Keywords
Tail fat; Isoflurane; Biotransformation; Sheep
Introduction
Isoflurane has been used frequently as an inhalant anesthetic in humans and animals because of its physical and chemical properties, such as low blood gas partition coefficient, and it does not cause cardiac arrhythmogenic effects. Approximately 0.2% of inhaled isoflurane is metabolized in humans by the cytochrome 450 2E1 enzyme, which mediates reactions to acyl chloride, carbon dioxide, trifluoroacetic acid, fluoride ions and water. Inorganic fluoride concentrations can be detected in the urine and blood of humans after isoflurane has been administered by inhalation [1-16]. All halogenated inhalation anesthetics can cause liver toxicity in various animal species and humans [15]. Liver damage manifestation might range from a moderate increase in liver enzymes to liver failure, especially in humans. Different studies have shown that decreased level of oxygen prior to activation of liver enzymes or prolonged decline in arterial blood pressure might increase the risk of liver damage post-anesthesia. It is believed that the intermediate and final metabolites of inhalation anesthetics are involved in triggering liver toxicity. Inorganic fluoride ion is a common metabolite of halogenated inhalation anesthetics. Metabolism of methoxyflurane, halothane, enflurane, isoflurane and sevoflurane leads to an increase in fluoride level in the hair of rat, guinea pig, dog and human [6,10,17-21]. Trifluoroacetic acid and Difluoroethylene are excreted via expiration since they are evaporative. Serum fluoride concentration (SFC) is an accessible factor for measuring the metabolism rate of the inhalation anesthetics as well as a measurable indicator for assessing the activity of the liver enzymes and blood concentration of the inhalation anesthetics [1,2,22-27].
Previous studies have suggested that the metabolism rate of inhalation anesthetics is increased in obese individuals compared to those with normal body mass index (BMI) [20,28,29]. SFC during and post-anesthesia in obese patients is higher than that detected in normal patients. Although the mechanism underlying the increased metabolism rate of inhalation anesthetics in obese patients is yet to be determined, it has been suggested that the increased amount of fat tissue in these patients is a key factor in this process. Changes in the function of cardiopulmonary system, fat distribution in the liver, impaired oxygen release in hepatocytes, increased activity of hepatic microsomal enzymes and diet affect the metabolism of inhalation anesthetics in obese patients [20,29].
The circumstances affecting selection of a proper inhalation anesthetics to maintain anesthesia in obese patients are still under investigation [8,12]. Risk of airway obstruction and pulmonary aspiration after waking up from the anesthesia is higher in obese patients compared to normal individuals [13,30]. Therefore, it is essential to assure that the post-anesthesia side effects of anesthetics are reduced by inducing coughing in these patients [22]. The difference in metabolism of some drugs in obese patients compared to that in normal patients depends on the obesity and the applied drugs [4]. In obese individuals, blood flow per each gram of fat is reduced compared to that in normal or non-obese individuals [11,17]. All types of inhalation anesthetics accumulate in adipose tissue which could prolong the process of recovery from anesthesia. The accumulated anesthetics in adipose tissue might re-enter blood flow via veins in this tissue and affect other tissues, such as the omentum, mesentery, small intestine and liver [9,26]. These factors might seriously affect obese patients, especially in prolonged anesthesia. Rapid recovery from anesthesia can reduce the risk of breathing problems, apnea and hypoxia in obese patients [9,26]. The solubility of isoflurane in the blood and tissues is almost 4 times higher than that of desflurane. Therefore, the blood-gas partition coefficient of the isoflurane is lower than that of desflurane and the time of recovery from isoflurane-induced anesthesia is 4 times longer than that of the desflurane-induced anesthesia [9,26].
Anesthetizing the overweight or obese patients could be an obstacle to anesthetists since they encounter various challenges, including tracheal intubation, increased airway resistance and increased capacity of metabolizing anesthetics, such as halothane and enflurane [3,7,28]. The accumulation rate of anesthetics in tissue, their solubility in the blood and tissues as well as the rate of drugs elimination from arterial blood circulation determine the recovery time from anesthesia [22]. If the solubility of an anesthetic is very low, the majority of it is excreted through lungs and the rest re-enters the blood circulation and tissues causing a delay in recovery from anesthesia. Healthy overweight/ obese patients, who are anesthetized using desflurane, recover from the anesthesia quicker than those anesthetized using either isoflurane or Propofol [22].
