Keywords
malaria prophylaxis, malaria chemoprophylaxis, atovaquone - proguanil, tafenoquine, primaquine, doxycycline, mefloquinehe
Introduction
Malaria is a highly contagious disease [1]. Humans contract malaria through the bite of an infected female anopheline mosquito. The responsible organism, is the Plasmodium spp. There are 4 different plasmodium species, able to cause disease in humans. P. falciparum malaria is the most serious, as its mortality may reach as high as 10%. Other more benign plasmodium species are P. vivax, P. malariae and P. ovale. (Table 1). P. f alciparum prevails in the sub-Saharan area, Africa, East Asia, Oceania and the Amazon. It poses a serious threat to the residents and the travelers of these areas as well.
In the last decades, war, natural disasters, unemployment and tourism have brought many population changes in malaria endemic countries. Moreover, the emerging resistance of plasmodium species to antimalarial drugs, dictate constant vigilance, in order to avoid new cases or epidemic outbreaks in malaria free countries. Despite all efforts, malaria still remains one of the most important causes of morbidity and mortality in tropical and sub-tropical areas. According to W.H.O., there are 300 – 500 million cases of malaria annually and every year, approximately 1.5 -2.7 million people die from the disease [2,3]. What’s more, malaria incidence is expected to increase due to climatological changes.
In the United States, the mean of imported malaria cases is 1300 patients annually (Table 2, appendix). The risk for travelers, who do not receive chemoprophylaxis varies according to the destination, but lies among 24 / 1000 travelers per month in West Africa, 2.5 / 1000 travelers per month in India and 0.5 / 1000 travelers per month in South America [4].
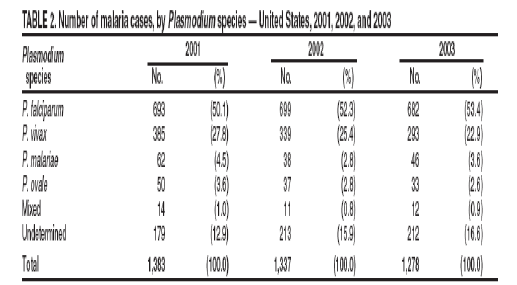
Unfortunately, the emerging resistance against the malaria chemoprophylactic drugs Table 2 renders many of them, inefficient. While, some of the new drugs can cause serious adverse effects and may be more expensive than former therapies.

Figure 1: Number of malaria cases among U.S. and foreign civilians, by year United States, * 1973-2003†
In order to tackle malaria, many regimens are being employed [5,6]. (Table 3, Appendix). Drugs or drug combinations that are currently suggested for malaria prophylaxis are chloroquine, atovaquone/proguanil, mefloquine, doxycycline and primaquine [7,8,9].
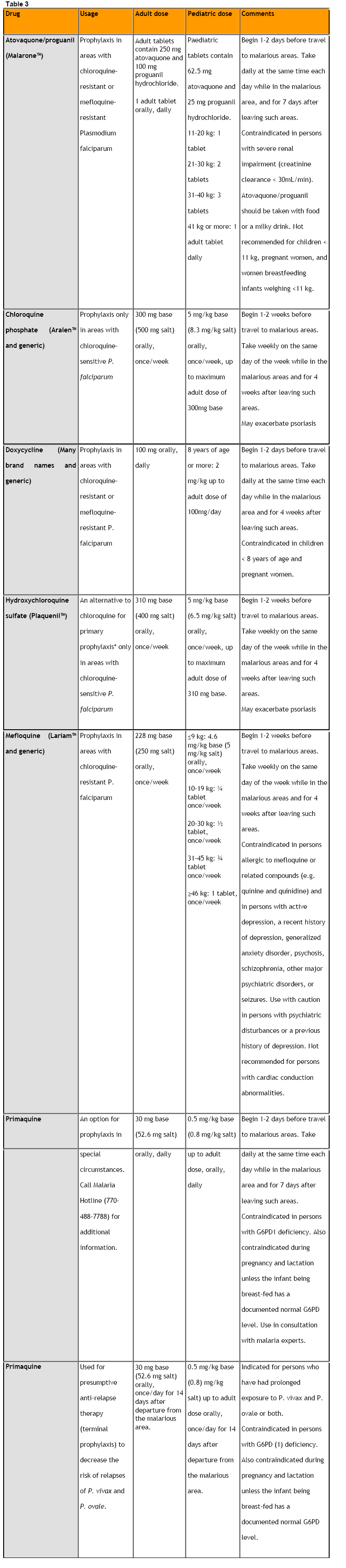
Resistance has been developed against most of these drugs, especially in South East Asia, where doxycycline and perhaps primaquine are the only efficient drugs. Unfortunately, they have been correlated with frequent adverse effects. Thus, new, efficient and safe chemoprophylactic antimalarial regimens are necessary [10,11,12].
