al., 2011) on Shabut. It was concluded that these differences may be attributed to the different abiotic and biotic factors such as season, the type and amount of feed available, water temperature, pH, salinity and fish age.
Omega-6 and omega-3 fatty acids are essential because humans, like all mammals, cannot synthised them and must obtain them in their diet. Humans and other mammals, except for carnivores such as lions, can convert linoleic acid (C18:2 n-6) to arachidonic acid (C20:4 n-6) and α-linolenic acid (C18:3 n-3) to EPA and DHA, but it is slow. The results showed that Shabut contained comparatively good level of n-3 polyunsaturated fatty acids (PUFA). In the present study, docosahexaenoic acid (C22:6 n-3, DHA) was the primary fatty acids in all months followed by eicosapentaenoic acid (C20:5 n–3 EPA). This result was similarly reported by (Olgunoglu et al., 2011). The proportion of DHA and EPA were highest in March.
Among n-6 series of the fatty acids, the amount of gamma- Linolenic acid (C18:3 n-6) higher in the muscle of Shabut throughout the average four month. Σn-6 was found the highest in the muscle of Shabut in March and there was statistically significance different between the other months. Smillary, the findings showed that Σn-3 was found the highest in the muscle of Shabut in March and there was statistically significance different between the other months.
In the present study, the ratio of PUFA to SFA was found between 0.94 and 0.98 throughout four months and there was no statistically significance different between months (P>0.05). According to the general nutritional guidelines of the Department of Health of the United Kingdom, this ratio should be 0.4 or more for a balanced fatty acid intake on a healthy diet (Wood et al., 2004). Therefore, the PUFA/SFA ratio results obtained in the present study was within the recommended range.
Several sources of information suggest that human beings evolved on a diet with a ratio of omega-6 to omega-3 essential fatty acids (EFA) of ~1. In a lower ratio of omega-6/omega-3 fatty acids is more desirable in reducing the risk of many of the chronic diseases of high prevalence in Western societies, as well as in the developing countries (Artemis, 2008). Some clinical studies (Lands, 1992; Okuyama, 2001) indicate that the ingested ratio of omega−6 to omega−3 (especially linoleic vs alpha-linolenic) fatty acids is important to maintaining cardiovascular health. The lower omega-6/omega-3 ratio in women with breast cancer was associated with decreased risk. A ratio of 2–3/1 suppressed inflammation in patients with rheumatoid arthritis, and a ratio of 5/1 had a beneficial effect on patients with asthma (Artemis, 2008). Both omega−6 and omega−3 fatty acids are essential; i.e., humans must consume them in the diets. Omega−6 and omega−3 eighteen-carbon polyunsaturated fatty acids compete for the same metabolic enzymes, thus the omega−6:omega−3 ratio of ingested fatty acids has significant influence on the ratio and rate of production of eicosanoids, a group of hormones intimately involved in the body's inflammatory and homeostatic processes which includes the prostaglandins, leukotrienes, and thromboxanes, among others. Altering this ratio can change the body's metabolic and inflammatory state. Tribole et al. (2006) healthy ratios of omega−6:omega−3, according to some authors, range from 1:1 to 1:4 (an individual needs more omega−3 than omega−6). Lands (2005) other authors believe that ratio 4:1 (when the amount of omega-6 is only 4 times greater than that of omega-3) is already healthy. However reported that excessive amounts of omega-6 polyunsaturated fatty acids (PUFA) and a very high omega-6/ omega-3 ratio, as is found in today’s Western diets, promote the pathogenesis of many diseases, including cardiovascular disease, cancer, and inflammatory and autoimmune diseases, whereas increased levels of omega-3 PUFA (a lower omega-6/ omega-3 ratio), exert suppressive effects (Artemis, 2008).
In the present study, n-3/n-6 proportion value was found between 0.25 and 0.39 throughout four months. According to a research (Lands, 2005) this lower omega-6/omega-3 ratio value could constitute a healthy human diet.
Fat-soluble vitamins and cholesterol content
The fat-soluble vitamins are essential nutrients controlling a diversity of biologically important processes in human body. Vitamin A, also called retinol, takes place in photoreception and regulates gene expression and cell division, bone growth, teeth development, reproduction etc. Vitamin D3 (cholecalciferol) plays crucial role in the regulation of calcium – phosphate balance stimulating calcium absorption by the small intestine and thus regulating bone metabolism. The biologically active isomer of vitamin E - alpha-tocopherol (a-TP) acts as an antioxidant, protecting membrane structures, essential fatty acids, and vitamins A and C from oxidation. Being one of the major sources of omega-3 PUFA and fat-soluble vitamins A, D3 and E, fish production by fish farming attains great economical importance. On the other hand, the worldwide decline of ocean fisheries stocks has provided impetus for rapid growth in fish and shellfish farming, or aquaculture (Rosamond L,et al.,2000).