In order to study the effects of body fat and eliminate other factors which might be involved in increasing the metabolism of inhalation anesthetics in overweight/obese patients, fat-tailed sheep has been used as a model for human in the present study. The body of most of Iranian sheep consists of a considerable amount of fat in their fat-tails which constitutes up to 28% of their total body weights [14,31,32]. Blood circulation of fat-tail occurs through the MSA which is a branch of the abdominal aorta [5]. The present study has investigated the biotransformation of isoflurane in fat-tailed sheep before and after ligation of MSA.
Materials and Methods
A total of twelve healthy fat-tailed Bakhtiyari ewe lambs, 9-12 month old, with a body score of 3 (on a scale of 0 to 5) (27) and with a mean ± SD weight of 25.3 ± 1.1 kg were included in the current study. The animals were kept in the livestock unit and were acclimatized to the experimental conditions for 21 days. The animals were fed lucerne and concentrate.
Prior to each anesthesia, a complete blood count and biochemical analysis were carried out for each ewe lamb in order to ensure their health. The ewe lambs were then randomly divided into two groups of six. Experiments were conducted in two steps:
Step 1: Food was withheld for 18 hrs before induction of the anesthesia. Sheep in two experimental groups were anesthetized for 3 hrs using isoflurane (Rhodia Co, UK) in oxygen. For induction of anesthesia sheep were administered with 4.5% isoflurane in oxygen (4 L min-1) via a fitted facemask without using any pre-anesthesia medication. To administer subsequent fluid and collecting venous blood samples, right jugular vein was catheterized prior to anesthesia. Induction of anesthesia in both groups was carried out using anesthesia masks, specifically prepared for this purpose. Isoflurane at the concentration of 4.5% and oxygen flow rate of 4 L per minute were given to the sheep using a small animal anesthesia device (Ohio Co, USA). Lidocaine 10% spray (Pasture institute, Iran) was used to anesthetize the larynx prior to tracheal intubation. Following intubation, sheep were connected to a re-breathing system and a medium plane of anesthesia, as determined by palpebral and pedal reflexes, and maintained for 3 hr using isoflurane (1.0 to 1.2%) in oxygen (1.5 L min-1).
Step 2: Sheep in the experimental group were injected, under sterile condition, with 5-4 ml of 2% lidocaine epidural in between the connection of the last lumbar to sacral. To assure that the needle was in place, losing the resistance to air injection applying a glass syringe was used. Following the epidural anesthesia, sheep were positioned in dorsal recumbency to ligate median sacral artery (In domestic mammals except horse median sacral artery runs beyond the sacrum passing ventrally along the coccygeal vertebrae and continuing as the median caudal artery) [23]. A 4 cm midline incision was made at the base of the tail in order to access MSA which was then ligated at its most proximal location using chromic catgut no. zero. To ensure that the cessation of blood flow in MSA was complete, a Doppler device was used in a place lower than the ligation site. After the ligation of MSA was ensured, the subcutaneous tissue was closed with no. 0 chromic catgut and skin with no. 1 polypropylene in a simple continuous pattern. The same procedure was carried out in the control group. However, MSA was not ligated after the subcutaneous dissection. At this step, similar to the first step, sheep were anesthetized for 3 hrs using halothane.
