I-Chen Sandra Chao1, Luan Jie2, and Jian-Kang Chao3,4*
1Department of Ophthamology, BenQ Hospital, Nanjing, China
2Zhongda Hospital, Southeast University Affiliated Hospital, China
3Department of Psychiatry, Pingtung Branch, Kaohsiung Veterans General Hospital, Pingtung, Taiwan
4Department of Social Work, National Pingtung University of Science & Technology, Pingtung, Taiwan
*Corresponding Author:
Jian-Kang Chao, MD, Ph.D.
Department of Psychiatry, Pingtung branch
Kaohsiung Veterans General Hospital
Pingtung, Taiwan
Tel: +886-8-7703232
Fax: +886-8-7700689
E-mail: jiankangchao2000@yahoo.com.tw
Received Date: November 10, 2017; Accepted Date: November 30, 2017; Published Date: December 08, 2017
Citation: Chao CS, Jie L, Caho J (2017) The Effect of Neovascularization on Human Retinal Pigment Epithelium after Hyperbaric Oxygen Therapy. Health Sci J. Vol. 11 No. 6: 535.
Copyright: © 2017 Chao CS, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Keywords
Hyperbaric oxygen; Human retinal pigment epithelial cells; Pigmented epithelium derived factor; Tumor necrosis factor alpha; Neovascularization
Introduction
Oxygen is a vital element in all metazoan life forms, it is essential for utilization of metabolic processes, such as energy production, intracellular signaling and regulation of gene expressions [1]. In high energy demanding circumstances such as in a dark condition, oxygen becomes one of the most limited metabolites in the retina, where the retina becomes highly sensitive to the fluctuation of oxygen tension such as in hypoxia [2]. Oxygen tension is critical for normal vascular growth and retinal neurogenesis. The study showed that senescent eye may be particularly prone to oxidative damage as exemplified by conditions such as macular degeneration and cataract. Because of its high consumption of oxygen, the retina is particularly susceptible to oxidative stress, which plays a major role in retinopathy [3]. The human retina is constantly at risk of hypoxia and ischemia, retina must be able to respond to the threat of pathological conditions where the oxygen tension may vary. The cells have different factors in action to respond to these changes. These factors include vascular endothelial growth factor (VEGF), fibroblast growth factors (FGFs), transforming growth factor alpha and beta (TGF-α, TGF-β), TNF-α, PEDF [4]. Pigment epithelium-derived factor (PEDF) has anti-angiogenic, anti-tumorigenic and neurotropic functions. PEDF is one of the most potent angiogenesis inhibitors. PEDF also inhibits endothelial cell migration via down regulating angiogenic inducers, plateletderived growth factor, interleukin-8, acidic fibroblast growth factor and lysophosphatidic acid. During Hypoxia PEDF is proteolytically degraded by matrix metalloproteinase (MMPs) [5].
Tumor necrosis factor alpha is a cell signaling protein mainly involved in systemic inflammation. Tumor necrosis factor alpha (TNF-α) is a cell signaling protein mainly involved in systemic inflammation. When TNF-α binds to TNFR1 (tumor necrosis factor receptor 1) in the retina it leads to the dissociation of inhibitory protein SODD (silencer of death domains), which enables the adaption protein TRADD (Tumor necrosis factor receptor type 1-associated death domain protein) and initiates the activation of NF-κB pathway and mitogen-activated protein kinase (MAPK) pathway. NF-κB pathway activates cell survival, proliferation and cell inflammatory responses.
