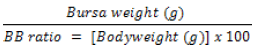Keywords
Berberine treatment; Clostridium perfringens; bodyweight; Gastroenteritis; Cholera; Water consumption; Antimicrobial activity
Abbreviations
BW: Bodyweight; BB ratio: Bursa-to-Bodyweight Ratio; FCR: Feed Conversion Ratio; NE: Necrotic Enteritis; SPSS: Statistical Package for the Social Sciences
Introduction
Berberine is an isoquinoline quaternary alkaloid, and has been identified as the major active component of many plants such as Coptidis rhizome, Huanglian and Phellodendri cortex [1,2]. It has been used for thousands of years in traditional herbal remedies in China and North America for the treatment of intestinal infections including acute gastroenteritis, cholera and bacillary dysentery [3]. This natural compound has drawn extensive attention as a scaffold for drug design with extensive literature and on-going clinical trials against a multitude of diseases [4].
The commercial poultry industry has been facing increasing pressure to reduce the use of antimicrobial growth promoters due to concerns that the use of antibiotics in the feed contributes to the spread of antibiotic-resistant genes by promoting the selection of antibiotic-resistant bacteria in animals [5-7]. Consequently, diseases such as Necrotic Enteritis (NE) have increased in prevalence, with NE related costs in the international poultry industry estimated to be in the region of two billion US dollars annually [8-10]. It is understood that the disease is typically caused by toxins produced by the bacterium Clostridium Perfringens [11]. Clinically, NE is characterized by a sudden increase in flock mortality, often without warning [12]. Subclinically, C. perfringens has been found to cause chronic damage to the intestinal mucosa, resulting in decreased digestion and absorption, reduced weight gain and increased feed-conversion ratio [13,14]. As C. perfringens spores are ubiquitous in nature in the environment and are ingested on a continuous basis via poultry feed, predisposing factors such as mucosal damage caused by coccidiosis are generally accepted to be required for this bacterium to cause disease [12,15,16].
Previous studies demostrate that Berberine is non-lethal in chickens up to dosages of 2000 mg/kg/ bodyweight and was effective in controlling against experimentally induced coccidial infection in chicken [17,18]. This is evident in the significant reduction of sporulated coccidial oocysts found in the faeces of treated birds. However, bloody diarrhea was observed, suggesting the absorptive mucosal surface was still damaged and does not disallow the notion of a C. perfringens outbreak [18]. In view of this, the potential use of Berberine in experimentally induced C. perfringens infection in broiler chickens is investigated for the first time. In addition to the importance of C. perfringens infection in livestock animals, the Clostridia genus is also associated with toxin-related infections in human patients [19,20]. Thereby, this study can also form the basis for further studies in drug discovery and development.
Materials and Methods
Source of material and animals
Berberine was purchased from the Sichuan Yuxin Pharmaceutical Industry Limited Company (Chengdu, China). Day-old Cobb 500 broiler chickens were obtained from Baiada Country Road Hatchery, Tamworth, NSW, Australia.
Phase 1 experimental design
The trial was performed using one hundred and fifty (150) broiler chicks. Chicks were vaccinated and initially handled as described by Wu et al. [21], before placed into positive pressure isolators for the duration of the trial. Each isolator has a floor space of 1.35 m2 and a positive pressure HEPA filtered (virus free) air supply from outside of the building. Water and food were provided ad libitum. An experimental ration formulated to resemble a commercial starter ration without any feed additives of either antimicrobiole or anticoccidial activity was fed throughout the study. The treatment groups are depicted in (Table 1). This allowed us to compare, for the first time, the dose response, efficacy, tissue residues and safety of the naturally occurring plant compound Berberine when administered prophylactically to birds in a C. perfringens utilizing proven experimental model [22].
| Group |
Bird |
Challenge |
Treatment |
Dosage |
Route |
Treatment |
No. |
| |
type |
|
|
|
|
Days |
Birds |
| 1 |
Broiler |
Nil |
Nil |
- |
- |
- |
15 |
| 2 |
Broiler |
Nil |
Nil |
- |
- |
- |
15 |
| 3 |
Broiler |
Nil |
Berberine |
1.0 g/L |
In-water |
1-16 |
15 |
| 4 |
Broiler |
Nil |
Berberine |
1.0 g/L |
In-water |
1-16 |
15 |
| 5 |
Broiler |
NE |
Nil |
- |
- |
1-16 |
15 |
| 6 |
Broiler |
NE |
Nil |
- |
- |
1-16 |
15 |
| 7 |
Broiler |
NE |
Berberine |
0.1 g/L |
In-water |
1-16 |
15 |
| 8 |
Broiler |
NE |
Berberine |
0.1 g/L |
In-water |
1-16 |
15 |
| 9 |
Broiler |
NE |
Berberine |
1.0 g/L |
In-water |
1-16 |
15 |
| 10 |
Broiler |
NE |
Berberine |
1.0 g/L |
In-water |
1-16 |
15 |
Table 1: Phase 1 Experimental Design: Challenge and Berberine in-water Treatment Regime.
