Keywords
Undifferentiated ovarian carcinoma; Immunohistochemistry
Abbreviations
CA: Cancer Antigen; CEA: Carcinoembryonic Antigen; CK: Cytokeratin; EMA: Epithelial Membrane Antigen; GCDFP-15: Gross Cystic Disease Fluid Protein 15; H&E: Hematoxylin/Eosin; IHC: Immuno-histoChemistry.
Introduction
Ovarian cancer is the 6th most common tumor in women. Worldwide more than 200,000 new cases are diagnosed each year are around 4% of all cancers diagnosed in women and 6.6 new cases per 100,000 women per year [1]. Tumors become more poorly differentiated as part of tumor progression; about 5% of ovarian carcinomas are poorly differentiated and difficult to classify that are designated undifferentiated [2]. Undifferentiated carcinomas are characterized by a pattern less solid, sheet-like growth of tumor cells, with an aggressive clinical course. In undifferentiated carcinomas there are no glands, nests, trabeculae, papillae, or spindled patterns, and also there is no squamous or mucinous metaplasia, and no/ minimal neuroendocrine differentiation [3]. If areas of a differentiated component are found, the tumor is called dedifferentiated carcinoma. It is necessary to exclude metastatic carcinomas and non-epithelial neoplasms [4].
Malignant epithelial tumors of the ovary result from the coelomic epithelium that covers the surface of the ovary and more than 75% of the patients with malignant ovarian tumors showed, at the time of diagnosis, an extension of the tumor beyond the ovary; to the pelvis and abdominal cavity [5]. There are 10% from undifferentiated ovarian tumors cases cannot be identified only on the basis of morphological criteria, and 8% of the ovarian tumors are metastasis from cancer of other organs as the gastrointestinal tract or the mammary gland. Their prognosis and management depend on their origin; therefore, it is essential to detect the origin of these tumors [6].
So, this study was conducted to use antibodies, which helped detect the epithelial origin of these undifferentiated ovarian tumors from the ovarian surface epithelium (CK AE1/ AE3, CK7, CK20, EMA, and PAX8). Along with it, we also used antibodies that help in differentiation between undifferentiated ovarian tumors and peritoneal mesotheliomas with ovarian extension (CK7, CK20, CA125, CEA, EMA, calretinin, inhibin-, and PAX 8) and from ovarian metastasis that originating in the gastrointestinal tract (CK7, CK20, CA125, CEA, and PAX 8) or from breast (GCDFP-15 and PAX 8).
Material and Methods
Tissue specimens
Paraffin-embedded blocks of 20 female patients diagnosed to have undifferentiated ovarian tumor were retrieved from the archives of Pathology Laboratory of Suez Canal University, in the period between January 2010 and December 2016. None of the patients recruited in this study were undergone pre-operative chemotherapy or radiotherapy. This study was approved by the Ethics Committee. All participants gave written informed consent before start this study. The study was performed in adherence to the tenets of the Declaration of Helsinki.
For all studied cases, first H&E-stained sections of the lesions were examined for histopathology review to confirm the diagnosis of undifferentiated carcinoma of the ovary. Then all cases stained with a panel of antibodies to detect the epithelial origin of the tumors were chosen (cytokeratin AE1/ AE3, EMA, vimentin antibodies). Since there is no specific marker indicate the ovarian origin of the tumors it was necessary to differentiation the studied tumors from possible ovarian metastasis as from gastrointestinal tract or breast, thus, the anti-calretinin antibody was used in the present study to help, together with CA125 and CEA antibodies, to exclude mesotheliomas. CK7 and CK20 staining allowed the separation of these tumors from ovarian metastasis originating in the gastrointestinal tract. While GCDFP-15 help to exclude breast cancer metastasis. PAX8 and inhibin- used to confirm the origin of ovarian tumor is from epithelial or from sex-cord elements.
Immunohistochemical staining
IHC was performed on formalin-fixed, paraffin-embedded sections using standard streptavidin–biotin–peroxidase complex (ABC) methods. The sections of 4 μm thickness were made and mounted on positively charged adhesive slides. The paraffin sections were immersed in three changes of xylene and they were hydrated using a graded series of alcohol solutions. Fresh citric buffer solution of pH 6 was used for all markers. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 minutes. Antigen retrieval was carried out using a microwave oven for 15 minutes (temperature according to target antigen) using buffer solution. Primary antibodies (Santa Cruz, Thermo Fisher and Cell Marque) were applied and incubated followed by secondary antibody and peroxidase-antiperoxidase complex. The sections were incubated with primary antibody for 1 hour at room temperature. The primary antibodies used as shown in Table 1 and staining was developed using 3′3- diaminobenzidine. Slides were counterstained for 3 min with Meyer’s hematoxylin and then they were mounted. We used a panel of primary antibodies, vimentin, panCKA1/A3, CK7, CK20, EMA, calretinin, CEA, CA125, inhibin-PAX8, and GCDFP-15. Negative controls were obtained by omitting the primary antibodies. External positive controls were used according to every primary antibody.