Hyperbaric oxygen therapy (HBO) is used a medical treatment, where it delivers 100% oxygen, that is higher than the atmosphere gas mixture of 21% oxygen, the 100% pure oxygen is delivered at a level that is higher than the atmospheric pressure (0.101325 MPa) [6]. HBO induces a state of hyperoxia, which is beneficial at a molecular, cellular and biochemical level. HBO caused changes in the pressure gradient due to the increase of oxygen in the blood, which allowed oxygen to distribute throughout the whole body and maintained the tissues in a hyperoxygenated state. The increased in oxygen delivering pressure has many therapeutic effects that is used in different areas of medicine, such as, decompression sickness, air embolism, carbon monoxide poisoning, enhance in healing, delay radiation injury. HBO has the ability to drastically increase the partial pressure of oxygen in the tissues of the body. The oxygen partial pressures achievable using HBO is much higher than those achievable while breathing pure oxygen at normobaric conditions (i.e., at normal atmospheric pressure, 0.101325 MPa) [7]. In ophthalmology HBO is beneficial for diabetic retinopathy and CRAO patients [8,9]. HBO has been one of the most studied alternative treatments for many ocular diseases. HBO increases the oxygen tension in the ocular system, which is proven to interfere the production of angiogenic factors-TNF-α and angiogenic inhibitors –PEDF. This study used ARPE-19 cells and hyperbaric oxygen intervention to detect the expression of PEDF and TNF-α by western blot to observe the neovascularization effect on RPE cells after HBO intervention and find the relationship between duration, HBO pressure and the production value between these factors can highly benefit future treatment and allowed individualized treatment for different types of ocular diseases for maximum benefits.
People can breathe 100% oxygen continuously for 24-36 hours at one standard atmosphere (101325 MPa), any longer can cause pulmonary oxygen toxicity symptoms to manifest. Pressure greater than one standard atmosphere can cause a faster development of the toxicity. When pressure is greater than two standard atmospheres, cerebral oxygen toxicity can lead to the development of seizure. The risk of oxygen toxicity can be reduced if patients were to breathe air during the HBO intervals for 5 minutes every 20-25 minutes of treatment [10]. During HBO, the excess production of reactive oxygen, nitrogen species and lack of antioxidant supplements can result in pathological oxidative stress, affecting the enzymatic antioxidant defense system [11]. Hence, prolonged HBO treatment can cause failure in the antioxidant defense system, leading to oxidative damages to ocular structures and cause the development of myopia, nuclear cataracts and keratoconus [12-14].
The retinal pigment epithelium (RPE) is a highly specialized neuroepithelium of the eye. It plays a critical role in retinal homeostasis; it is positioned between the choriocapillaries and the retinal photoreceptor cells [15]. The presence of tight junction between cells allowed the RPE to serve as a major part of the blood-retinal barrier and to function as a transporting and absorbing epithelium, controlling the milieu of the photoreceptor elements. Any disruption with the neural retina could cause photoreceptor cell pathology and thus adversely affect the visual process [16].
In the absence of disease, the vasculature of the mammalian eye is quiescent, as the angiogenic inhibitors prevent blood vessels from invading the cornea and vitreous. The eye consists of inhibitors responsible for the avascularity of the ocular compartments. Interruption toward the balance between the angiogenic factors and the inhibitors can cause retinal neovascularization, which is one of the most common complications in ocular diseases such as Dr. HBO has been one of the most studied alternative treatments for many ocular diseases. HBO increases the oxygen tension in the ocular system, which has being proven to interfere with the production of angiogenic factors- TNF-α and angiogenic inhibitors -PEDF. Finding the relation between duration, HBO pressure and the production value between these factors can highly benefit future treatments and allow individualized treatment for different types of ocular diseases for maximum benefits.
Materials and Methods
ARPE-19 cells were obtained from the American Type Culture Collection (ATCC), (USA). The study was submitted for Ethics committee approval and has ruled that approval was not required for the study. Well grown ARPE cells was selected, when the cells had cover about 80% of the Petri dish, Change into bovine serum free broth 12 hours before intervention, The cells were then divided into control and experimental groups randomly and treated with different HBO pressures (0.15 MPa, 0.2 MPa, and 0.25 MPa) under normal condition with DMEM/F12 broth and hypoxic condition with with CoCl2 DMEM/F12 broth.
Experimental material
Object of study: ARPE-19 cells were obtained from American Type Culture Collection, ATCC (USA).
Experimental procedures: Select well grown ARPE cells which were obtained from the American Type Culture Collection, ATCC (USA), the cells had to cover about 80% of the petri dish, Change into bovine serum free broth 12 hours before intervention, the cells were then divided into control and experimental groups randomly and treated with different HBO pressures (0.15 MPa, 0.2 MPa, and 0.25 MPa) under normal conditions with DMEM/F12 broth and hypoxic condition with CoCl2 DMEM/F12 broth.