Necrotic enteritis challenge protocol
The disease model used was based on previously validated studies [15,23,24]. The challenge groups were infected at 9 days of age via oral gavage with 5,000 wild-type strain sporulated oocysts each of E. maxima and E. acervulina and 2,500 sporulated oocysts of E. brunetti in 1 mL of 1% (w/v) sterile saline. At 14 days of age, a known pathogenic strain of C. perfringens was administered (type A strain EHE-NE36, CSIRO Livestock Industries, Geelong, Australia), i.t. (~8.0 log10 cfu/chicken). Whenever a challenge treatment was given, control chickens were administered the diluent or vehicle minus the agent, in the same manner as the challenged birds. All birds were sacrificed and autopsied at 16 days of age.
Assessment of effects
Feed and water intake, bodyweight (BW), feed conversion ratio (FCR) as well as NE lesion scores at autopsy were recorded and compared between groups to determine treatment effects. Bodyweight was recorded on day 1 and 16. The mean initial weight of the chicks for all groups was recorded as not significantly different. The NE lesion score was determined according to Prescott et al. [25]. Birds that died prior to autopsy were examined for NE lesion scores. Mortalities determined to be due to NE was recorded. FCR was calculated by the following formulae [26].

Organs and body systems of chickens from all groups were examined for gross visual pathological changes. Bursa of fabricius were collected, visually examined and gross weight recorded. BW and bursa weight were used to calculate the bursa-to-bodyweight (BB) ratio [27].
Phase 2 experimental design
A follow-up study was conducted to determine the feed palatability, water consumption and bird productivity following incorporation of Berberine in-feed at 2.0 g/kg in ninety (90) commercial broiler chicks. Chicks were vaccinated as in Phase 1, before placed into four individual floor pens, each of 22 or 23 chicks. Water and food were provided ad libitum. An identical experimental ration to Phase 1 was fed throughout the study. The treatment groups are depicted in (Table 2), and allowed us to evaluate the effects of Berberine on BW, feed and water consumption and FCR.
| Group |
Bird |
Challenge |
Treatment |
Dosage |
Route |
Treatment |
No. |
| |
type |
|
|
|
|
Days |
Birds |
| 1 |
Broiler |
Nil |
Nil |
- |
- |
- |
22 |
| 2 |
Broiler |
Nil |
Nil |
- |
- |
- |
23 |
| 3 |
Broiler |
Nil |
Berberine |
2.0 g/kg |
In-feed |
1-20 |
22 |
| 4 |
Broiler |
Nil |
Berberine |
2.0 g/kg |
In-feed |
1-20 |
23 |
Table 2: Phase 2 Experimental Design: Berberine in-feed Treatment Regime.
Assessment of effects: Feed and water intake, BW, FCR were recorded and compared between groups. The mean initial weight of the chicks of all groups was recorded as not significantly different. Birds were examined for gross visual pathological changes.
Statistical analyses
All values were expressed as means + SEM. Repeated measures one-way ANOVA was used to analyse all the data in Phase 1 Trial. Student’s t-test was used to analyse all the date in Phase 2 Trial. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) 10.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Phase 1 trial
Mortality and lesion scores: Table 3 summarizes the effects of Berberine against C. perfringens. Results show significant efficacy of Berberine at 1.0 g/L in controlling the disease compared to untreated groups based on significantly reduced mortality and lesion scores (Figure 1). No mortalities and NE lesions were observed in the negative control groups and the unchallengedtreated groups; groups 1, 2 and 3, 4 respectively. In NE-challenged birds, untreated groups 5 and 6 resulted in 83% mortality prior to autopsy compared to 0% mortality found in the 1.0 g/L Berberine groups 7 and 8. The lower dose 0.1 g/L Berberine groups 9 and 10 resulted in a 79% mortality rate. This dose-response effect is reflected in the lesion scores, with the untreated and low dose Berberine groups having lesion scores of nearly 4 compared to 1 in the high dose Berberine groups.
| Group |
1,2 |
3,4 |
5,6 |
7,8 |
9,10 |
| Bird type |
Broiler |
Broiler |
Broiler |
Broiler |
Broiler |
| Challenge Details |
Nil |
Nil |
NE |
NE |
NE |
| Treatment |
Nil |
Berberine |
Nil |
Berberine |
Berberine |
| Concentration in-water |
- |
1.0g/L |
- |
0.1g/L |
1.0g/L |
| No. Days Treatment |
- |
16 |
16 |
16 |
16 |
| No. Birds |
30 |
30 |
30 |
30 |
30 |
| Mortality % prior to autopsy |
0 |
0 |
83.5 |
79.5 |
0 |
| |
Median Lesion Scores |
|
|
|
|
| Duodenal Lesion Score (0 absent to 4 severe) |
0 |
0 |
4 |
4 |
1 |
| Jejunal Lesion Score (0 absent to 4 severe) |
0 |
0 |
4 |
4 |
1 |
| Ilial Lesion Score (0 absent to 4 severe) |
0 |
0 |
4 |
4 |
1 |
Table 3: Effects of Berberine in-water on Mortality prior to autopsy and NE Lesion Score Summary Data.