After anesthesia was induced, a catheter No. 22 was used to draw arterial blood from ear arteries and Lactated Ringer’s solution (10 ml kg-1 per hr) was administered throughout anesthesia. Pulmonary ventilation was performed mechanically with a tidal volume of 15 ml/ Kg of body weight and respiratory rate was 12-10 per minute. Arterial blood samples were collected at 30, 60 and 120 minutes post-anesthesia for blood gas analysis using a blood gas analyzer. The samples were sent to laboratory for analysis after measuring the rectal temperature and hemoglobin level. The venous blood samples were obtained from jugular vein at 0 (before anesthesia as the baseline), 1, 3 6, 12, 24, 48 and 72 hrs following the induction of anesthesia. The blood samples were left to clot at room temperature and serum was separated by centrifugation. Serum fluoride concentration (SFC) was measured using a potentiometer (Puls, 1988). The venous blood samples were collected at time zero (before induction of anesthesia) and 72 hrs after anesthesia in order to measure the activity of aspartate aminotransferase and gamma glutamyltransferase, total protein and albumin [31]. Anesthesia induction time (time from the administration of isoflurane to tracheal intubation), extubation time (time from the disconnection of isoflurane to swallowing reflex), time to sternal recumbency (time from the discontinuation of isoflurane to sternal recumbency) and time of standing up (time from the discontinuation of isoflurane to time of standing on limb) were recorded carefully.
Data analysis
Experimental results were expressed as mean ± standard deviation (SD). Since all the obtained data in the first stage of anesthesia were similar in experimental and control groups, they are all first combined and then statistically analyzed. The SFC, blood biochemical factors and the recovery time before and after anesthesia are analyzed using ANOVA and Duncan's new multiple range test (MRT). Statistical analysis was carried out using SPSS V. 17(SPSS Inc., Chicago, USA) and a value of P<0.05 was considered as statistically significant.
Results
There was no significant difference in body weight of animals in the two groups. MSA was easily exposed at the base of the tail and its ligation in the experiment group was performed without any problem. No complication occurred during or after surgery. Induction of anesthesia, using facemask, was performed easily, without any resistance and discomfort. Following the first stage of anesthesia, using isoflurane, SFC was significantly increased 3 and 6 hrs post-anesthesia compared to pre-anesthesia (time zero) P<0.05 (Figure 1). Highest levels of flurane ions were detected 3 and 6 hrs after induction of anesthesia (Figure 1A). Given no significant difference in SFC between the experimental and control groups at the first step of anesthesia, the obtained data from this stage were combined.
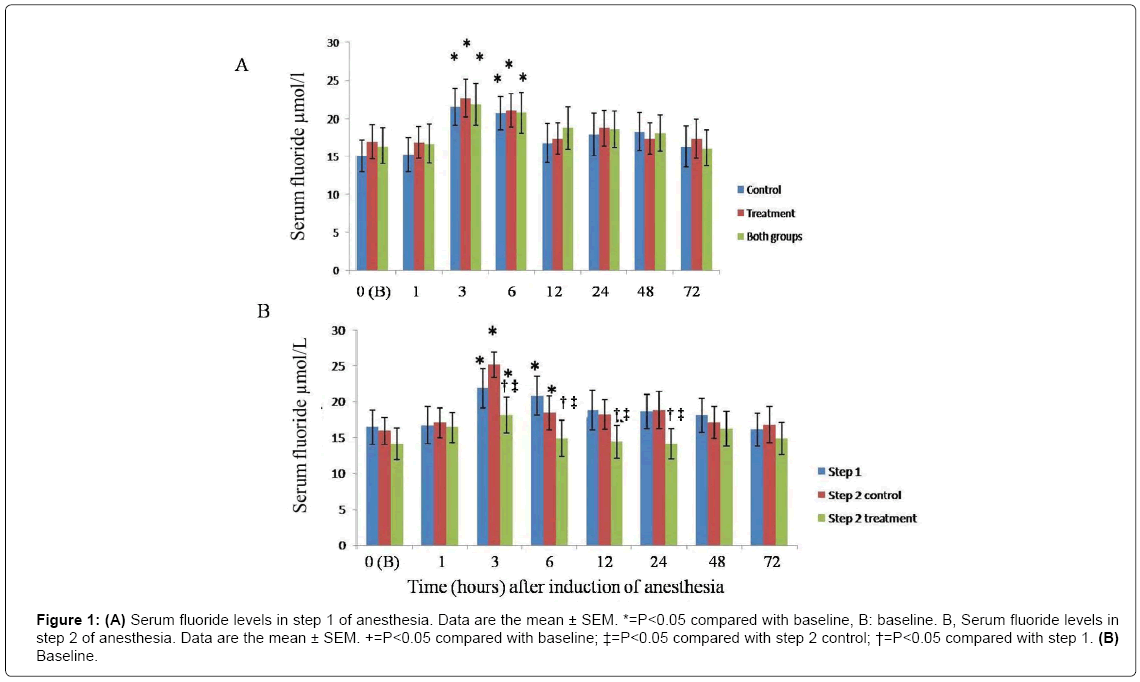
Figure 1: (A) Serum fluoride levels in step 1 of anesthesia. Data are the mean ± SEM. *=P<0.05 compared with baseline, B: baseline. B, Serum fluoride levels in step 2 of anesthesia. Data are the mean ± SEM. +=P<0.05 compared with baseline; ‡=P<0.05 compared with step 2 control; †=P<0.05 compared with step 1. (B) Baseline.
