Keywords
Cancer/Testis antigens; Bladder cancer; Kidney cancer; Gene expression; Quantitative polymerase chain reaction
Introduction
A major challenge of tumor immunology is to identify and target tumor-associated antigens (TAA) which can induce a tumor specific immune response [1]. Certain criteria have been posited as necessary in the search for such a TAA. The TAA should be expressed by cancer cells or normal cells that are not exposed to the host immune system. Secondly, the TAA should be highly immunogenic to induce an immune response upon vaccination. Finally, the TAA should be critical to tumorigenesis such that antigen loss under immune pressure should result in the interruption of tumor function [2]. The search for TAA with the aforementioned characteristics has prompted great interest in cancer/testis antigens (CTA) which are TAA expressed in immunoprivileged testis tissue and in certain malignancies.
MAGE-1 was the first TAA to be cloned in 1991 [3]. To date, 70 families of CTA including over 200 members have been isolated and compiled in a database (https://www.cta.Incc.br/). CTA can be classified into two groups: X-CTA found on the X chromosome and autosomally coded non-X-CTA. Nearly half of CTA are found on the X chromosome1 and it is estimated that about 10% of the X chromosome codes for CTA [4]. X-CTA are generally expressed on proliferating immature spermatogonia and non-X-CTA are usually expressed in the later stage of spermatocytes once proliferation has concluded [1,5]. Given their expression on developing germ cells, immune tolerance is not induced against CTA. The blood-testis barrier – structurally maintained by Sertoli cell junctions, the absence of HLA class I expression on germ cells, and the microenvironment prevents interactions between germ cells and the host immune system [6,7].
Since their discovery, aberrant expression of several CTA have been found in urologic malignancies including bladder, prostate, testis and kidney cancers [8]. However, despite a great number of studies showing expression of CTA in urologic malignancies, reported expression levels have been variable. For example, in renal neoplasms, CTA expression ranges from absent [9,10] or poor [11,12] to robust [13-15]. In the case of bladder cancer, an early study showed expression of MAGE antigens (a large class of CTA) in muscle-invasive bladder cancer [10]. Several studies have since found variable CTA expression in bladder tumors with higher expression observed in muscle-invasive cancer compared to T1 disease [16,17].
While there has been great interest in CTA as possible immunogenic targets, clinical trials looking for anti-tumor activity of a CTA-specific immune response have been oftentimes disappointing [18]. One possible proposed explanation could be down regulation of CTA by tumor cells to avoid immune surveillance. In our related work in prostate cancer, we have found several CTA which appear to be downregulated in tumor cells compared to normal tissues. This study aims to analyze the expression of 13 of these cancer/ testis antigens found to be most down regulated in our human prostate cancer gene array (data not shown) in 10 RCC and 10 bladder cancer specimens to determine whether expression is also decreased in other urologic malignancies. We used quantitative PCR to compare CTA expression between ribonucleic acid (RNA) samples isolated from neoplastic tissues and adjacent normal, non-diseased tissue from the same patient. The expression level of the CTA in the normal tissue is defined as 1.0.
Materials and Methods
RNA from human tissue (from consented patients, from an IRB-approved protocol) was provided by the Virginia Commonwealth University (VCU) Tissue and Data Acquisition and Analysis Core (TDAAC) Facility, supported, in part, by the funding from NIH-NCI Cancer Center Core Support Grant P30 CA016059, as well as through the Department of Pathology, School of Medicine, and Massey Cancer Center of VCU. Each residual tumor sample was snap-frozen at -80°C. Frozen tissue sections (5 μm) were used for histopathological assessment after staining with hematoxylin and eosin and adjacent sections were used for RNA isolation. All samples contained more than 70% tumor and less than 20% necrosis.
Total RNA was extracted from multiple 10 μm tissue sections using the MagMAXTM-96 for Microarrays Total RNA Isolation Kit (Life Technologies, Carlsbad, CA), in an automated fashion using the Mag-MAXTM Express magnetic particle processor. RNA purity was judged by NanoDrop One spectrophotometer (Thermo Scientific, Waltham, MA) at 260, 270 and 280 nm. RNA integrity was assessed by running 1 μL of every sample in RNA 6000 Nano LabChips on the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Using the ThermoScriptTM RT-PCR System (Invitrogen, Carlsbad, CA) from 1 μg of total RNA using random primer, cDNA was synthesized. Total of 13 CTA were evaluated (SSX2, SPA17, CEP55, TEX14, TEX15, ODF4, AKAP4, ACRBP, PAGE4, MAGEA4, BORIS, CCNA1 and TSGA10). Their primer sequences are presented in Table 1. Out of these 13 CTA examined, 4 are X-CTA (SSX2, AKAP4, PAGE4, MAGEA4) and the rest are non-XCTA (SPA17, CEP55, TEX14, TEX15, ODF4, ACRBP, BORIS, CCNA1, TSGA10).