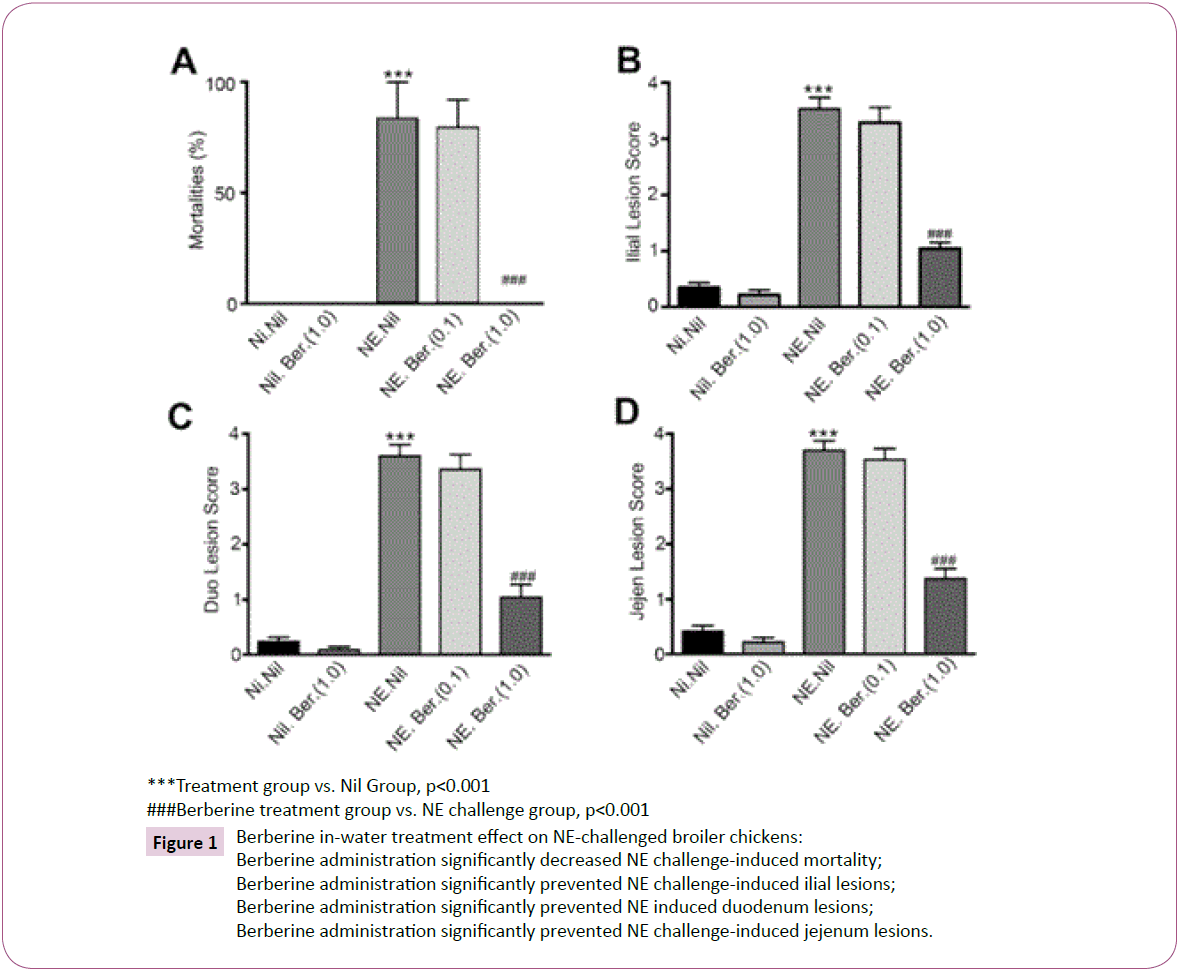
Figure 1:Berberine in-water treatment effect on NE-challenged broiler chickens:
Berberine administration significantly decreased NE challenge-induced mortality;
Berberine administration significantly prevented NE challenge-induced ilial lesions;
Berberine administration significantly prevented NE induced duodenum lesions;
Berberine administration significantly prevented NE challenge-induced jejenum lesions.
BW, feed and water consumption, FCR and BB ratio: The impact of C. perfringens and Berberine at 0.1 g/L and 1.0 g/L on BW, feed and water consumption and FCR is summarized in (Table 4). BW was observed to be adversely affected by both disease and treatment compared to negative control groups (Figure 2). Negative control groups recorded a mean final BW of birds of 595.3 ±16.26 g, compared to 407.6 ±7.706 g of challengeduntreated groups. Unchallenged-high dose Berberine was found to have similar final BWs at 408.0 ± .73 g, while challenged-high dose Berberine showed the worst result at 290.5 ± 10.16 g despite the perceived efficacy of Berberine against C. perfringens. Feed and water consumption exhibited similar trends, with groups treated with high dose Berberine recorded to have consumed the least feed and water per bird. This translated to highest FCR in challenged-treated groups and lowest FCR in negative control groups. Water consumption was most affected by Berberine, with groups treated with the high dosage drinking less than 50% of the total water consumed on average by the negative control groups; 856.6 ± 79.34 ml and 1796 ± 147 ml respectively. Relevant histopathological lesions were not observed in the bursa of the birds. BB Ratio (Figure 3) was slightly decreased in the challenged groups, apart from groups treated with high dose Berberine.