In the second step of anesthesia, the increase in SFC in sheep with unligated MSA was similar to that detected in the first step of anesthesia. However, increase in SFC, in sheep whose MSA was ligated, was only significant 3 hrs post-anesthesia compared to that measured pre-anesthesia. Concentration of this ion was significantly decreased 3, 6, 12 and 24 hrs post-anesthesia in experimental croup compared to the control group and the first step of the anesthesia (Figure 1B). Following 3 hrs of anesthesia using isoflurane, SFC in the first step of anesthesia was respectively 22.7 ± 2.5 and 21.6 ± 2.4 in experimental and control groups. At the same time points in the second step, SFC was 18.1 ± 2.1 and 25.1 ± 1.8 in experimental and control groups, respectively (Figure 1A and 1B). No significant difference was observed in induction time between the two groups in the first and second steps of the anesthesia (P<0.05). However, extubation time, time to sternal recumbency and attempts to stand time were significantly prolonged in sheep with unligated MSA, compared to those in the experimental group (Figure 2, P<0.05). Blood gases, pH and bicarbonate levels were not significantly different at 30 min, 1 and 2 hrs post-anesthesia. Furthermore, neither hypoxia (PaO2 less than 60 mm Hg) nor severe hypercarbia (PaCO2 more than 50 mm Hg) were detected (Table 1, P<0.05). No significant differences were observed in blood biochemical factors pre-anesthesia and 72 hrs post-anesthesia in 3 hr-long anesthesia using isoflurane group (Table 2, P<0.05). Furthermore, no liver damage was observed following the first and second steps of the experiment. Although a significant decrease was detected in rectal temperature in both groups, there were no significant changes in the heart rate and rhythm. Salivation was increased in most of the sheep during anesthesia using isoflurane. All the sheep easily recovered from anesthesia. No adverse reaction, side effect or complication were observed during or after 3 hr anesthesia using isoflurane.
| Time after the induction of anesthesia (min) |
Stages |
PaO2 (mmHg) |
PaCO2 (mmHg) |
pH (Units) |
HCO3 (mmol/L) |
| 30 |
Stage 1 |
241.33 ± 49.85 |
47.50 ± 12.65 |
7.30 ± 00.08 |
24.10 ± 2.29 |
| Stage 2 (intact): |
251.33 ± 59.75 |
49.38 ± 13.12 |
7.31 ± 00.09 |
26.24 ± 2.96 |
| Stage 2 (MSAL): |
261.43 ± 52.77 |
39.98 ± 14.32 |
7.39 ± 00.13 |
22.15 ± 1.85 |
| 60 |
Stage 1 |
271.41 ± 62.85 |
49.64 ± 13.81 |
7.35 ± 00.12 |
25.42 ± 2.34 |
| Stage 2 (intact): |
245.29 ± 59.37 |
50.13 ± 18.45 |
7.41 ± 00.09 |
26.63 ± 1.89 |
| Stage 2 (MSAL): |
263.32 ± 72.29 |
39.75 ± 9.92 |
7.38 ± 00.19 |
25.17 ± 2.31 |
| 120 |
Stage 1 |
247.43 ± 43.35 |
48.67 ± 15.92 |
7.32 ± 00.05 |
27.35 ± 1.76 |
| Stage 2 (intact): |
254.36 ± 57.44 |
39.62 ± 15.45 |
7.31 ± 00.07 |
25.42 ± 2.36 |
| Stage 2 (MSAL): |
231.65 ± 48.97 |
48.19 ± 10.21 |
7.44 ± 00.10 |
26.71 ± 1.98 |
Intact, intact (control) sheep; MSAL, median sacral artery ligated sheep.