Divided into groups: We divided to group A 60 minutes and group B 90 minutes two groups.
1. Control group Normoxic (C1) (does not undergo HBO treatment)
2. Control group Hypoxic (C2) (does not undergo HBO treatment)
3. Experimental group (A1/B1) 0.15 MPa 60/90 minutes with DMEM/F12 broth
4. Experimental group (A2/B2) 0.20 MPa 60/90 minutes with DMEM/F12 broth
5. Experimental group (A3/B3) 0.25 MPa 60/90 minutes with DMEM/F12 broth
6. Experimental group (A4/B4) 0.15 MPa 60/90 minutes with CoCl2 DMEM/F12 broth
7. Experimental group (A5/B5) 0.20 MPa 60/90 minutes with CoCl2 DMEM/F12 broth
8. Experimental group (A6/B6) 0.25 MPa 60/90 minutes with CoCl2 DMEM/F12 broth
HBO exposure method: Disinfect the chamber with ultraviolet light for 20 minutes, and wipe down the surface with 75% alcohol. Place the petri dishes inside the chamber, flush the chamber with 100% pure oxygen for 5 minutes to flush out the air (none pure oxygen) and then close the chamber. Slowly increase the pressure to desired amount (0.15 MPa, 0.2 MPa, 0.25 MPa). Normalized pressure for 60 minutes and 90 minutes. After the cells have been exposed to HBO for the desired duration, slowly decompress. The decompression process should be controlled within 5 minutes, after 24 hours interval repeat process again. After complete HBO exposure, the cells were then tested for PEDF and TNF-α expression with western blot.
Statistical analysis
All experiments were repeated three times and western blots were scanned using a gel documentation system, Band Scan 5.0 western blot analysis software and PEDF, TNF-α relative level were normalized to beta-actin values. Results are expressed as mean and standard error of mean, statistical analysis were performed by comparing the means using a standard two way ANOVA.
Results
The changes in the PEDF expression after ARPE-19 cells had under gone HBO intervention for normoxic condition and hypoxic condition.
After normoxic and hypoxic ARPE-19 cells had undergone different HBO pressures (0.15 MPa, 0.20 MPa, 0.25 MPa), the PEDF protein expressions are as follow. For 60 minutes with HBO intervention, the PEDF protein expression increased as the pressure increased with statistical significance (P=0.0149) (Figures 1 and 2). When compared to hypoxic cells with nonhypoxia cells, the normoxic cells had a higher PEDF protein expression. After normoxic and hypoxic ARPE-19 cells had undergone different HBO pressures (0.15 MPa, 0.20 MPa, 0.25 MPa), the PEDF protein expressions are as follow. For 90 minutes with HBO intervention, the PEDF protein expression increased as the pressure increased with statistical significance (P=0.0208) (Figures 1 and 2). When compared to hypoxic cells with non-hypoxia cells, the non- hypoxic cells had a higher PEDF protein expression.
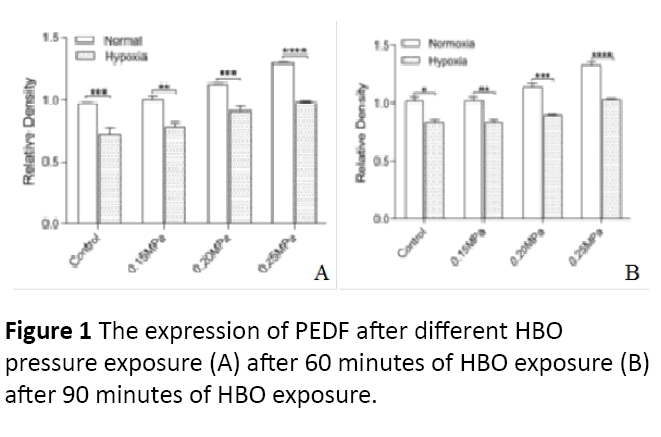
Figure 1: The expression of PEDF after different HBO pressure exposure (A) after 60 minutes of HBO exposure (B) after 90 minutes of HBO exposure.