The purpose of this critical review is to conclude which regimen is best suited for recommendation to travelers of malaria endemic areas.
Purpose
This critical review has been conducted in order to address the following questions:
1. Which antimalarial regimens are more efficient?
2. Which regimens are safer for travelers in endemic regions?
3. Which regimen is best tolerated by the travelers?
Methodology
The literature research for this critical review included the Internet and the library of the Department of Health Sciences of the National and Capodistrean University of Athens. The Medline and Cinahl databases have been used, as well as the search engines google, altavista and lycos. Data were also found in the sites of W.H.O. and C.D.C.
The articles researched, were clinical trials studies.
Keywords were: malaria prophylaxis, malaria chemoprophylaxis, atovaquone/proguanil, tafenoquine, primaquine, doxycycline, mefloquine
The time frame involved articles, which were published between the years 1999 and 2006. The chosen articles were clinical trials, which were not conducted on pregnant women or children. Articles written prior to 1999, in languages other than English and abstracts only, were excluded.
In the end, 8 articles remained.
Results
The study of Dennis Shanks, et.al, with the title, “A New Primaquine Analogue, Tafenoquine (WR 238605), for Prophylaxis against Plasmodium falciparum Malaria (2001)”.
This study assessed the ability of tafenoquine (WR 238605) to prevent malaria in a P. falciparum holoendemic area. It is a double–blind,placebo–controlled, randomized clinical trial in West Kenya.
The study population included healthy male and female volunteers, aged between 18 and 55. They were all residents of a highly malarious area, in West Kenya. In the statistical analysis, failure of prophylaxis rates were compared by calculating point estimates ant 95% Confidence Intervals for the protective efficacy of each tafenoquine dose relative to placebo.
However, despite the 95% Confidence Intervals, the researchers do not explain whether the sample size has been selected based on statistical power or significance level. Thus, it is uncertain, whether the results are statistically significant.
Furthermore, as the authors point out, the participants were all residents of a highly endemic region and they might have developed stronger immunity against malaria compared to the general population, therefore they might not be a representative sample.
The study of Alper Sonmez, et.al, with the title, “The Efficacy and Tolerability of Doxycycline and Mefloquine in Malaria Prophylaxis of the ISAF Troops in Afghanistan”.
This study took place in Afghanistan, one of the endemic regions for chloroquine resistant P. falciparum. Mefloquine and doxycycline are recommended for chemoprophylaxis. The purpose of the study was to compare the efficacy and tolerability of the regimens in Turkish soldiers in Kabul, Afghanistan.
The duration of the chemoprophylaxis was approximately 12 weeks for each soldier. The side effects and the compliance were evaluated with questionnaires in the 2nd and 8th week of the chemoprophylaxis.
The SPSS 10 for windows was used for the statistical analysis. The comparisons between groups used the x2 test and the Fischer exact x2 test. Only alpha values <0.05 were considered statistically significant.
However, it is undetermined whether the results should be considered statistically significant. The sample is purposive and the authors do not clarify whether the size has been selected based on the statistical power, although there is a significance level. Moreover, there was a large subject withdrawal and there was no control group. Additionally, we are not certain if the questionnaires used in order to evaluate the compliance and the adverse effects, have been estimated for their reliability. Finally, as the authors indicated, the region was malarious hypoendemic and therefore, the prophylactic efficacy may not be as high as estimated.
The study of Bertrand Lell, et. al., with the title, “Malaria chemoprophylaxis with tafenoquine: a randomised study”.
The authors conducted a randomized, double blind study, where they evaluated the prophylactic efficacy and the safety of tafenoquine in different doses. The study took place between February and July, 1999, in Gabon, a region highly endemic for P. falciparum malaria. Individuals, aged between 12 – 20 years old, were invited, from 3 different secondary schools.
The 95% C.I. for protective efficacy were calculated as the ratio of two Poisson variables. The reported data were obtained by per protocol analysis. The pair Student’s t test for continuous laboratory data was used for the statistical analysis. The x2 test was used in order to calculate differences in the number of individuals reporting adverse effects.
This study had a good external, as well as internal validity. However, there was a large subject withdrawal. Furthermore, the participants had increased immunity due to the highly endemic region, they resided.