Table 1: Blood gas indices during 3 h of halothane anaesthesia in sheep stages 1 and 2 of the experiment (see text for details).
| Group |
Time |
AST (U/L) |
GGT (U/L) |
Total protein (g/dL) |
Albumin (g/dL) |
| Stage 1 (n=12) |
Baseline (0) |
125.12 ± 31.15 |
39.42 ± 8.32 |
2.56 ± 0.32 |
7.35 ± 0.69 |
| 72 h |
115.25 ± 32.54 |
43.36 ± 6.52 |
1.94 ± 0.24 |
7.54 ± 0.71 |
Stage 2
Intact (n=6) |
Baseline (0) |
117.65 ± 31.12 |
37.89 ± 5.14 |
1.96 ± 0.49 |
8.12 ± 0.83 |
| 72 h |
121.34 ± 33.19 |
41.65 ± 6.29 |
2.09 ± 0.29 |
7.48 ± 1.09 |
| MSLA (n=6) |
Baseline (0) |
122.36 ± 30.45 |
38.54 ± 7.65 |
1.84 ± 0.45 |
7.28 ± 0.98 |
| 72 h |
112.98 ± 39.19 |
42.09 ± 7.78 |
2.07 ± 0.25 |
7.74 ± 0.93 |
Data are the mean ± SD; Intact, Intact (control) sheep; MSAL, Median sacral artery ligated sheep; AST, Aspartate aminotransferase; GGT, Glutamyl transferase
Table 2: Serum chemistry values before and 72 h after 3 h of halothane anaesthesia sheep at stages 1 and 2 of the experiment (see text for details).
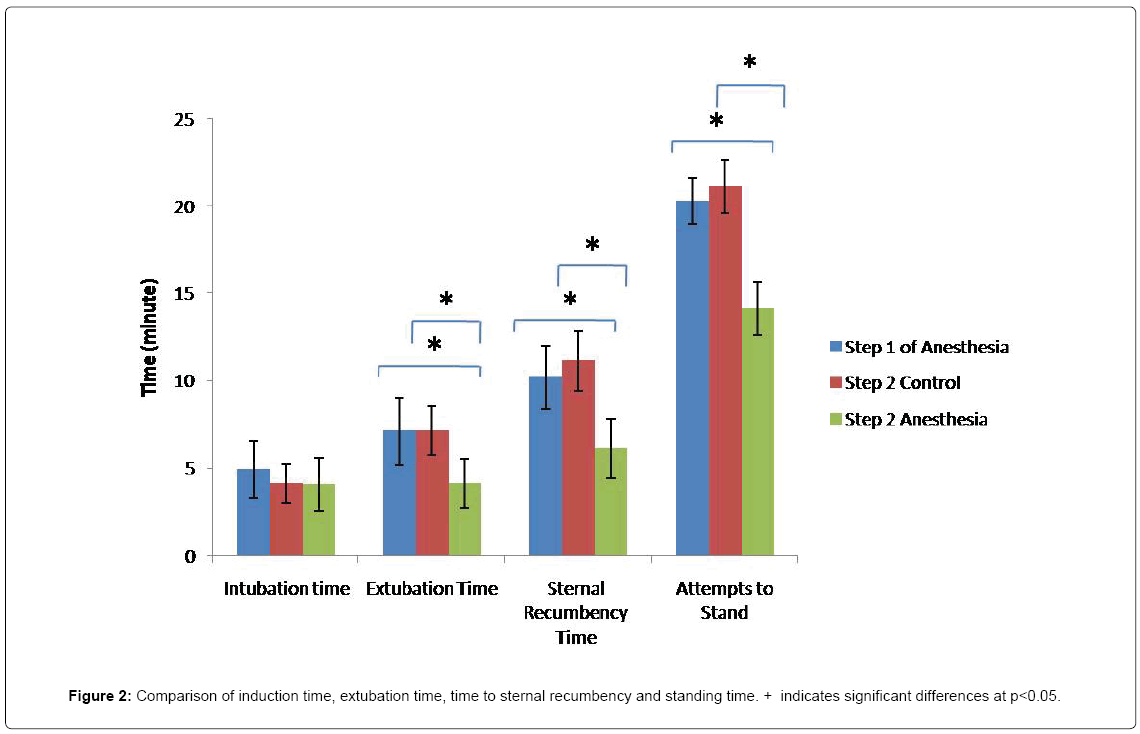
Figure 2: Comparison of induction time, extubation time, time to sternal recumbency and standing time. + indicates significant differences at p<0.05.