| Group |
1,2 |
3,4 |
|
5,6 |
7,8 |
|
9,10 |
|
| Bird type |
Broiler |
Broiler |
|
Broiler |
Broiler |
|
Broiler |
|
| Challenge Details |
Nil |
Nil |
|
NE |
NE |
|
NE |
|
| Treatment |
Nil |
Berberine |
|
Nil |
Berberine |
|
Berberine |
|
| Concentration in-water |
- |
1.0g/L |
|
- |
0.1g/L |
|
1.0g/L |
|
| No. Days Treatment |
- |
16 |
|
16 |
16 |
|
16 |
|
| No. Birds |
30 |
30 |
|
30 |
30 |
|
30 |
|
| Feed Consumption (Total) (g) |
20,517 |
12,381 |
|
17,189 |
14,788 |
|
10,503 |
|
| Mean Feed Consumption (g/bird) |
707.3 ±4.471 |
507.3 |
±85.39 |
573.0 ±23.57 |
524.5 |
±50.61 |
433.4 |
±47.56 |
| Water Consumption (Total) (ml) |
52,223 |
21,752 |
|
40,807 |
36,086 |
|
20,861 |
|
| Mean Water Consumption (ml/bird) |
1,796.0 ±147 |
870.3 |
±1.1 |
1,360.0 ±17.1 |
1,281.1 ±103.5 |
|
856.7 |
±79.34 |
| Mean Bodyweight (g) |
595.3 ±16.26 |
408 |
±10.73 |
407.6 ±7.71 |
379.8 |
±15.85 |
290.5 |
±10.16 |
| Feed Conversion Ratio |
1.287 ±0.03 |
1.464 |
±0.20 |
1.580 ±0.07 |
1.602 |
±0.11 |
1.771 |
±0.10 |
| Mean Bursa Weight (g) |
0.857 |
0.604 |
|
0.542 |
0.531 |
|
0.449 |
|
| Bursa-to-Bodyweight Ratio |
0.1425 ±0.009 |
0.1454 ±0.007 |
|
0.1354 ±0.006 |
0.1368 ±0.006 |
|
0.1563 ±0.008 |
|
Table 4: Effects of Berberine in-water on BW, Feed and Water Consumption, FCR and BB Ratio Summary Data.
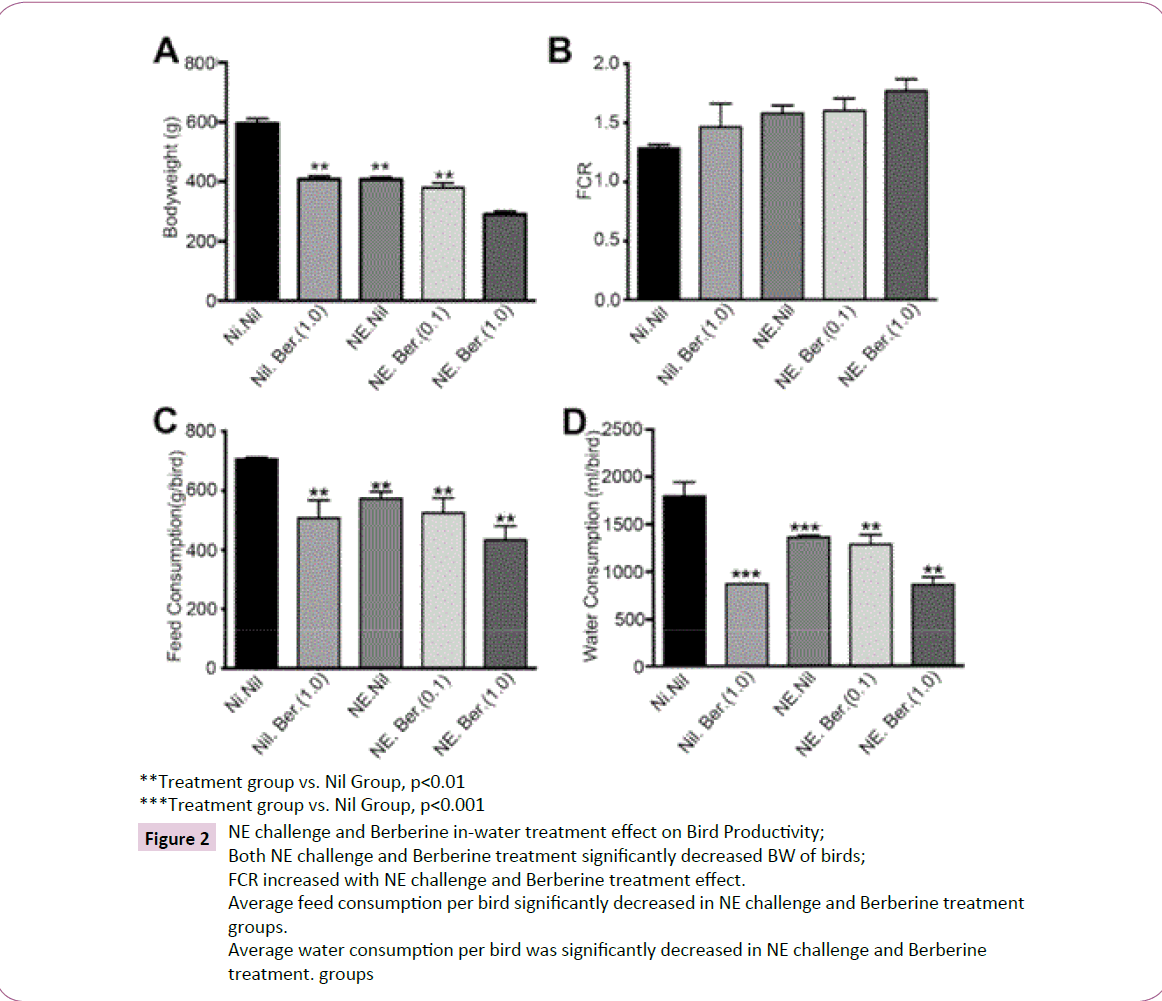
Figure 2: NE challenge and Berberine in-water treatment effect on Bird Productivity;
Both NE challenge and Berberine treatment significantly decreased BW of birds;
FCR increased with NE challenge and Berberine treatment effect.