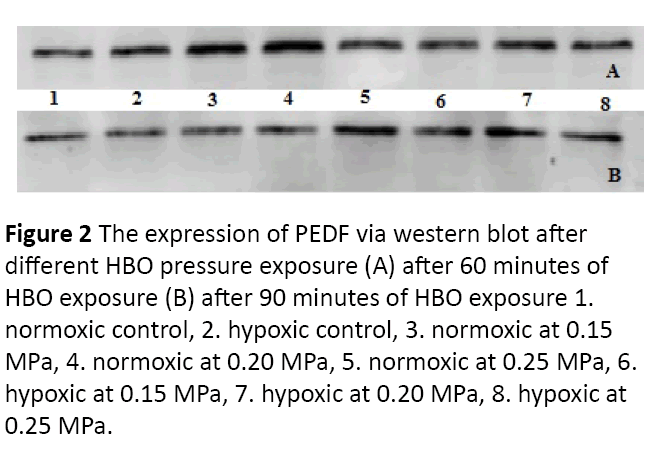
Figure 2: The expression of PEDF via western blot after different HBO pressure exposure (A) after 60 minutes of HBO exposure (B) after 90 minutes of HBO exposure 1. normoxic control, 2. hypoxic control, 3. normoxic at 0.15 MPa, 4. normoxic at 0.20 MPa, 5. normoxic at 0.25 MPa, 6. hypoxic at 0.15 MPa, 7. hypoxic at 0.20 MPa, 8. hypoxic at 0.25 MPa.
The changes in the TNF-α expression after ARPE-19 cells had undergone HBO intervention for normoxic condition and hypoxic condition.
After non-hypoxic and hypoxic ARPE-19 cells had undergoing different HBO pressures (0.15 MPa, 0.20 MPa, 0.25 MPa), the TNF-α protein expressions are as follows. For 60 minutes with HBO intervention, the TNF-α protein expression decreased as the pressure increased with statistical significance (P=0.0131) (Figures 3 and 4). When compared to hypoxic cells with nonhypoxia cells, the hypoxic cells had a higher PEDF protein expression.
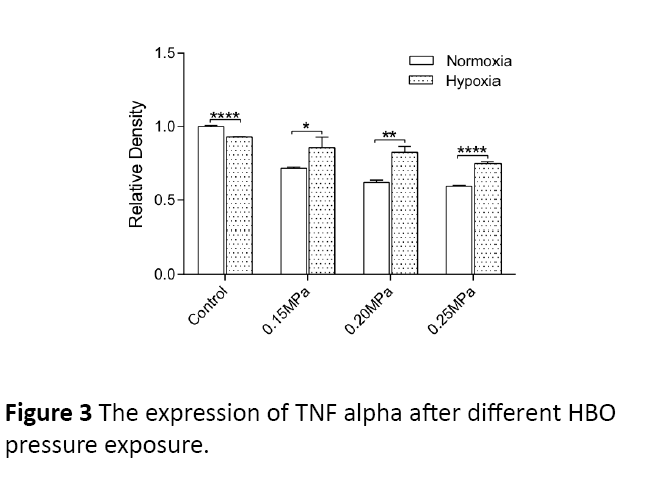
Figure 3: The expression of TNF alpha after different HBO pressure exposure.

Figure 4: The expression of TNF alpha via western blot after different HBO pressure exposure 1. normoxic control, 2. hypoxic control, 3. normoxic at 0.15 MPa, 4. normoxic at 0.20 MPa, 5. normoxic at 0.25 MPa, 6. hypoxic at 0.15 MPa, 7. hypoxic at 0.20 MPa, 8. hypoxic at 0.25 MPa.