| Primary antibody |
Catalog No. |
Source |
Clone |
Dilution |
Location |
| Vimentin |
sc-6260 |
Santa Cruz |
V9 |
1:50 |
cyt |
| Pan-CK AE1/AE3 |
sc-81714 |
Santa Cruz |
AE1& AE3 |
1:50 |
cyt |
| EMA |
sc-53377 |
Santa Cruz |
E29 |
1:50 |
mem/cyt |
| CK 7 |
MA5- 11986 |
Thermo Fisher |
OV-TL 12/30 |
1:100 |
cyt/mem |
| CK 20 |
sc-52320 |
Santa Cruz |
IT-Ks20.8 |
1:100 |
cyt/mem |
| CA 125 |
325M-16 |
Cell Marque |
OC 125 |
1:50 |
cyt/mem |
| pan CEA |
sc-73455 |
Santa Cruz |
Antibody (161) |
1:100 |
cyt/mem |
| Calretinin |
sc-135853 |
Santa Cruz |
Antibody (34) |
1:50 |
nuc/cyt |
| Inhibin |
MA5-15315 |
Thermo Fisher |
4A2 |
1:100 |
cyt |
| PAX-8 |
MA1-117 |
Thermo Fisher |
1F8-3A8 |
1:100 |
nuc |
| GCDFP-15 |
MA5-11633 |
Thermo Fisher |
23A3 |
1:50 |
nuc/cyt |
Note: *All antibodies were mouse monoclonals. nuc: Nuclear; mem: Membranous; cyt: Cytoplasmic; CA: Cancer Antigen; CEA: Carcinoembryonic Antigen; CK: Cytokeratin; EMA: Epithelial Membrane Antigen; and GCDFP-15: Gross Cystic Disease Fluid Protein 15.
Table 1: Summary of antibodies information*.
IHC results were evaluated in a semiquantitative manner and scored according to the percentages of positively staining cells. Cases were divided into the following groups: (negative): no staining and only few scattered positive cells <5% was considered to be negative; (1+): 5-25% of cells stained; (2+): 25-50% of cells stained; (3+): 50-75% of cells stained; (4+): 75-100% of cells stained. Only tumor cells stained in the appropriate cytoplasmic/membrane/nuclear position were scored.
Results
Revision of the H&E stained slides of the studied group revealed all the twenty cases had undifferentiated ovarian carcinomas (Figure 1A).
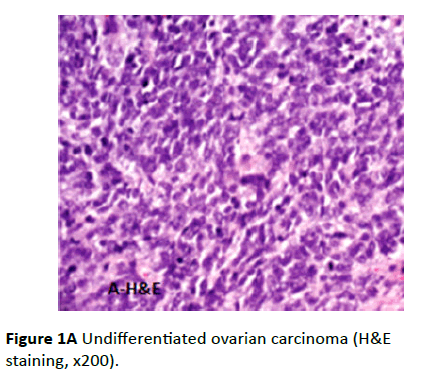
Figure 1A: Undifferentiated ovarian carcinoma (H&E staining, x200).
Vimentin antibody
The entire twenty cases showed positive tumor stromal reaction with vimentin antibody IHC with presence of internal positive control. The positive staining was uniformly distributed in the cytoplasm of tumor stromal cells and it ranged from moderate to strong positive (Figure 1B). The tumor epithelial cells showed weak scattered positive reaction with paranuclear concentration only two of the studied cases showed moderate positive immunostaining at the epithelium.
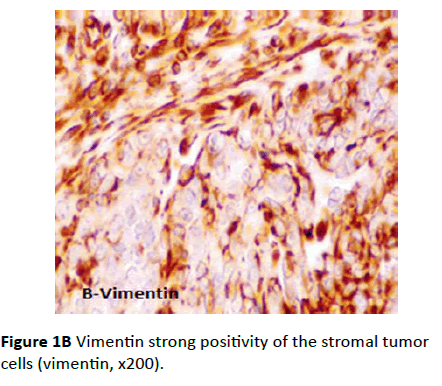
Figure 1B Vimentin strong positivity of the stromal tumor cells (vimentin, x200).
Cytokeratin AE1/AE3 antibody
Eighteen cases showed positive epithelial tumor cells reaction while it was negative reaction in the stroma. The immunostaining pattern was diffuse and the intensity ranged from weak, moderate to strong positive. The staining in all the eighteen cases was uniformly cytoplasmic and frequently associated with a “pericellular pattern” (Figure 1C). Only two cases were CK AE1/AE3 negative.
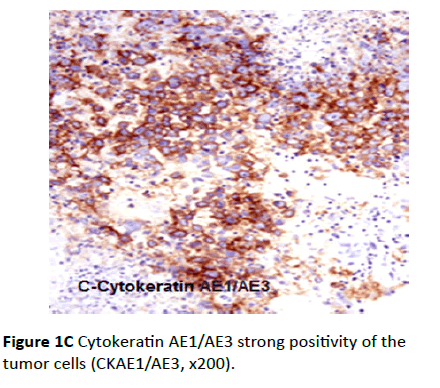
Figure 1C Cytokeratin AE1/AE3 strong positivity of the tumor cells (CKAE1/AE3, x200).