The values of A (retinol), D (D2, D3), E (α-tocopherol), K (K1, K2) vitamins are shown in Table 2. Among the fat-soluble vitamins (A, D, E and K) analysed in four months, the vitamin E content was highest followed by K, D and A.
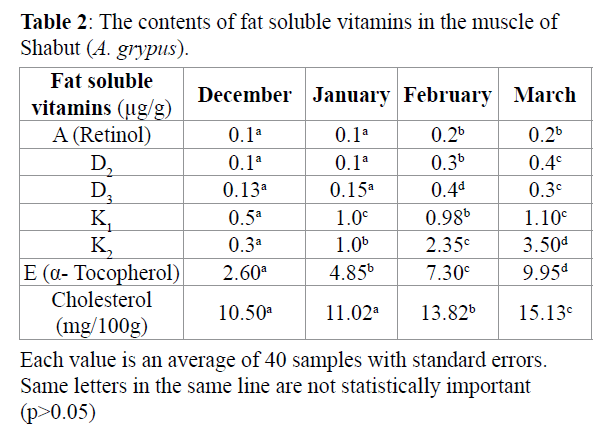
Table 2: The contents of fat soluble vitamins in the muscle of Shabut (A. grypus).
The findings showed that there was a significant difference in vitamins throughout months.
We have not found a study related with the vitamins content of Shabut. For different species, a study on the vitamin content of fish and fish products consumed in Portugal was undertaken by Dias et al. (2003), who found that vitamins A, D and E in eel meat were 887, 16 and 2400 μg/100g respectively. In salmon these were found to be 33, 11 and 4000 μg/100g respectively. In rainbow trout these values were determined as 8.8, 19 and 130 μg per 100 g respectively (Dias et al., 2003). In addition, vitamin D (D2 + D3) content was 23.3 μg/100 g, and vitamin E (alpha-tocopherol) content was 4 mg/100 g in Anguilla sp. (USDA, 2005). In a different study (Stancheva et al., 2010) the retinol (vitamin A) content in the edible tissue of rainbow trout (Oncorhynchus mykiss) was 22.3±2.0 μg/100g; cholecalciferol (Vitamin D3) 6.0±0.29 μg/100g and α-tocopherol (Vitamin E) 809.1± 56.0 μg/100g. In the another study on freshwater spiny eel (Mastacembelus simack) vitamin A content was 0.533 μg/g, vitamin E (alpha-tocopherol) content was 1.89 μg/g , vitamin D2 content was 0.79 μg/g and vitamin D3 content was 1.36 μg/g respectively (Harl?oglu et al., 2010).
Vitamin content can vary in different parts of the same tissues, and among animals collected at different times and locations. Indeed, geographic availability, seasonality, and physiological state/maturity are known to affect variability in nutrient composition, particularly for vitamins (Greenfield & Southgate, 2003).
We have not found a study related with the cholesterol content of Shabut. For different species, the cholesterol content of M. simack was found to be 52.60 ± 4.36 mg/100 g in the a study (Harl?oglu et al., 2010). In a study on the cholesterol content of selected marine fishes in Malaysian waters, Osman et al. (2001) noted that the cholesterol content was 37.1 in Gymnura spp., 40.3 in Pampus argenteus, 41.1 in Scomberomorus commersonii, 41.8 in Clupea fimbriata, 45.9 in Eleutheronema tradactylum, 46.6 in Magalapsis cordyla, 46.8 in Parastromateus niger, 46.9 in Plotosus spp., 47.3 in Selarides leptolejus and 49.1 mg/100 g in Rastrelliger kanagurta.
In a different study, cholesterol content of some fish species caught from Turkish seas were investigated by ?mre and Sa?l?k (1998). they found cholesterol content (mg/100g) as 43.4 in the sardine (Sardina pilchardus) and 40.3 mg per 100 g in pandora (Pagellus erytrinus), 75.3 in sargo (Diplodus sargus), 63.4 in mackerel (Scomber scombrus) and 58.4 in sole (Solea solea). Cahu et al. (2004) were found the cholesterol content in channel catfish (58mg/100g) and rainbow trout (60 mg/100g)
In the present study, the cholesterol content (mg/100g) of the Shabut muscle was found to be between 10.50 and 15.13 in four months. The results showed that cholesterol level of Shabut is low. Osman et al. (2001) stated that the cholesterol content in fish is influenced by several factors, among them the PUFA content, and that an increase in PUFA content will be followed by a decrease in the cholesterol level.