Average feed consumption per bird significantly decreased in NE challenge and Berberine treatment
groups.
Average water consumption per bird was significantly decreased in NE challenge and Berberine
treatment. groups
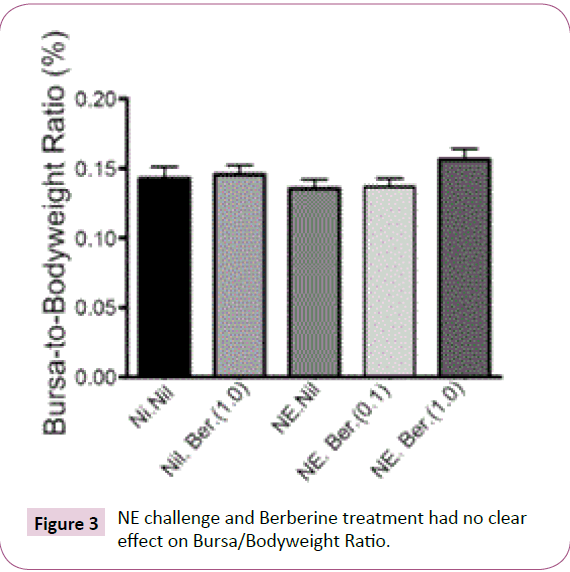
Figure 3: NE challenge and Berberine treatment had no clear effect on Bursa/Bodyweight Ratio.
Phase 2 trial
BW, feed and water consumption and FCR: Table 5 summarizes the effect of Berberine in-feed at 2.0 g/kg against C. perfringens. Bird productivity overall was observed to not be affected by Berberine in-feed. BW was observed to not be significantly different in treated groups and control groups; 628.0 ±14.22 g and 614.9 ±14.48 g respectively. Similarly, average feed consumption per bird and FCR were also not affected. Only water consumption was varied, increasing significantly in treated groups at 3,423 ± 59.09 ml/bird compared to 2,330.0 ± 34.88 ml/bird of control groups.
Discussion
C. perfringens-associated NE is an economic burden for the poultry industry due to the associated mortality, decreased bird productivity and associated increased FCR. This is projected to increase with the reduction of antimicrobial growth promoter use [28]. This study hoped to find a natural alternative to already known antimicrobials for the control of NE, especially with the emergence of drug resistance as a public health concern [29]. The Phase 1 in vivo trial demonstrated Berberine was extremely effective in controlling C. perfringens induced mortality and lesions. A clear dose-response relation was evident with the high concentration showing greatest reduction in lesion score and death. This suggests that the activity can be attributed to the Berberine itself, which is in accordance with previous studies involving treating broilers with Berberine [17,18,30]. The reduction in severity of observed clinical signs in the treated groups compared to the untreated groups is likely to be associated with decreased toxin secretion into the intestine, resulting in decreased C. perfringens induced damage to the gut. This is supported by the inhibitory intestinal secretory response of Berberine [31-33]. In addition, studies demonstrating the antimicrobial activity of Berberine against Clostridia bacterium is well-documented and suggests a direct inhibition of C. perfringens overgrowth [34-37].
Recently, there has also been accumulating evidence that modulation of gut microbiota confers beneficial effects in both humans and animal trials [38]. Berberine has been shown to significantly promote restoration of the intestinal microbiota by countering effects of intestinal damage triggered by antibiotics through the inhibition of Proteobacteria overgrowth [39]. Zhang et al. [40] reports that Berberine enriched short chain fatty acid (SCFA) producing genera of Blautia and Allobaculum by approximately 10-fold, where SCFAs are reported to alleviate inflammation and improve gut barrier function [41,42]. Similarly, Jeong et al. [43] reported that Berberine significantly suppressed pro-inflammatory genes in mice, while another study demonstrated reduction in lipopolysaccharides (LPS)-induced intestinal damage and decreased serum levels of downstream inflammatory cytokines [44]. The acute phase response induced by LPS in broiler chickens is indicated to be largely mitigated by Berberine [30]. Intestinal inflammatory cascades has been associated with NE, however this may be due to the intercurrent nature of coccidiosis and NE disease [12,45]. Other studies have shown that Berberine reduces smooth muscle contraction and intestinal motility and delays intestinal transit time in humans [46]. Therefore, it is likely that Berberine acts in a multitude of ways in the control of experimentally induced NE.