Discussion
The amount of fluoride detected pre-anesthesia in the present study was similar to that reported by Puls in a study conducted on sheep. The reported rate of fluoride in sheep blood was reported 2.6 to 21.1 μM/L [18]. In the current study, anesthesia was induced applying isoflurane which was delivered using a facemask. Neither pre-anesthesia medication nor injectable anesthetics were used in this study in order to prevent any drug interaction with metabolism of isoflurane [1,27]. Although the concentration rate of isoflurane which was inhaled by each animal was not measured in the present study, the setting of evaporator in the anesthetic machine and the depth of anesthesia were the same in both groups. Some studies have reported that SFC level was increased in human and rats during and after halothane and methoxy flurane-induced anesthesia [27,28]. An increase in SFC level up to 48 hrs after halothane-anesthesia compared to pre-anesthesia (baseline, time zero) has also been reported [25].
No significant difference was detected in SFC level in first and second steps of anesthesia in sheep with unligated MSA which could indicate that repeating anesthesia applying isoflurane after two weeks induces no effect on isoflurane metabolism in sheep. The average SFC level in sheep with ligated MSA was 27% lower 3 and 24 hrs post-anesthesia, compared to that detected at the same time intervals in the control group. It has been reported that SFC level in overweight/obese patients in prolonged anesthesia is higher than that measured in patients with normal range BMI [19,24]. Since inhalation anesthetics tend to be accumulated in adipose tissue, increased liver fat may result in an increased absorption of inhalation anesthetics which consequently leads to amplified metabolism of these drugs. As anesthetics are more diffused in adipose tissue of overweight/obese patients, they are longer exposed to hepatic microsomal enzymes which results in faster increase of fluoride ions and augmentation of average concentration of this ion [24,29]. Oxygen release disruption also occurs in the liver of obese individuals. Metabolism of isoflurane via reduction reaction occurs more in hypoxia. Therefore, due to the disruption of oxygenation of liver cells in obese patients and higher occurrence of hypoxia, the amount of fluoride ions resulted from metabolism via reduction reaction is higher in these patients [24,29].
Based on blood gases analysis during anesthesia, oxygen and carbon dioxide levels maintained at the normal range in all the sheep. Therefore, sheep did not suffer from hypoxia hypercapnia at any stages of anesthesia. As mentioned above, hypoxia can affect metabolism of isoflurane. Since each sheep has also been considered as its own control, other factors (including function of the liver, kidney, cardiovascular and respiratory systems) were not variable and the only difference between the groups was the amount of the adipose tissue of fat-tail. The average weight of the fat-tail in Iranian sheep is 9.8% of the total body weight [33]. Therefore, fat-tail forms a significant amount of the total body fat of the Iranian sheep. On average, the amount of fat in sheep with ligated MSA, was 2.4 kg less than that of the control group. Adipose tissue is highly capable of storing inhalation anesthesia and acts as a storage source of these drugs in overweight/obese patients. This can explain the higher partial pressure of isoflurane in the blood stream of sheep with unligated MSA compared to those whose MSA was ligated. Extubation time and time to sternal recumbency were significantly shorter in sheep with ligated MSA. Shorten recovery time from isoflurane-induced anesthesia after cessation of blood flow of fattail has previously been reported [24]. Faster recovery of sheep with ligated MSA (sheep experimented in the second stage of anesthesia) can be the result of faster decrease of partial pressure of alveolar isoflurane in these animals. Possibly, the rate of alveolar drain is higher in the sheep with ligated MSA. Increased recovery time from anesthesia has also been observed in overweight/obese individuals [24]. As revealed, no significant difference was in liver enzymes subsequent the first and second steps of anesthesia. This indicates that repeating anesthesia applying isoflurane with a two week interval does not affect the level of hepatic enzymes in sheep.