The study of J. Dirk van der Berg, et.al, with the title, “Safety and Efficacy of Atovaquone and Proguanil Hydrochloride for the Prophylaxis of Plasmodium falciparum Malaria in South Africa”.
The aim of this study was to determine the safety and efficacy of the atovaquone and proguanil hydrochloride combination therapy for the prophylaxis of P. falciparum malaria. The trial took place in South Africa during the main season of malaria transmission, February through July. The participants were 175 healthy, non immune volunteers. They were all South African National Defense Force personnel, male and female, aged between 16 and 65 years old and they had a risk for malaria infection. A written informed consent was provided. The mean duration of exposure to the drug was 8.9 weeks. The proportion of prophylactic success was summarized using a 95% Confidence Interval.
The study was not randomized, thus the results can not be applied to the general population. Furthermore, it is not clear, whether the sample size was selected based on statistical power or significance level. Nevertheless, there was a 95% Confidence Interval. Therefore, the results should not be considered statistically significant. Moreover, there was no control group and the subject withdrawal was large. These factors have a negative effect in the internal validity of the trial.
The study of J. Kevin Baird, et.al., with the title, “Randomized, Parallel Placebo-Controlled Trial of Primaquine for Malaria Prophylaxis in Papua, Indonesia”.
This study is a randomized, parallel placebo- controlled trial. The efficacy of primaquine for malaria protection was compared to the placebo. The participants were residents of three villages in the northeastern Papua. Their age was between 12 and 65 years old, their weight was >40 kg and they lived in Papua for a period between 3 and 26 months. All the participants had lived in malaria free areas for more than 2 years. They provided written informed consent. The researchers used a randomization code assigning sequential numbers to either inclusion or exclusion for the trial in a 3:1 ratio.
The statistical analysis was carried out using the statistical package SPSS 9.0 and Epi Info (version 6.04 Center for Disease Control and Prevention). The differences in means were assesed using the paired or unpaired Student’s t test or Mantel Haenszel test. P values ≤ 0.05 were considered significant.The integral validity of the study was adequate. It is not clear whether the sample size was chosen size based on statistical power, although there was a significance level. Thus the results should not be considered statistically significant. Moreover, the sample may not be representative, because all the participants were residents of the area.
The study of Judith Ling, et.al., with the title, “Randomized, Placebo-Controlled Trial of Atovaquone/ Proguanil for the Prevention of Plasmodium falciparum or Plasmodium vivax Malaria among Migrants to Papua, Indonesia”.
This is a randomized double- blind study on 297 individuals from a non endemic area in Indonesia, who migrated to Papua, where malaria is endemic. Atovaquone/ proguanil is compared to the placebo in order to determine the prophylactic efficacy of the regimen for P. falciparum and P. vivax infection. The age of the volunteers was between 12 and 65 years old and their weight was over 40 kg. They had migrated from a non endemic area during a period of 3 to 26 months. Every participant signed a written informed consent. Subjects were randomized in a 3:1 ratio to continue or discontinue the study. The 95% Confidence Interval was calculated from the binomial distribution. The Yates’s corrected x2 test was used in the statistical analysis.
Apart from the participant withdrawal in the control group, mainly due to infection, the trial had adequate internal and external validity.
The study of Eli Schwartz and Gili Regev-Yochay, with the title “Primaquine as Prophylaxis for Malaria for Non immune Travelers: A Comparison with Mefloquine and Doxycycline”.
This is a comparative study among primaquine, mefloquine and doxycycline for their ability to prevent malaria. It is a retrospective study during 1995 -1998. The travelers joined rafting trips to Ethiopia. The study population consisted of 158 Israelis who joined rafting trips to the river Omo in Ethiopia, for a duration of 12 to 20 days. The trips took place during 1995 – 1998. All travelers were followed up in different clinics for malaria symptoms and their compliance was assessed. In addition, 50 participants who took primaquine filled out questionnaires on side effects.
The binomial distribution with correction for continuity was used for the comparison of efficacy of primaquine with that of the other drugs. The x2 test and the Fisher exact test were used for the statistical analysis.
The sample of the study was not randomized and we do not know if the size was estimated based on statistical power or significance level. Therefore the results should not be considered statistically significant Furthermore, it is not clarified whether the reliability of the questionnaires was estimated. Finally, the internal validity of the study is threatened because there was no control group.
The study of T. Y. Sukwa, et.al., with the title “A Randomized, Double-Blind, Placebo-Controlled Field Trial to Determine the Efficacy and Safety of Malarone, (Atovaquone/ Proguanil) for the Prophylaxis of Malaria in Zambia”.