EMA antibody
EMA reaction was diffusely positive in eighteen cases. The immunostaining was predominantly membranous in the tumor cells (Figure 1D), few cells showed cytoplasmic staining, while the stromal cells were negative. The intensity of immunostaining ranged from moderate to strong positive. Again the same two negative cases for CK AE1/AE3 were also negative for EMA, which suggested it is not epithelial in origin. The pattern of vimentin+ve/CK-ve/EMA-ve suggests sex-cord stromal tumor especially granulosa cell tumor.
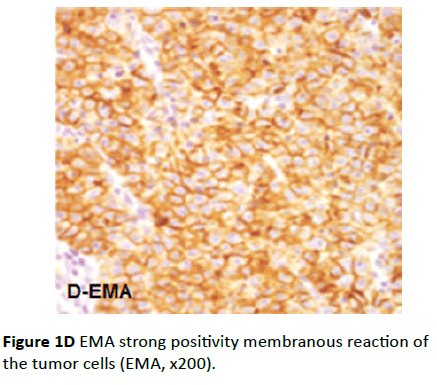
Figure 1D EMA strong positivity membranous reaction of the tumor cells (EMA, x200).
CA125 antibody
Twelve cases showed positive CA125 antibody reaction at epithelial tumor cells. In some positive cases, the distribution of CA125 staining was focal, heterogeneous type intensity and the pattern of staining was membranous. While in other positive cases the distribution of CA125 staining was diffuse, strong intensity with membranous and cytoplasmic pattern (Figure 1E).
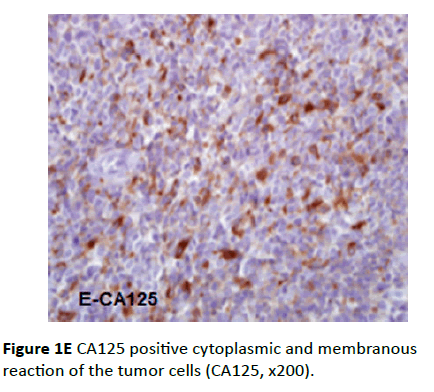
Figure 1E CA125 positive cytoplasmic and membranous reaction of the tumor cells (CA125, x200).
CEA antibody
IHC analysis for CEA antibody showed that only the CA125 negative cases were CEA positive (eight cases). The distribution of CEA staining was focal, weak intensity and the pattern was cytoplasmic for all the positive specimens (Figure 1F).
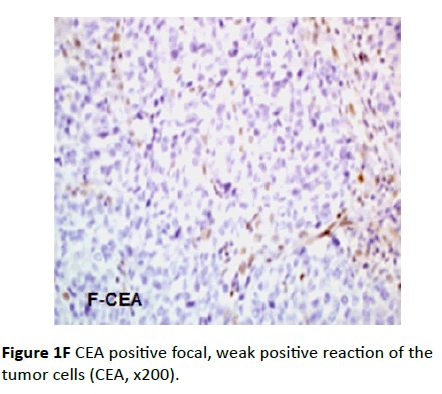
Figure 1F CEA positive focal, weak positive reaction of the tumor cells (CEA, x200).
CK7 antibody
Eighteen cases from all studied group were positive for CK7 antibody. The intensity of the reaction ranged from moderate to intense. The distribution of the staining was diffuse and the pattern was cytoplasmic, frequently associated with a “pericellular” pattern, which characterizes the epithelial tumors (Figure 1G), while the tumor stroma was negative.
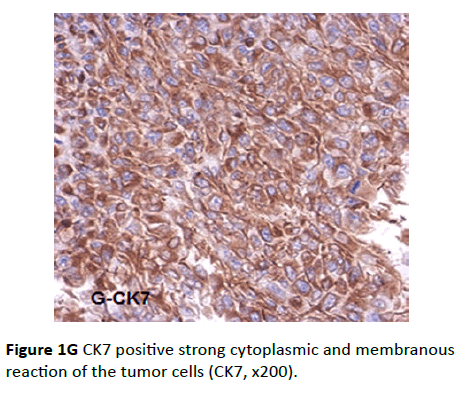
Figure 1G CK7 positive strong cytoplasmic and membranous reaction of the tumor cells (CK7, x200).
CK20 antibody
All the studied cases were CK20 negative in comparison with the positivity of external control (colon).
Calretinin antibody
Four cases were positive for this marker; two cases of these were also positive for vimentin, CK AE1/AE3, CK7, and EMA and negative for CK20 and inhibin antibodies, suggesting malignant mesothelioma. In these two positive cases, calretinin positive cells showed diffuse moderate to intense staining. The pattern of the staining was cytoplasmic or cytoplasmic and nuclear (Figure 1H), frequently associated with a “pericellular” pattern, which characterizes the epithelial tumors. The other two cases were positive for vimentin and inhibin, and negative for CK AE1/AE3, CK7, CK20, and EMA.