The study results also show decreased BW and increased FCR in all groups compared to negative control groups. The significant impairment in BW in challenged birds is in accordance to previous NE studies [21,23], where it is believed the chronic damage to the intestinal mucosa caused by C. perfringens leads to decreased digestion and absorption, and increased FCR [13,14]. However, surprisingly Berberine also proved to be highly detrimental to BW and feed and water consumption. Water consumption decreased by more than 50% in high dose Berberine groups. Thereby the results show a negative effect on bodyweight and FCR from both the disease and Berberine, although the lack of statistical evidence means the FCR data should be taken with a grain of salt. It is hypothesized that rather than Berberine having an adverse effect on the birds systemically, it was more likely that it was a palatability issue where the chickens did not like the taste of Berberine at high dosages in-water. This is supported by the significantly decreased water consumption and the innate bitterness of the Berberine [47,48]. Toxicity studies conducted by the National Toxicology Program have also demonstrated lack of acute, short-term, developmental and genetic toxicity of Berberine [49]. In fact, a broiler study conducted by Zhang et al. [50] suggests dietary supplementation with Berberine infeed can improve growth performance by enhancing immunity, reducing oxidative stress, and promoting intestinal colonization.
This is reinforced by the BB ratio. The bursa is a primary lymphoid organ in birds and plays a key role in the differentiation of B-lympocytes and BB ratio is generally accepted as a key indicator of immune system health [51]. Cazaban et al. [52] shows that an ideal BB ratio potential of 0.11 or above should be observed in healthy male Cobb 500 commercial broilers from 7 to 42 days of age housed in isolated conditions. All birds in the present study had BB ratios >0.11, with slightly increased BB ratio in birds treated with high dose Berberine, suggesting Berberine may positively impact the immune system.
Furthermore, the results of the Phase 2 in vivo trial reaffirm that the route of administration played a key factor in the adverse bird productivity results of the first trial. Unlike the first trial where Berberine was administered via water, the BW, feed consumption, and FCR were not affected by Berberine in-feed compared to the control groups. Although similarly, there is a lack of statistical evidence for FCR. In addition, water consumption was observed to have significantly increased in treated groups compared to control groups. This suggests Berberine in-feed stimulated thirst in the birds, which may be due to Berberine promoting glucose metabolism [53-55].
The limitations of this study include the experimental design. Increasing the number of birds or adding groups to each treatment would have allowed for statistically significant conclusions to be drawn regarding FCR. Additional control groups receiving the standard treatment regime used for NE control in Phase 1 would have provided comparative data for Berberine efficacy. There should also have been groups treated with a third concentration of Berberine in-water for a more convincing dose-response argument. Similarly, for Phase 2, additional groups with varying concentrations of Berberine in-feed would have confirmed that route of administration was a crucial factor in bird productivity. A challenge model using Berberine in-feed would also have been a welcome addition. Finally, the strain type A EHE-NE36 is uncommon in industry. As such, the results of the present study are not reflective of the disease in commercial farms as the strain used is considerably more virulent. Although this may be indicative that a lower dose would still be efficacious in practice.