The authors conducted a randomized, double–blind, placebo-controlled study, in order to determine the efficacy and safety of Malarone (250 mg atovaquone/ 100 mg proguanil hydrochloride per tablet) for malaria chemoprophylaxis and especially P. falciparum malaria in Zambia.
The study population included healthy male and female volunteers, aged between 18–65 years old, who resided in a highly malarious endemic region in Zambia All the participants provided written informed consent forms and the research protocol was approved by the Zambia’s Institute of Tropical Diseases.
The prophylactic efficacy for the two groups were compared by considering the 2 * 2 frequency table and performing a Fisher’s exact test. The mean difference was estimated with Hodges-Lehmann and there was 95% C.I. for the biochemical and hematological factors.
The study had good internal and external validity.
Research Results
The prophylactic efficacy of atovaquone/ proguanil was 97% against P. falciparum. Two more studies showed that the prophylactic efficacy of the regimen against all Plasmodium spp. is 95% and 93% with 95% C.I.
The prophylactic efficacy of tafenoquine against P. falciparum was 68% with a 400mg/ day for 3 days, 86% with 200mg / day for 3 days and consequently 200 mg weekly and 89% with 400 mg/ day for 3 days and consequently 400mg weekly. In another study, none of the participants who received 250 mg tafenoquine daily for 3 days contracted malaria.
The prophylactic efficacy of primaquine against malaria was 93% for all Plasmodium spp.. One study simply refers to the regimen as more efficient compared to mefloquine and doxycycline. Finally, in one trial none of the participants who received mefloquine or doxycycline contracted malaria.
As far as the safety of the regimens is concerned, tafenoquine has been related to hemolytic incidences in patients with G6PD deficiency and mild gastrointestinal disorders. Primaquine was related to hemolytic incidences, toxinemia, gastrointestinal disorders, headache, cough, sore throat, malaise, dizziness and back pains. The complains for the atovaquone/ proguanil regimen regarded stomatitis, headache, gastrointestinal disorders, back pain, flue like syndrome and exfoliative skin rash. Doxycycline was related to gastrointestinal disorders, rash, malaise, headache, insomnia, and neurological disorders. Finally, mefloquine was associated with gastrointestinal and neurological disorders.
As far as the tolerance of the regimen is concerned, in one trial 2 out of 223 participants discontinued tafenoquine use, due to toxinemia and hemolysis. In a study of 158 travelers one had to discontinue primaquine prophylaxis due to gastrointestinal disorders and another one doxycycline prophylaxis due to a rash. In another trial, 12.5 % and 4.6% of the participants withdrew from doxycycline and mefloquine use, respectively, due to adverse effects. Furthermore, in a study on 175 participants, 3 discontinued atovaquone/ proguanil treatment due to headache and dizziness, while in another one, 4 out of 150 participants had to withdraw due to abdominal pain and exfoliative skin rash.
Conclusions
The most efficient regimens were atovaquone/ proguanil, tafenoquine and primaquine, while mefloquine and doxycycline were less efficient. As for the safety, tafenoquine and primaquine should not be prescribed to patients with G6DP deficiency, due to the risk of hemolysis. All the regimens were well tolerated and most withdrawals were, due to adverse effects in patients on doxycycline and mefloquine. However, we should be cautious with these results. Apart from the trials on atovaquone/ proguanil, by Judith Ling et.al. and T. Y. Sukwa et al., and that on tafenoquine, by Bertrand Lell et.al, all the other studies faced methodological inconsistencies. Thus, more reliable studies have to be conducted, in order to provide valid results for the protection and the safety of the travellers.
Directions
This review leads to the following directions for future research:
• More trials should be conducted, on non-immune individuals.
• Safer regimens have to be employed for high risk groups such as children and pregnant women.
An appropriate antimalarial drug should be demonstrated, in order to reduce the risk of emerging resistant Plasmodium spp
5292
References
- World Health Organization, 1990. Practical Chemotherapy of Malaria. World Health Organ Tech Rep Ser 805: 1–141.
- WHO, 1996. Investing in Health Research and Development: Report of the Ad Hoc Committee on Health Research Relating to Future Intervention Options. Geneva, Switzerland: World Health Organization. TDR/Gen/96.1.
- WHO, 1993. Implementation of the global malaria control strategy—Report of a WHO study group on the implementation of the global plan of action for malaria control 1993–2000. Geneva, Switzerland: World Health Organization. WHO Technical Report Series 839.