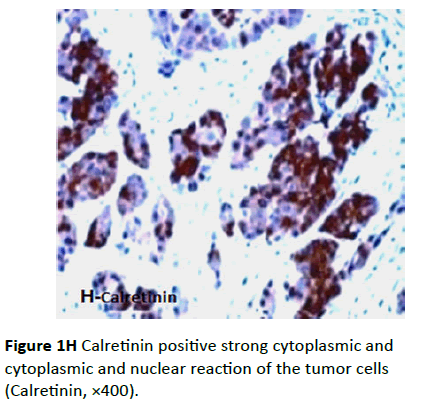
Figure 1H Calretinin positive strong cytoplasmic and cytoplasmic and nuclear reaction of the tumor cells (Calretinin, ×400).
Inhibin antibody
Two cases were positive for inhibin-antibody; these two cases were also positive for vimentin and calretinin and negative for AE1/AE3, CK7, CK20, and EMA antibodies. Positive tumor cells reaction toward inhibin showed diffuse moderate to intense staining. The pattern of the staining was cytoplasmic (Figure 1I).
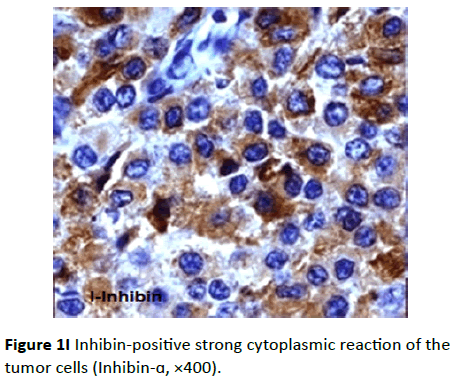
Figure 1I Inhibin-positive strong cytoplasmic reaction of the tumor cells (Inhibin- ×400).
PAX8 antibody
Sixteen cases were positive for this marker. The intensity of the reaction ranged from moderate to intense. The distribution of the staining was diffuse and the pattern was nuclear (Figure 1J). The other negative cases were the four cases showed calretinin positivity (two cases of malignant mesothelioma and the two cases of granulosa cell tumor).
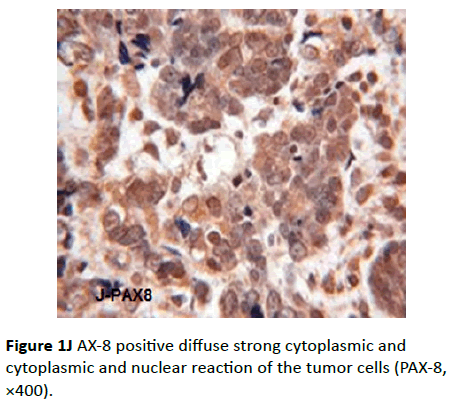
Figure 1J AX-8 positive diffuse strong cytoplasmic and cytoplasmic and nuclear reaction of the tumor cells (PAX-8, ×400).
GCDFP-15 antibody
All the studied cases were negative to this marker in comparison with the positivity of the external control (mammary tissue). The correlation of the results obtained after the other immunostaining with above markers allowed the exclusion of ovarian metastasis from breast. The reaction of each individual case with all used panel of markers is shown in Table 2.
| No. |
Vimentin |
panCKA1/A3 |
CK7 |
CK20 |
CK125 |
pan CEA |
EMA |
Calretinin |
Inhibin |
PAX-8 |
GCDFP-15 |
| Case 1 |
+ve |
+ve |
+ve |
-ve |
+ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 2 |
+ve |
+ve |
+ve |
-ve |
+ ve |
- ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 3 |
+ve |
+ve |
+ve |
-ve |
-ve |
-ve |
+ve |
+ve |
-ve |
-ve |
-ve |
| Case 4 |
+ve |
+ve |
+ve |
-ve |
-ve |
-ve |
+ve |
-ve |
-ve |
+ ve |
-ve |
| Case 5 |
+ve |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
+ve |
+ve |
- ve |
-ve |
| Case 6 |
+ve |
+ve |
+ve |
-ve |
-ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 7 |
+ve |
+ve |
+ve |
-ve |
-ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 8 |
+ve |
+ve |
+ve |
-ve |
+ ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 9 |
+ve |
+ve |
+ve |
-ve |
+ ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 10 |
+ve |
+ve |
+ve |
-ve |
-ve |
-ve |
+ve |
+ve |
-ve |
-ve |
-ve |
| Case 11 |
+ve |
-ve |
-ve |
-ve |
-ve |
-ve |
-ve |
+ve |
+ve |
-ve |
-ve |
| Case 12 |
+ve |
+ve |
+ve |
-ve |
+ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 13 |
+ve |
+ve |
+ve |
-ve |
+ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 14 |
+ve |
+ve |
+ve |
-ve |
+ve |
+ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 15 |
+ve |
+ve |
+ve |
-ve |
+ve |
+ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 16 |
+ve |
+ve |
+ve |
-ve |
-ve |
+ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 17 |
+ve |
+ve |
+ve |
-ve |
-ve |
+ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 18 |
+ve |
+ve |
+ve |
-ve |
+ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 19 |
+ve |
+ve |
+ve |
-ve |
+ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
| Case 20 |
+ve |
+ve |
+ve |
-ve |
+ve |
-ve |
+ve |
-ve |
-ve |
+ve |
-ve |
Note: +ve: Positive; -ve: Negative; CA: Cancer Antigen; CEA: Carcinoembryonic Antigen; CK, Cytokeratin; EMA: Epithelial Membrane antigen; and GCDFP-15; Gross Cystic Disease Fluid Protein-15.