Discussion
The retina is a ten-layered structure consisting of several layers of neurons interconnected by synapses. In the retina, the only neurons that are sensitive to the light directly are the photoreceptors [17]. Fluctuating oxygen concentrations are primarily responsible for retinal neovascularization [18]. The cells in the retina consume a large amount of oxygen to convert light energy into readable neuronal signals for the brain to translate. Sufficient amount of blood flow through the retina and choroid to ensure the sufficient amount of oxygen is delivered. In the retina, the oxygen is used to generate ATP by mitochondria to provide the necessary energy for proper cell functioning [17]. In the human eye, there are two separate oxygen delivery systems, the retina and the choroid vasculatures [18]. The two systems provide oxygen to different parts of the retina [18]. The choroid vasculature, which is highly vascularized, with little auto-regulatory response to oxygen fluctuation provides the necessary oxygen for the outer retinal layers, including the photoreceptors and the greater portion of the outer plexiform layer. The retina vasculature, which are the branches of the central retinal artery, are sparser than the choroid vasculature, it has a more sensitive auto- regulatory response toward oxygen fluctuation, and provides oxygen to the inner retinal layers [19]. The two vasculatures create a complex change of oxygen tension in the eye. Oxygen tension is the highest near the choroid vasculature, providing about 50 mmHg of oxygen to the RPE layer. The oxygen tension falls dramatically toward the photoreceptor layer, where it requires large amounts of oxygen as energy. In the inner retina, which is supplied by the retinal vasculature, the oxygen tension is lowest in the inner plexiform layer and highest at the region where the retinal vasculature is found [16]. In diseases such as diabetic retinopathy, branched retinal vein occlusion or retinopathy of prematurity, the retinal tissue develops pathological changes after experiencing tissue ischemia. Ischemia develops when the blood supply is inadequately provided to allow sufficient amount of oxygen and nutrients for proper cell functioning [20]. Ischemia deprives tissues of three survival requirements: oxygen, metabolic substrates and removal of waste products. During the initial loss of these three requirements, the cells will lower the homeostatic responses to cope with the environmental changes, but prolonged ischemia will induce injury to the tissues. Ischemia can result from local or systemic circulatory defects, retinal ischemia causing local impairment, such as central retinal artery occlusion and branch retinal artery occlusion [21]. As the retinal system has retinal vasculature and the choroidal vasculature, impairment to the retinal vasculature normally only affects the inner retinal layers as the outer retinal layers is metabolically supported by the choroidal vasculature. The separate choroidal vasculature serves a protective function during ischemia, as hypoxia occurs in the inner layer of the retina causing neuronal damage. The photoreceptors also gain oxygen and nutrients from the choroidal vasculature, it does not suffer a great deal during ischemia, thus protecting the visual acuity [22]. However, when the choroidal vasculature is also affected, both the inner and the outer retinal layers will suffer from hypoxic damage [23]. When total arterial blood occlusion occurs, it can lead to the development of ischemic edema in the inner layers of the retina and that eventually can lead to inner retinal atrophy [24]. Whereas when partial arteriolar occlusions occur, it can lead to hemorrhage, micro-aneurysm formation and retinal edema in the affected areas. When total or partial venous outflow occlusion occurs, the retina may develop microaneurysms, hemorrhages, exudates and edema [25,26]. Diabetic retinopathy (DR), is a common complication in patients with diabetes mellitus, most patients with 20 years of diabetes mellitus and with the disease onset before the age of 30, show signs of DR [27]. 50% of DR is proliferative diabetic retinopathy. DR is the most common microangiopathy in the retina and it is also one of the leading causes of blindness and visual impairment. During proliferative diabetic retinopathy (PDR), the retinal cells go through bi-phasic progression with an initial vaso-obliterative phase and subsequent uncontrolled vaso-proliferative period [27]. In patients with diabetes mellitus, the insulin levels or/and activity is decreased resulting in abnormal glucose metabolism and increased levels of blood glucose. The increase in blood glucose can cause pathological structural and physiological effect on the retinal capillaries and result in incompetence, both functionally and anatomically.
Conclusion
Hyperbaric oxygen has been one of the most studied alternative treatments for many ocular diseases. Hyperbaric oxygen increases the oxygen tension in the ocular system, which is proven to interfere with the production of angiogenic factors TNF-α and angiogenic inhibtors–PEDF. The study showed, in this experiment the duration of HBO intervention does not affect the release of PEDF and TNF- α by RPE cells, which is contradictory to the hypothesis, this may be due to the limited sessions of HBO treatment given to the cells. HBO can raise the level of PEDF and decrease the level of TNF-α in RPE cells, and has an antiangiogenic effect that can suppress the formation of neovascularization.