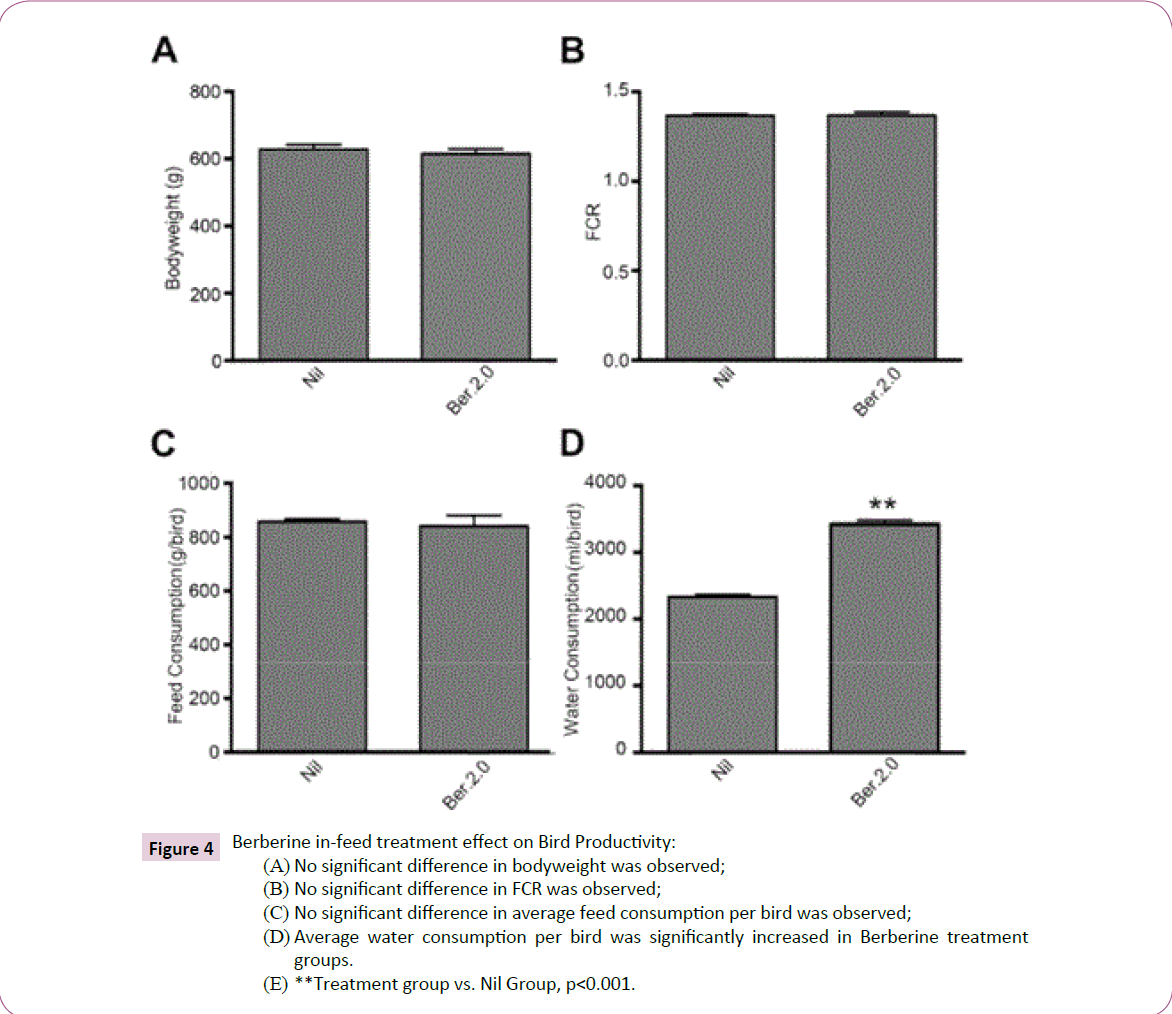
Figure 4: Berberine in-feed treatment effect on Bird Productivity:
(A) No significant difference in bodyweight was observed;
(B) No significant difference in FCR was observed;
(C) No significant difference in average feed consumption per bird was observed;
(D) Average water consumption per bird was significantly increased in Berberine treatment
groups.
(E) **Treatment group vs. Nil Group, p<0.001.
In conclusion, our data suggests that the addition of Berberine in-water at high dose can protect broiler chickens against C. perfringens induced NE. We provide experimental evidence that Berberine in-water protects against mortality and effectively improves the histopathological scores of chickens in the NE disease model. We hypothesize that Berberine acts as an antimicrobial and a modulator of the gut microbiota. Our data also demonstrates that administration of Berberine in-feed alleviated the bird productivity concerns, and surmise that this is due to the feed masking the inherently bitter taste of Berberine. Overall, Berberine is a promising, potential alternative for the control and treatment of NE.
Acknowledgements
This research was conducted with the help of the University of New England, Armidale, NSW, Australia and Veterinary Health Research Ltd, Armidale, NSW, Australia. We are thankful to Professor Steve Walkden-Brown, Associate Professor Shubiao Wu and Bruce Chick for their assistance and expertise. Any errors are our own and should not tarnish the reputations of these esteemed professionals.
Funding
This research was supported by iRiccorgpharm Pty Ltd in its initiative to reduce antibiotic usage in livestock.
Competing and Conflicting Interests
No declaration of competing and conflicting interests.
19249
References
- Imanshahidi M, Hosseinzadeh H (2008) Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytotherapy research 22: 999-1012.
- Xu JL, Wang,Xu B (2004) Research development of Coptischinensis. Zhongguoyixuekexueyuanxuebao.ActaAcademiaeMedicinaeSinicae 26: 704-707.
- Cordell GA, Quinn‐Beattie ML, Farnsworth NR (2001) The potential of alkaloids in drug discovery. Phytotherapy Research15: 183-205.
- Tillhon M (2012) Berberine: new perspectives for old remedies. Biochemical pharmacology 84: 1260-1267.
- Castanon J (2007) History of the use of antibiotic as growth promoters in European poultry feeds. Poultry science86: 2466-2471.
- Dibner J (2005) J. Richards, Antibiotic growth promoters in agriculture: history and mode of action. Poultry science 84:634-643.
- No ER (2003) European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 21: 1831.
- Van der Sluis W (2000)Clostridial enteritis is an often underestimated problem. World Poultry 16: 42-43.
- Van der Sluis W (2000)Clostridial enteritis-a syndrome emerging world wide. World Poultry 16: 56-57.
- Dahiya J (2006) Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Animal Feed Science and Technology129:60-88.
- SongerJG, Meer RR (1996) Genotyping ofClostridiumperfringensby Polymerase Chain Reaction is a Useful Adjunct to Diagnosis of Clostridial Enteric Disease in Animals. Anaerobe2: 197-203.
- Williams R (2005)Intercurrentcoccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathology 34:159-180.
- Elwinger KA (1991) Factors affecting the incidence of necrotic enteritis caecal carriage of Clostridium perfringens and bird performance in broiler chicks. ActaVeterinariaScandinavica 33:369-378.