Conclusion
The average SFC was decreased 27% in sheep with ligated MSA indicating the important role of body fat in pharmacokinetics of isoflurane in sheep. The fat in the tail absorbs more isoflurane which is slowly released into the general circulation after administration of anesthetic is discontinued, resulting in prolonged elevated concentration of isoflurane and, therefore, prolonged recovery. But the influence of removing the uptake into fat could explain the F concentration being lower-because less is absorbed and consequently less to be metabolized. Regarding the ligation of MSA in the treatment group, the animals exhibited no evidence of tail feature.
Acknowledgements
The authors would like to thank Shahrekord University. Special thanks also go to Hassan Khajehei, who undertook language editing of the manuscript.
21815
References
- Anderson BJ (2015) Pharmacokinetics and Pharmacodynamics in the Pediatric Population. In: Pediatric Sedation Outside of the Operating Room,pp: 173-193.
- Anderson JS, Rose NR, Martin JL, Eger EI, Njoku DB (2007) Desflurane hepatitis associated with hapten and autoantigen-specific IgG4 antibodies.Anesthesia and Analgesia104: 1452.
- Antoniou SA, Antoniou GA, Koch OO, KÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂöhler G, Pointner R, et al. (2015) Laparoscopic versus open obesity surgery: a meta-analysis of pulmonary complications.Digestive Surgery 32: 98-107.
- Chahraski H, Radhmer B (1975) Particularites anatomiques de l'organe graisseux(adipeux) caudal du mouton chez les races d'Iran.Cahiers de Medecine Veterinaire.
- Cheasser C,Stoelting R (1973) Serum inorganic fluoride concentrations during and after halothane, fluroxene, and methoxyflurane anesthesia in man.The Journal of the American Society of Anesthesiologists 39: 537-539.
- Defresne A, Hans G, Goffin P, Bindelle S, Amabili P, et al. (2014)Recruitment of lung volume during surgery neither affects the postoperative spirometry nor the risk of hypoxaemia after laparoscopic gastric bypass in morbidly obese patients: A randomized controlled study.British Journal of Anaesthesia 113: 501-507.
- Demirel I, Bolat E, Altun AY ( 2017) Obesity and Anesthesia Management. Current Topics in Anesthesiology.InTech.
- El Azab SR, Bayomi Z, El Gaby SS, Abdelgaber FM (2015) Effect of sevoflurane versus isoflurane on middle ear pressure during tonsillectomy operation.Ain-Shams Journal of Anaesthesiology 8: 535.
- Jones RS, Auer U, Mosing M (2015)Reversal of neuromuscular block in companion animals.Veterinary Anaesthesia and Analgesia42: 455-471.
- Lee MJ, Wu Y, Fried SK (2013)Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications.Molecular Aspects of Medicine 34: 1.
- Liu FL, Cherng YG, Chen SY, Su YH, Huang SY, et al.(2015) Postoperative recovery after anesthesia in morbidly obese patients: a systematic review and meta-analysis of randomized controlled trials.Canadian Journal of Anesthesia 62: 907-917.
- Maher DP, Lee J, Woo P, Zhang X, White PF, et al. (2016) Ritonavir Use in Human Immunodeficiency VirusÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
âÂÂPositive Surgical Patients is Not Associated with an Increase in Postoperative Critical Respiratory Events.Journal of Pain & Palliative Care Pharmacotherapy30: 25-30.