Table 2: Expression characteristics of immunohistochemical markers in the study.
Discussion
Histopathology diagnosis of cancer and categorization of the proper tumor type are very important steps in patient's diagnosis and essential for the adequate treatment. In our practice, sometimes it is difficult to make the correct diagnosis because of atypical clinical presentation of the patient or because of the presence of undifferentiated histological features. With the help of IHC It is possible to subtype malignant tumors accurately [7]. Undifferentiated malignant tumors are either metastasis of unknown origin or a primary neoplasia without obvious cell line of differentiation. So, the undifferentiated tumor generally implies a high-grade malignancy, frequently associated with pleomorphic to anaplastic appearance [8].
IHC is very important applications in ovarian cancer diagnosis as it has many applications of different markers that may assist in the diagnosis of a primary ovarian malignancy or in tumor subtyping. The distinction between a primary ovarian carcinoma and metastatic carcinoma from various sites may be problematic [9]. Generally, using panels of markers are better than depending on an individual marker, as there is no marker is totally specific or sensitive for any tumor type. Clinical data, gross and microscopic findings are important along with IHC results to reach the proper diagnosis as unexpected positive and negative staining reactions may occur in IHC [10,11].
In the present study, twenty cases diagnosed as undifferentiated carcinoma of the ovary were studied using a panel of IHC markers to detect the origin of the tumor. The selected antibodies were chosen to help in differentiation between primary ovarian tumors and other tumor metastasis.
In present study, 18/20 cases showed positive reactivity to CK AE1/AE3, only 2 cases were negative, also those 2 cases were negative for others epithelial markers (CK7, CK20, CK125, EMA, and calretinin). Immunostaining for cytokeratin AE1/AE3 is essential to confirm the epithelial origin of the studied tumors. This “pericellular pattern” which was noticed in the studied cases suggested the epithelial origin of these tumors. The pericellular pattern, described as being of higher intensity at the periphery of tumor cells, is not observed in mesenchymal tumors [12].
Eighteen cases of the studied group co-expressed cytokeratin AE1/AE3 and vimentin. The co-expression of vimentin and cytokeratin is frequently found in several types of carcinomas while sex cord stromal tumors (such as the granulosa cell tumor with which is made the differential diagnosis of some undifferentiated ovarian carcinoma) are vimentin positive but usually cytokeratin negative [13]. For a correct differential diagnosis between undifferentiated ovarian carcinomas and diffuse type of granulosa cell tumor it is necessary to use inhibin-antibody which is negative in undifferenstiated ovarian carcinomas but positive in granulosa cell tumor [14].
EMA used as a supplement marker to CKs for detection of epithelial differentiation, especially in sarcomatoid carcinoma or in undifferentiated carcinomas that are negative or only focally positive for CKs [15]. In the current study, 18/20 cases showed strong positive reaction toward EMA antibody. The results are in accordance with the studies that reported that all the analyzed undifferentiated ovarian carcinomas were strongly positive for the EMA antibody [5].
All the studied cases showed vimentin positive reaction. Vimentin/CK coexpression helps to focus on certain types of epithelial tumors as possible primary sites in the evaluation of metastatic tumors. Co-expression of vimentin with CK is frequently seen in some carcinomas, as papillary and anaplastic thyroid, renal cell, and endometrial, ovarian carcinomas. While the absence of vimentin is observed in colonic, small intestinal adenocarcinomas or in transitional cell carcinomas [16,17]. Vimentin, cytokeratin AE1/AE3 and EMA positivity in the eighteen cases confirmed the epithelial origin of these tumors and excluded sex cord stromal tumors.
All the studied cases showed vimentin positive reaction. Vimentin/CK coexpression helps to focus on certain types of epithelial tumors as possible primary sites in the evaluation of metastatic tumors. Co-expression of vimentin with CK is frequently seen in some carcinomas, as papillary and anaplastic thyroid, renal cell, and endometrial, ovarian carcinomas. While the absence of vimentin is observed in colonic, small intestinal adenocarcinomas or in transitional cell carcinomas [16,17]. Vimentin, cytokeratin AE1/AE3 and EMA positivity in the eighteen cases confirmed the epithelial origin of these tumors and excluded sex cord stromal tumors.