Conclusions
It appears that no enough data on the vitamin and cholesterol compositions and fatty acid profiles of Shabut are available. Therefore the results of the present study will form the basis for further research in this field of fish chemistry for the benefits of human beings.
In conclusion, it can be stated that Shabut (A. grypus) has comparatively good fatty acid composition, Σn-6/Σn-3 ratio, fatsoluble vitamins content and low cholesterol level. Shabut (A. grypus) can therefore be recommended for human consumption as a comparatively good source of nutrition. Finally, we recommend Shabut (A. grypus) especially obtained in March for healthy human diet.
5816
References
- nAkpınar MA. Cyprinus carpio L. (Osteichthyes: Cyprinidae)nkaraciğer ve kasındaki total lipid ve total yağ asidininnmevsimsel değişimi. Çukurova Üniversitesi Fen BilimlerinDergisi. 1986;4:33-42. Turkish
- nArtemis PS. The importance of the omega-6/omega-3 fatty acidnratio in cardiovascular disease and other chronic diseases.nExp Bio Med. 2008;233:674-688
- nAtar HH, Ates M. The effects of commercial diets supplemented with mannanoligosaccharide (MOS) and vitamin B12 onnthe growth and body composition of the shabbout fries (Torngrypus H. 1843). J Food Agr Env. 2010;8(1):281-284
- nCahu C, Salen P, Lorgeril MD. Farmed and wild fish in thenprevention of cardiovascular diseases: assessing possiblendifferences in lipid nutritional values. Nut Met Card Dis.n2004;14:34-41
- nChristie WW. Gas chromatography and lipids. The Oil Pres:nGlaskow; 1992
- nCoad BW. A provisional, annotated check-list of the freshwaternfishes of Iran. J Bombay Nat Hist Soc. 1996;76(1):86 -105
- nDias, M. G.; Sanchez, M. V.; Bartolo, H.; Oliveira, L., 2003:nVitamin content of fish and fish products consumed innPortugal. Electron. J. Environ. Agric. Food Chem. 2, 510–n513
- nDorostghoal M, Peyghan R, Papan F, Khalili L. Macroscopic andnmicroscopic studies of annual ovarian maturation cycle ofnShirbot Arabibarbus grypus in Karon river of Iran. Iran J VetnRes. 2009;27:172-179
- nEder K. Gas chromatographic analysis of fatty acid methyl esters.nJ Chrom B: Biom Sci App. 1995;671:113-131
- nEpler R, Sokolowska-Mikolajczk M, Popek W, Bieniarz K, BartelnR, Szczerbowski JA. Reproductive biology of selected fishnspecies from Lakes Tharthar and Habbaniya in Iraq. ArchnFish Pol. 2001;9:199-209
- nGeldiay R, Balık S. Freshwater fishes of Turkey. 3rd ed. İzmir:nEgean University; 1988
- nGreenfield, H., & Southgate, D. A. T. (2003). Food compositionndata: Production, management and use (2nd ed.). Rome:nFAO
- nHalver EJ. 2002: The vitamins. In: Halver EJ, Hardy RW, editors.nFish nutrition. London: Academic Press; 2002. p. 62-141
- nHara A, Radin NS. Lipid extraction of tissues with a low-toxicity.nAnal Biochem. 1978;90(1): 420-426
- nHarlioğlu AG, Gölbaşi S. Changes in fatty acid composition,ncholesterol and fat-soluble vitamins during development ofneggs and larvae in shabbout (Arabibarbus grypus, Heckeln1843). J App Icht. 2013;29(6);1357-1360
- nHarlioglu, A.G and Yilmaz, O., Fatty acid composition, cholesterolnand fat-soluble vitamins of wild-caught freshwater spiny eel,nMastacembelus simack (Walbaum, 1792) J. Appl. Ichthyol.n27 (2011), 1123–1127
- nHenderson RJ, Tocher DR. The lipid composition and biochemistrynof freshwater fish. Prog Lip Res. 1987;20:281-346
- nInhamuns AJ, Franco MRB. EPA and DHA quantification in twonspecies of freshwater fish from Central Amazonia. FoodnChem. 2008;107:587-591
- nImre, S.; Sag˘ lık, S., 1998: Fatty acid composition and cholesterolncontent of some Turkish fish species. Turk. J. Chem. 22,n321–324