- Mangombi J, Brouat C, Loiseau A, Banga O, Leroy EM, et al. (2016) Urban population genetics of the invasive black rats in Franceville, Gabon.Journal of Zoology 299: 183-190.
- Mohseni M, Safari S, Alavian SM (2014) Volatile anesthetics in ischemic liver injury: enemy or friend?Hepatitis Monthly, p: 14.
- Natalini CC, Krahn CL, Serpa PB, Griffith JE, de Almeida RM (2017) Intravenous 15% isoflurane lipid nanoemulsion for general anesthesia in dogs.Veterinary Anaesthesia and Analgesia.
- Oliveira AL, Azevedo DC, Bredella MA, Stanley TL, Torriani M (2015)Visceral and subcutaneous adipose tissue FDG uptake by PET/CT in metabolically healthy obese subjects.Obesity 23: 286-289.
- Puls R ( 1988) Mineral levels in animal health. Diagnostic data. Sherpa International.
- Rietbrock I (2013) Presented at the Inhalationsanaesthetika: Neue Aspekte 2 Internationales Symposium.
- SharifiS,Dehghan A (2015) Review of the effect of pulmonary ventilation on the blood flow velocity of abdominal aorta at the time of inhalation anesthesia with the isofluran gas in dogs. International Journal of Biology, Pharmacy and Allied Sciences4: 573-585.
- Scapellato ML, Carrieri M, MaccÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂàI, Salamon F, Trevisan A, et al. (2014)Biomonitoring occupational sevoflurane exposure at low levels by urinary sevoflurane and hexafluoroisopropanol.Toxicology Letters231: 154-160.
- Schroeder CA (2014) Hepatobiliary disease.Canine and Feline Anesthesia and Co-Existing Disease, pp: 82-92.
- Schummer A, Wilkens H, Vollmerhans B, Hebermehl KH (1981) The circulatory system the skin and cutaneous organs of the domestic mammals,p: 159.
- Sharifi S, Sarteshnizi AR, Sharifi F, Yousefian E (2015) Presented at the Veterinary Research Forum.
- Sharifi S, Vesal N (2005)Effects of tail fat on halothane biotransformation in fat-tailed sheep.Clin Exp Pharmacol Physiol32: 531-535.
- Steffey EP, Mama KR, Brosnan RJ (2015)Inhalation anesthetics.Lumb & Jones' Veterinary Anesthesia3: 297-323.
- Tavana M, Peighambarzadeh SZ, Savojbolaghi SH (2015)Comparison between intraosseous and subcutaneous excretory urography in Persian squirrels.Research Opinions in Animal & Veterinary Sciences, p: 5.
- Tonidandel A, Booth J, DÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¾ÃÂâangelo R, Harris L, Tonidandel S (2014)Anesthetic and obstetric outcomes in morbidly obese parturients: a 20-year follow-up retrospective cohort study.International Journal of Obstetric Anesthesia23: 357-364.
- von Opium M, Hyoscyamin M (2013) 6 Pharmakologie der InhalationsanÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂästhetika.AnÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂästhesiologie, p: 125.
- XarÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂá D, Santos A, Abelha F (2015)Adverse respiratory events in a post-anesthesia care unit.Archivos de BronconeumologÃÂÃÂÃÂâÂÂÃÂâ â≢ÃÂÃÂââ¬Ã
¡ÃÂâÂÂÃÂÃÂa(English Edition)51: 69-75.
- Young SR, Stoelting RK, Peterson C, Madura JA (1975)Anesthetic biotransformation and renal function in obese patients during and after methoxyflurane or halothane anesthesia.Anesthesiology42: 451-457.
- Zafar S, Younas N, Correia S, Shafiq M, Tahir W, et al. (2017)Strain-specific altered regulatory response of Rab7a and Tau in Creutzfeldt-Jakob disease and AlzheimerÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¾ÃÂâs disease. Molecular Neurobiology54: 697-709.
- Zamiri M, Izadifard J (2017)Relationships of fat-tail weight with fat-tail measurements and carcass characteristics of Mehraban and Ghezel rams.Small Ruminant Research26: 261-266.