- Neal R. Gross, Court reporters and transcribers,1323 Rhode Island Ave., N.W.(202) 234-4433 Washington, D.C. 2005
- Hoffman SL, 1992. Diagnosis, treatment, and prevention of malaria. Med Clin North Am 76: 1327–1355.
- National Center for Infectious Diseases, Division of Global Migration and Quarantine,2006
- WHO, 1995. Model Prescribing Information: Drugs Used in Parasitic Diseases. Geneva, Switzerland: World Health Organization.
- Bradley DJ, Bannister B on behalf of the Advisory Committee on Malaria Prevention for UK Travellers. Guidelines for malaria prevention in travellers from the United Kingdom for 2001. Comm Dis Public Health 2001; 4 (2): 84-101.Available from: https://www.phls.co.uk/publications/CDPHVol4/No%202/malaria%20guidelines.pdf
- Shanks GD, Gordon DM, Klotz FW, Aleman GM, Oloo AJ, Sadie D, Scott TR, 1998. Efficacy and safety of atovaquone/ proguanil for suppressive prophylaxis against Plasmodium falciparum malaria. Clin Infect Dis 27: 494–499.
- Bradley D, Warhurst D C, on behalf of an expert group of doctors, nurses, and pharmacists. Guidelines for the prevention of malaria in travellers from the United Kingdom. Commun Dis Rep CDR Rev 1997; 7 (10): 138-52.
- Shapiro TA, Ranasinha CD, Kumar N, Barditch-Crovo P. Prophylactic activity of atovaquone against Plasmodium falciparum in humans. Am J Trop Med Hyg 1999; 60 (5): 831-836.
- Sukwa TY, Mulenga M, Chisdaka N, Roskell NS, Scott TR. A Randomized, Double-Blind, Placebo-Controlled Field Trial to Determine the Efficacy and Safety of Malarone, (Atovaquone/ Proguanil) for the Prophylaxis of Malaria in Zambia. Am J Trop Med Hyg. 1999, 60(4):521-5.
- Schwartz E, Regev-Yochay G. Primaquine as Prophylaxis for Malaria for Nonimmune Travelers: A Comparison with Mefloquine and Doxycycline. Clin Infect Dis. 1999; 29(6):1502-6.
- Ling J, Baird JK, Fryauff DJ, Sismadi P, Bangs MJ, Lacy M, Barcus MJ, Gramzinski R, Maguire JD, Kumusumangsih M, Miller GB, Jones TR, Chulay JD, Hoffman SL; Naval Medical Research Unit 2 Clinical Trial Team. Randomized, Placebo-Controlled Trial of Atovaquone/ Proguanil for the Prevention of Plasmodium falciparum or Plasmodium vivax Malaria among Migrants to Papua, Indonesia. Clin Infect Dis. 2002 1;35(7):825-33.
- Baird JK, Lacy MD, Basri H, Barcus MJ, Maguire JD, Bangs MJ, Gramzinski R, Sismadi P, Krisin , Ling J, Wiady I, Kusumaningsih M, Jones TR, Fryauff DJ, Hoffman SL; United States Naval Medical Research Unit 2 Clinical Trials Team. Randomized, Parallel Placebo-Controlled Trial of Primaquine for Malaria Prophylaxis in Papua, Indonesia. Clin Infect Dis. 2001 Dec 15;33(12):1990-7.
- van der Berg JD, Duvenage CS, Roskell NS, Scott TR. Safety and Efficacy of Atovaquone and Proguanil Hydrochloride for the Prophylaxis of Plasmodium falciparum Malaria in South Africa. Clin Ther. 1999; 21(4):741-9.
- Lell B, Faucher JF, Missinou MA, Borrmann S, Dangelmaier O, Horton J, Kremsner PG. Malaria chemoprophylaxis with tafenoquine: a randomised study. Lancet. 2000 10;355(9220):2041-5
- Sonmez A, Harlak A, Kilic S, Polat Z, Hayat L, Keskin O, Dogru T, Yilmaz MI, Acikel CH, Kocar IH. The Efficacy and Tolerability of Doxycycline and Mefloquine in Malaria Prophylaxis of the ISAF Troops in Afghanistan. J Infect. 2005, 51(3):253-8
- Shanks GD, Oloo AJ, Aleman GM, Ohrt C, Klotz FW, Braitman D, Horton J, Brueckner R. A New Primaquine Analogue, Tafenoquine (WR 238605), for Prophylaxis against Plasmodium falciparum Malaria. Clin Infect Dis. 2001, 15;33(12):1968-74.