Conflict of Interest
The authors declare that there is no conflict of interest.
21412
References
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. (1998) Role of HIF-1alpha in hypoxiamediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485-490.
- Banasiak KJ, Xia Y, Haddad GG (2000) Mechanisms underlying hypoxia induced neuronal apoptosis. Prog Neurobiol 62: 215-249.
- McMonnies CW (2015) Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin Exp Optom 98: 122-125.
- Kimble EA, Svoboda RA, Ostroy SE (1980) Oxygen consumption and ATP changes of the vertebrate photoreceptor. Exp Eye Res 31: 271288.
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, et al. (1999) Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 285: 245-248.
- Gesell, Laurie B (2008) Hyperbaric oxygen therapy indications. The hyperbaric oxygen therapy committee report (12th edn.). Undersea and Hyperbaric Medical Society, Durham, NC.
- Gokce G, Metin S, Erdem U, Sobaci G, Durukan AH, et al. (2014) Late hyperbaric oxygen treatment of cilioretinal artery occlusion with nonischemic central retinal vein occlusion secondary to high altitude. High Alt Med Biol 15: 84-88.
- Sharkey LS (2000) Current indications for hyperbaric oxygen therapy. ADF Health 1: 64-72.
- Yan L, Liang T, Cheng O (2015) Hyperbaric oxygen therapy in China. Med Gas Res 5: 3.
- Erecinska M, Silver IA (2001) Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 28: 263-276.
- Darley-Usmar V, Halliwell B (1996) Blood radicals: Reactive nitrogen species, reactive oxygen species, transitionalmetal ions, and the vascular system. Pharm Res 13: 649-656.
- Tibbles PM, Edelsberg JS (1996) Hyperbaric-oxygentherapy. N Engl J Med 334: 1642-1648.
- Palmquist BM, Philipson B, Barr PO (1984) Nuclear cataract and myopia during hyperbaric oxygentherapy. Br J Ophthalmol 68: 113-117.
- Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM (2008) Oxidative stress indiseases of the human cornea. Free Rad Biol Med 45: 1047-1055.
- Boyer MM, Poulsen GL, Nork TM (2000) Relative contributions of the neurosensory retina and retinal pigment epithelium to macular hypofluorescence. Arch Ophthalmol 118: 27-31.
- El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai YN, Caldwell RB, et al. (2006) Neuroprotective and blood–retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 168: 235-244.
- Ames AR (1992) Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: A commentary based on studies on retina. Can J Physiol Pharmacol 70: 158-164.
- Ozgurtas T, Tekin S, Yesildal F, Umut K, Fevzi NA, et al. (2016) A novel model of retinopathy of prematurity in normobaric hyperoxic conditions. Int J Ophthalmol 9: 1265-1270.
- Flammer J, Mozaffarieh M (2008) Autoregulation, a balancing act between supply and demand. Can J Ophthalmol 43: 317321.
- Bek T (2009) Inner retinal ischaemia: Current understanding and needs for further investigations. Acta Ophthalmol 87: 362-367.
- Hayreh SS (2011) Acute retinal arterial occlusive disorders. Prog Retin Eye Res 30: 359-394.
- Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA (2007) Oxygen distribution andconsumption in the macaque retina. Am J Physiol Heart Circ Physiol 293: H1696-H1704.
- Hayreh SS, Zimmerman MB (2007) Fundus changes in central retinal artery occlusion. Retina 27: 276289.
- Pournaras CJ, Tsacopoulos M, Strommer K, Gilodi N, Leuenberger PM (1990) Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology 97: 13211328.
- Cai J, Boulton M (2002) The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond.) 16: 242260.
- Ferris FL, Davis MD, Aiello LM (1999) Treatment of diabetic retinopathy. N Engl J Med 341: 667678.
- Addison DJ, Garner A, Ashton N (1970) Degeneration of intramural pericytes in diabetic retinopathy. Br Med J 1: 264-266.