The pattern of coordinate expression of both CK7/CK20 assists in the distinguish between a primary ovarian tumor and a metastatic colorectal carcinoma. Although either marker can be positive in both tumors, primary ovarian neoplasms are often diffusely positive with CK7 while CK20 is variable; conversely metastatic colonic carcinoma is usually diffusely positive with CK20 and focally positive with CK7 when this marker is expressed [19]. It is also demonstrated by a comprehensive study of CK7 and CK20 for 384 primary tumors and their metastasis 120 and other studies focusing on CK20 expression by primary and metastatic colorectal carcinoma [20]. The CK7/CK20 phenotype can be especially useful in the differentiation between primary ovarian carcinoma from primary colonic carcinoma or primary endometrial, pulmonary, or mammary adenocarcinoma (CK7+/ CK20-) [20,21]. In the current study, all the cases were CK20 negative and 18 cases were CK7 positive, this result support that the primary tumors of the eighteen positive CK7 cases are ovarian, and excluding metastasis from GIT or breast carcinoma. The results of the present study correspond to those already registered by the specialists who have reported that all the undifferentiated ovarian carcinomas were CK7 positive (majority showing diffuse staining) and CK20 negative [22].
In the present study, only 11/20 cases showed positive CA125 reaction, which was focal and heterogeneous. Even if CA125 is not a specific marker of the ovarian origin of the tumors, the staining heterogeneously favors ovarian origin as noticed in many studies, which have shown that 70% of the undifferentiated carcinomas react to CA125 and malignant cells stained with CA125 frequently showed heterogeneous focal positivity [23].
In the studied group, only four cases were CEA positive with focal weak staining. CEA is an oncofetal glycoprotein overexpressed in a variety of adenocarcinomas and consistently by gastrointestinal adenocarcinomas [24,25]. Therefore it used in a panel for recognition of poorly differentiated colonic adenocarcinoma (CEA positive) from ovarian carcinoma (CEA negative) [25]. It is also frequently used as a negative marker in the mesothelioma panel. A weak or a focal staining for CEA supports the ovarian origin of the tumors while it is intense and diffusely positive for CEA in tumors with gastrointestinal origin [12].
In distinguishing between a metastatic breast carcinoma and ovarian carcinoma, markers which may be useful are PAX8 (usually positive in ovarian carcinomas and negative in breast carcinomas, and GCDFP-15 (usually negative in ovarian carcinomas and positive in breast carcinomas) [26-28]. In this study, all the cases were negative to GCDFP15 and 16/20 was positive to PAX8. PAX8 proved to be a specific and a sensitive marker for endometrioid ovarian carcinomas having 89% sensitivity [29].
Nonaka et al. [18] reported that Pax8 is very useful marker in distinguishing ovarian carcinomas from breast carcinomas. PAX8 has been demonstrated to be a highly sensitive and relatively specific. They studied 124 ovarian carcinomas cases (84 papillary serous, 18 endometrioid, 12 mucinous, 10 clear cell), and found that Pax8 was expressed typically in a diffuse fashion in 96% of the papillary serous tumors, 89% of the endometrioid tumors, 100% of the clear cell tumors, and 8% of the mucinous tumors. While 243 cases of breast cancer (178 ductal and 65 lobular) all were negative for Pax8 [27].
GCDFP-15 is a 15-kd secretory glycoprotein of various body fluids (saliva, milk, and seminal fluid). It is considered a marker of apocrine differentiation [30] with high specificity for breast carcinomas [31-33]. Kaufmann et al. studied 328 cases of metastatic adenocarcinoma and their results demonstrated that GCDFP-15 expression had a sensitivity of 83%, a specificity of 93%, and a predictive accuracy of 92% for breast carcinomas against all other types of carcinomas including ovarian carcinomas [33]. In another study including 105 breast cancers and 585 non-mammary malignancies, GCDFP-15 identified breast carcinomas with a sensitivity of 74% and a specificity of 95% [31].
In the current study, the presence of vimentin in the tumor epithelial cells required the introduction of other antibodies to help differentiate the studied tumors from tumors with the mesenchymal origin, especially from mesotheliomas. Thus, anti-calretinin antibody was also used (presently considered to be the best marker for mesotheliomas) together with cytokeratin, and EMA antibodies (epithelial cells markers).
So, in the present work 4/20 cases were positive to calretinin, two of them were also showed positive reactivity to inhibin- and vimentin, and negative for all other antibodies, suggesting sex-cord stromal tumor, especially granulosa cell tumor. While the other two positive calretinin cases were negative to inhibin-and PAX8 antibodies, and positive to panCK A1/A3, CK7, and EMA picture suggests strongly malignant mesothelioma. Calretinin is 29 kDa calcium binding protein, it is a good marker for malignant mesothelioma with a sensitivity approaching 100% [34] so, it is quite specific for differentiation between mesothelioma and adenocarcinomas, which are positive for it in only 8% to 11% of cases [35,36] Thus, calretinin antibody was used together with CKs, and EMA antibodies (epithelial cells markers) to detect malignant mesothelioma. Also, calretinin serves as a highly sensitive marker for sex cord–stromal tumors but it is not as specific as inhibin in this respect because it also labels ovarian epithelial neoplasms (22%) [37-39].