- nKahkesh FB, Yooneszadeh M, Amiri F, Nikpey M. Surveynof different hormones on final maturation in shirbutn(Arabibarbus grypus Heckel, 1843). W J Fish Mar Sci.n2011;3(6):548-552
- nKatsanidis E, Addis PB. Novel HPLC analysis of tocopherols andncholesterol in tissue. Free Radic Bio Med. 1999;27:1137-n1140
- nLands WEM. Biochemistry and physiology of n–3 fatty acids. FednAme Soc Exp Bio. 1992;6(8):2530-2536
- nLands WEM. Fish, omega 3 and human health. Amer Oil ChemnSoc. 2005; ISBN 978-1-893997-81-3
- nLovell T. Nutrition and feeding of fish. London: Kluwer AcademicnPublishers; 1998
- nL’opez-Cervantes G, S’anchez-Machado DI, R’ios-V’azqueznNJ. High performance liquid chromatography methodnfor the simultaneous quantification of retinol, tocopherolnand cholesterol in shrimp waste hydrolysate. J Chrom A.n2006;1105:135-139
- nMarammazi JG, Kahkesh F. Effects of dietary protein andnenergy levels on growth performance, feed utilization andnbody composition of juvenile shirbot Arabibarbus grypusn(Heckle, 1843). Iran J Fish Sci. 2011;10(3):461-474
- nMaghami SSG, Jafari BJ, Masoumian M. Myxobolusnnodulointestinalis intestinal parasite of barbus fishes innKhozestan, Iran. J Ani Veter Adv. 2008;7(3):231-234
- nOkuyama H. High n−6 to n−3 ratio of dietary fatty acids rathernthan serum cholesterol as a major risk factor for coronarynheart disease. Eur J Lip Sci Tech. 2001;103(6): 418-422
- nOlgunoğlu MP, Olgunoğlu IA. Seasonal variation of tracenelements in muscle tissues of two commercially valuablenfreshwater fish species (Silurus triostegus and Arabibarbusngrypus Heckel, 1843) from Atatürk Dam Lake (Turkey).nAfri J Biotech. 2011;10(34):6628-6632
- nOlgunoglu AI, Olgunoglu MP, Artar E. Seasonal changesnin biochemical composition and meat yield of Shabutn(Arabibarbus grypus, Heckel 1843). Iran J Fish Sci.n2011;10(1):181-187
- nOsman, H.; Suriah, A. R.; Law, E. C., 2001: Fatty acid compositionnand cholesterol content of selected marine fish in Malaysiannwaters. Food Chem. 73, 55–60
- nOymak SA, Dogan N, Uysal E. Age, growth and reproduction ofnthe shabut Arabibarbus grypus (Cyprinidae) in Atatürk DamnLake (Euphrates River), Turkey. Cybium. 2008;32 (2):145-n152
- nOymak SA, Akın HH, Doğan N. Heavy metal in tissues of Torngrypus from Atatürk Dam Lake, Euphrates River-Turkey.nBiologia. 2009;64(1):151-155.
- nRosamond L. Naylor, Rebecca J. Goldburg, Jurgenne H.nPrimavera, Nils ,Kautskysk, Malcolm C. M. Beveridge,nJason Clay, Carl Folkesk, Jane Lubchenco, Harold Mooneyn& Max Troell, NATURE, 405, 29 (2000)
- nStancheva M, Dobreva D, Merdzhanova A, Galunska B. Vitaminncontent and fatty acids composition of rainbow troutn(Oncorhynchus mykiss). Plovdiv Univ, Paisii Hilendarski,nBulg Sci Pp. 2010;37(5):117-123
- nSahinoz E, Dogu Z, Aral F. Embryonic and pre-larval developmentnof shabbout (Arabibarbus grypus H.) Isra J Aquacu.n2007;59(4);236-239
- nTribole EF, Thompson RL, Harrison RA, Summerbell CD, NessnAR, Moore HJ, et al. Risks and benefits of omega 3 fatsnfor mortality, cardiovascular disease, and cancer: systematicnreview. Brit Med J. 2006;332(7544):752-755
- nWood JD, Richardson GR, Nute GR, Fisher AV, Campo MM,nKasapidou E. et al. Effects of fatty acids on meat quality: anreview. Meat Sci. 2004;66:21-32
- nYılmaz Ö. Elazığ Hazar Gölü’nde yaşayan Capoeta capoetanumbla (Heckel, 1843)’ın total yağ asidi miktarı ve yağnasitleri cinslerinin mevsimlere göre değişimi. F.U. FennBilimleri Enstitüsü. PhD. Thesis. Elazığ. 1995. Turkish
- nZivotofskya AZ, Amar Z. Identifying the ancient shibuta fish.nEnvi Bio Fish. 2006;75:361-363
- nZlatanos S, Laskaridis K. Seasonal variation in the fatty acidncomposition of three Mediterranean fish-sardine (Sardinanpilchardus), anchovy (Engraulis encrasicholus) and picareln(Spicara smaris). Food Chem. 2007;103:725-728.