- Kaldhusdal M (2001) Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian diseases 2: 149-156.
- Gholamiandehkordi AR (2007) Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathology 36:375-382.
- Stanley D (2014) Differential responses of cecalmicrobiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One 9: e104739.
- Malik TA (2014) In vivo anticoccidial activity of berberine [18, 5, 6-dihydro-9, 10-dimethoxybenzo (g)-1, 3-benzodioxolo (5, 6-a) quinolizinium]–an isoquinoline alkaloid present in the root bark of Berberislycium. Phytomedicine21:663-669.
- Malik TA (2016) Synergistic approach for treatment of chicken coccidiosis using berberine–A plant natural product. Microbial pathogenesis93: 56-62.
- Bartlett JG (1978) Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. New England Journal of Medicine 298: 531-534.
- Hatheway CL (1990)Toxigenic clostridia. Clinical microbiology reviews 3: 66-98.
- Wu SB (2014) Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecalmicrobiota of broiler chickens. Veterinary microbiology169:188-197.
- Lanckriet A (2010)The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathology 39: 63-68.
- Wu S, Rodgers N,Choct M (2010) Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian diseases 54: 1058-1065.
- McReynolds J (2004) Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poultry science 83:1948-1952.
- Prescott J (1979)The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. Avian diseases 5: 1072-1074.
- Kalavathy R (2003) Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. British Poultry Science 44: 139-144.
- Rathgeber B (2008) Growth performance and spleen and bursa weight of broilers fed yeast beta-glucan. Canadian journal of animal science 88: 469-473.
- Immerseel FV (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathology 33: 537-549.
- Teuber M (2001) Veterinary use and antibiotic resistance. Current opinion in microbiology4: 493-499.
- Shen Y (2010)The effects of berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poultry science89:13-19.
- Akhter M,Sabir M, Bhide N (1977) Anti-inflammatory effect of berberine in rats injected locally with cholera toxin. The Indian journal of medical research 65: 133-141.
- Rabbani G (1987)Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. Journal of infectious diseases155: 979-984.
- Sack RB, Froehlich JL (1982)Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infection and immunity35: 471-475.
- Ahn YJ (2000)Cordycepin: Selective Growth Inhibitor Derived from Liquid Culture of Cordyceps m ilitaris against Clostridium spp. Journal of agricultural and food chemistry48: p. 2744-2748.
- BhadraKM,Maiti GSK (2008) Berberine–DNA complexation: New insights into the cooperative binding and energetic aspects. BiochimicaetBiophysicaActa (BBA)-General Subjects 1780: 1054-1061.
- Chae SH (1999) Growth-inhibiting effects of Coptis japonica root-derived isoquinoline alkaloids on human intestinal bacteria. Journal of agricultural and food chemistry 47: 934-938.
- Lim MY (2007) Antimicrobial activity of 5-hydroxy-1, 4-naphthoquinone isolated from Caesalpiniasappan toward intestinal bacteria. Food Chemistry100: 1254-1258.
- Delzenne NM (2011) Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nature Reviews Endocrinology 7:639-646.
- Lv Z(2015)Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment. Antimicrobial agents and chemotherapy59:3726-3735.
- Zhang X (2012) Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS one7: e42529.
- CaniPD (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091-1103.
- PengL (2009) Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of nutrition 139: 1619-1625.
- Jeong HW (2009)Berberine suppresses proinflammatory responses through AMPK activation in macrophages. American Journal of Physiology-Endocrinology and Metabolism296: E955-E964.
- Zhang Q (2011) Preventive effect of Coptischinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food and Chemical Toxicology 49: 61-69.
- Al-Sheikhly F, Al-Saieg A (1980) Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian diseases 8: 324-333.
- Yuan JX, Shen X, Zhu J (1994) Effect of berberine on transit time of human small intestine. ZhongguoZhong xi yijie he zazhiZhongguoZhongxiyijiehezazhi= Chinese journal of integrated traditional and Western medicine/ZhongguoZhong xi yijie he xuehui, ZhongguoZhongyiyanjiuyuanzhu ban 14): 718-720.
- Wang Y (2013) Sensory evaluation of the taste of berberine hydrochloride using an Electronic Tongue. Fitoterapia86: 137-143.
- Jahnke GD (2006) Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Research Part B: Developmental and Reproductive Toxicology 77:195-206.
- Program NT (2010) Toxicology and carcinogenesis studies of goldenseal root powder (Hydrastis Canadensis) in F344/N rats and B6C3F1 mice (feed studies). National Toxicology Program technical report series1: 562.
- Zhang H (2013)The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poultry science92:1981-1988.
- Schat KA, Skinner MA (2008)Avian immunosuppressive diseases and immune evasion. Avian immunology 6: 299.
- Cazaban C (2015) Proposed bursa of fabricius weight to body weight ratio standard in commercial broilers. Poultry Science94: 2088-2093.
- Xia X (2011)Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS one 6: e16556.
- Yin J (2013) Metformin and berberine promote glucose metabolism via inhibiting mitochondrial respiratory chain complex I independently of AMPK activation in DIABETOLOGIA. SPRINGER 233 SPRING ST, NEWYORK, NY 10013 USA 22: 1256.
- Zhang Q (2014)Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC complementary and alternative medicine14:188.