Inhibin- is a dimeric 32 kDa peptide hormone produced by ovarian granulosa cells and testicular Sertoli cells. It serves as a sensitive and highly specific marker for ovarian39 and testicular sex cord–stromal tumors [38]. Most ovarian sex cord stromal tumors stain positively with anti nhibin, and this might be of value in their distinction from other neoplasms that might mimic them [40]. To distinguish between an ovarian sex cordstromal neoplasm and an epithelial tumor, especially ovarian carcinoma, -inhibin is extremely useful when performed as part of a larger panel. Immunostains for -inhibin and calretinin are positive in most neoplastic granulosa and Sertoli cells [40,41].
Conclusion
In summary, in the current study, a panel of markers used to detect the primary site of the tumor in twenty cases of undiff36erentiated ovarian carcinoma. The majority of marker combinations had high specificity and sensitivity for diagnosing metastatic carcinomas, especially those originating in breast, colon, and malignant mesothelioma. Use of these additional markers along with clinical data could help the improvement of prediction rates. The designed combinations of immunostaining profiles are helpful in the diagnosis of tissue origin of metastatic adenocarcinomas and could offer a fast and correct prediction of the primary site. In the present study the expression patterns of panCKA1/A3, CK7, CK20, vimentin, EMA, calertinin, inhibin- PAX8, and GCDFP15, were sufficient for classification in most cases, whereas expression of CA125 and CEA may help in supporting the diagnosis.
So, in the twenty studied cases, 2 cases were malignant mesothelioma (calretinin +, panCKA1/A3 +, CK7 +, EMA +, vimentin +, CK20 -, CK125 -, CEA -, inhibin -, PAX8 -, GCDFP15 -). Two other cases were granulosa cell tumor (inhibin +, calertinin +, vimentin +, panCKA1/A3 -, CK7 -, EMA -, CK20 -, CK125 -, CEA -, PAX8 -, GCDFP15 -). Sixteen cases were undifferentiated ovarian carcinoma (PAX8 +, vimentin +, panCKA1/A3 +, CK7 +, EMA +, CK125 -/+, CEA-/+, calretinin -, inhibin -, PAX8 -, GCDFP15 -).
A panel approach which is composed of carefully selected antibodies is always recommended the antigenic profile of positive as well as negative markers will help in the characterization of the tumor. Though IHC is most rapid and cost-effective method for undifferentiated neoplasms.
19621
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2012) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: 359-386.
- Kurman RJ, Shih IeM (2010) The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol 34: 433-443.
- Tafe LJ, Garg K, Chew I, Tornos C, Soslow RA (2010) Endometrial and ovarian carcinomas with undifferentiated components: clinically aggressive and frequently underrecognized neoplasms. Mod Pathol 23: 781-789.
- Silva EG, Deavers MT, Bodurka DC, Malpica A (2006) Association of low-grade endometrioid carcinoma of the uterus and ovary with undifferentiated carcinoma: a new type of dedifferentiated carcinoma? Int J Gynecol Pathol 25: 52-58.
- Seidman JD, Russel P, Kurman RJ (2002) Surface epithelial tumours of the ovary. In: Kurman RJ (edn): Blaustein’s Pathology of the Female Genital Tract, (5th edn), Springer-Verlag, New York.
- Boerman OC, Van Niekerk CC, Makkink K, Hanselaar TG, Kenemans P, et al. (1991) Comparative immunohistochemical study of four monoclonal antibodies directed against ovarian carcinoma-associated antigens. Int J Gynecol Pathol 10: 15-25.
- Jambhekar NA, Chaturvedi AC, Madur BP (2008) Immunohistochemistry in surgical pathology practice: a current perspective of a simple, powerful, yet complex, tool. Indian J Pathol Microbiol 51: 2-11.
- Vege DS, Soman CS, Joshi UA, Ganesh B, Yadav JN (1994) Undifferentiated tumors: An immunohistochemical analysis on biopsies. J Surg Oncol 57: 273-276.
- McCluggage WG, Wilkinson N (2005) Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology 47: 231-247.
- McCluggage WG (2002) Recent advances in immunohistochemistry in gynaecological pathology. Histopathology 40: 309-326.
- McCluggage WG, Young RH (2005) Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol 22: 3-32.
- Hammond RH, Bates TD, Clarke DG, Grant AG, Haines AM, et al. (1991) The immunoperoxidase localization of tumour markers in ovarian cancer: the value of CEA, EMA, cytokeratin and DD9. Br J Obstet Gynaecol 98: 73-83.
- Dabbs DJ, Geisinger KR, Norris HT (1986) Intermediate filaments in endometrial and endocervical carcinomas. The diagnostic utility of vimentin patterns. Am J Surg Pathol 10: 568-576.
- Pelkey TJ, Frierson HF Jr, Mills SE, Stoler MH (1998) The diagnostic utility of inhibin staining in ovarian neoplasms. Int J Gynecol Pathol 17: 97-105.
- Pinkus GS, Kurtin PJ (1985) Epithelial membrane antigen--A diagnostic discriminant in surgical pathology: Immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol 16: 929-940.
- McNutt MA, Bolen JW, Gown AM, Hammar SP, Vogel AM (1985) Coexpression of intermediate filaments in human epithelial neoplasms. Ultrastruct Pathol 9: 31-43.
- Azumi N, Battifora H (1987) The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol 88: 286-296.
- Bahrami A, Truong LD, Ro JY (2008) Undifferentiated tumor: True identity by immunohistochemistry. Arch Pathol Lab Med 132: 326-348.
- Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, et al. (2006) Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol 30: 1130-1139.
- Shah KD, Tabibzadeh SS, Gerber MA (1987) Comparison of cytokeratin expression in primary and metastatic carcinomas. Diagnostic application in surgical pathology. Am J Clin Pathol 87: 708-715.
- Chu P, Wu E, Weiss LM (2000) Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol 13: 962-972.
- Wauters CC, Smedts F, Gerrits LG, Bosman FT, Ramaekers FC (1995) Keratins 7 and 20 as diagnostic markers of carcinomas metastatic to the ovary. Hum Pathol 26: 852-855.
- Neunteufel W, Breitenecker G (1989) Tissue expression of CA 125 in benign and malignant lesions of ovary and fallopian tube: a comparison with CA 19-9 and CEA. Gynecol Oncol 32: 297-302.
- Sheahan K, OBrien MJ, Burke B, Dervan PA, OKeane JC, et al. (1990) Differential reactivities of carcinoembryonic antigen (CEA) and CEA-related monoclonal and polyclonal antibodies in common epithelial malignancies. Am J Clin Pathol 94: 157-164.
- Berezowski K, Stastny JF, Kornstein MJ (1996) Cytokeratins 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol 9: 426-429.
- Tornos C, Soslow R, Chen S, Akram M, Hummer AJ, et al. (2005) Expression of WT1, CA 125, and GCDFP-15 as useful markers in the differential diagnosis of primary ovarian carcinomas versus metastatic breast cancer to the ovary. Am J Surg Pathol 29: 1482-1489.
- Nonaka D, Chiriboga L, Soslow RA (2008) Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol 32: 1566-1571.
- Liu H, Shi J, Wilkerson ML, Lin F (2012) Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 138: 57-64.
- Xiang L, Kong B (2013) PAX8 is a novel marker for differentiating between various types of tumor, particularly ovarian epithelial carcinomas. Oncol Lett 5: 735-738.
- Viacava P, Naccarato AG, Bevilacqua G (1998) Spectrum of GCDFP-15 expression in human fetal and adult normal tissues. Virchows Arch 432: 255-260.
- Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, et al. (1989) Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol 20: 281-287.
- Raab SS, Berg LC, Swanson PE, Wick MR (1993) Adenocarcinoma in the lung in patients with breast cancer. A prospective analysis of the discriminatory value of immunohistology. Am J Clin Pathol 100: 27-35.
- Kaufmann O, Deidesheimer T, Muehlenberg M, Deicke P, Dietel M (1996) Immunohistochemical differentiation of metastatic breast carcinomas from metastatic adenocarcinomas of other common primary sites. Histopathology 29: 233-240.
- Ordóñez NG (2006) The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: A comparative study. Mod Pathol 19: 417-428.
- Comin CE, Novelli L, Boddi V, Paglierani M, Dini S (2001) Calretinin, thrombomodulin, CEA, and CD15: a useful combination of immunohistochemical markers for differentiating pleural epithelial mesothelioma from peripheral pulmonary adenocarcinoma. Hum Pathol 32: 529-536.
- Miettinen M, Sarlomo-Rikala M (2003) Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: An immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol 27:150-158.
- McCluggage WG, Maxwell P (2001) Immunohistochemical staining for calretinin is useful in the diagnosis of ovarian sex cord-stromal tumours. Histopathology 38: 403-408.
- Movahedi-Lankarani S, Kurman RJ (2002) Calretinin, a more sensitive but less specific marker than alpha-inhibin for ovarian sex cord-stromal neoplasms: An immunohistochemical study of 215 cases. Am J Surg Pathol 26: 1477-1483.
- Deavers MT, Malpica A, Liu J, Broaddus R, Silva EG (2003) Ovarian sex cord-stromal tumors: An immunohistochemical study including a comparison of calretinin and inhibin. Mod Pathol 16: 584-590.
- McCluggage WG, Shanks JH, Whiteside C, Maxwell P, Banerjee SS, et al. (1998) Immunohistochemical study of testicular sex cord-stromal tumors, including staining with anti-inhibin antibody. Am J Surg Pathol 22: 615-619.
- Arora DS, Cooke IE, Ganesan TS, Ramsdale J, Manek S, et al. Immunohistochemical expression of inhibin/activin subunits in epithelial and granulosa cell tumours of the ovary. J Pathol 181: 413-418.