| Gene |
Accession number |
Primer sequence(5' to 3') |
Product length |
| CCNA1 |
NM_003914 |
CAGTGCACTTGCCAGTTGTTC GTCTCCATCCCAAGTGACGAG |
148 bp |
| MAGEA4 |
NM 001011548 |
GAGCTTCTGCGTCTGACTCG GGAATCCTGTCCTCCGGITG |
108bp |
| CEPSS |
NM 018131 |
TOCCCGCTCTGATAACAGTC GTCGCCAAGTCCAAAGAAGC |
108bp |
| TEX15 |
NM 031271 |
GTTOTCCCIGGTGATTOTGC AGTCCTCAGAGCCCATGTTG |
109bp |
| SPA17 |
NM 017425 |
ACGGTTACCCAGCAACTAGA AGTGOOTG170GAGAATOGA |
117bp |
| TEXI4 |
NM_198393 |
GTCGTATCCCGAGCATGGAG AACAGGACAGGGGACTGGAA |
116bp |
| AKAP4 |
NM_003886 |
AGGGTCCTACATGATGGCGTA TCTTGCTGTCCTTCTGOGTTG |
117bp |
| ACRBP |
NM_032489 |
GAAACCGCAATCGGAAGGTG GAACTCCTOGCTCCATCGAA |
110bp |
| ODF4 |
NM_153007 |
GAGAATCACACACAGCTTCCG GAGAAGGCCACGACCAATAGT |
93bp |
| BORIS |
NM_001269040 |
CCCATTGTGCCACCATCA AGCATGCAAGTTGCGCATAT |
64bp |
| SSX2 |
NM 175698 |
AACTCCCCTCAGGGATACGA GCCCATGTTCGTGAAAGGTC |
71bp |
| PAGE4 |
NM_007003 |
TCTTCCCTTCATTCTTCGCCAG TCTTGATCTCACTCGTGCACTC |
95bp |
| TSGA10 |
NM_182911.3 |
CTGTAAGGCAGGCAGGTAGAC TCAGAAACTGGAGCCCCTAGA |
138bp |
Table 1: CTA Primer sequences.
In each experiment, at least three independent reactions were performed to obtain the mean. Quantitative real time PCR was performed in triplicates using the Mx3000P system (Agilect Technologies, Inc. Santa Clara, CA), using SensiFAST SYBR Lo-ROX Kit (Bioline, Taunton, MA). PCR conditions were as follows: an initial denaturation step (10 min at 95°C), 40 cycles consisting of three steps (30 sec at 95°C, 1 min at 55°C, 30 sec at 72°C), and 1 cycle consisting of three steps (1 min at 95°C, 30 sec at 55°C and 30 sec at 95°C). The cycle threshold (CT) value was the PCR cycle number in which the fluorescence signal was significantly distinguishable from the baseline for the first time.
The housekeeping gene (GADPH) was used as an endogenous control for target gene expression evaluation. GAPDH primer were obtained from Qiagen (Valencia, CA). Data were presented by the relative amount of mRNA with the formula 2−ΔΔCT, which stands for the difference between the CT of a gene of interest and the CT of the housekeeping gene, GAPDH.
For a single comparison of 2 groups, the Student t test was used (SigmaPlot Software; SPSS, Chicago, IL). If data distribution was not normal, Mann-Whitney rank sum test was used. For all analysis, the level of significance was set at a probability of .05 or less to be considered significant. Data are presented as the mean ± standard error of the mean (SEM).
Results
We used quantitative PCR (qPCR) to study expression of 13 CTA in 10 bladder cancer specimens (Figure 1). The CTA analyzed were SSX2, SPA17, CEP55, TEX14, TEX15, ODF4, AKAP4, ACRBP, PAGE4, MAGEA4, BORIS, CCNA1, and TSGA10. All but two of the bladder specimens contained muscleinvasive cancer and all specimens demonstrated high grade morphology. One specimen had a sarcomatoid component and another had a small cell component. The rest were high grade muscle-invasive urothelial cell carcinoma. In the bladder cancer specimens analyzed, expression of the CTA studied was significantly less than in the tumor-adjacent control samples. CCNA1 was found to be decreased in all 10 samples. When comparing across all specimens, the change was statistically significant in 8 of the CTA tested – SSX2 (P=0.018), CEP55 (P=0.005), TEX14 (P=0.005), TEX15 (P<0.001, ODF4 (P<0.001), MAGEA4 (P=0.005), CCNA1 (P<0.001) and TSGA10 (P=0.005). For each of these CTA, expression was decreased in tumor samples. The difference in expression of the other CTA studied did not reach statistical significance (SPA17, AKAP4, ACRBP, PAGE4, and BORIS).
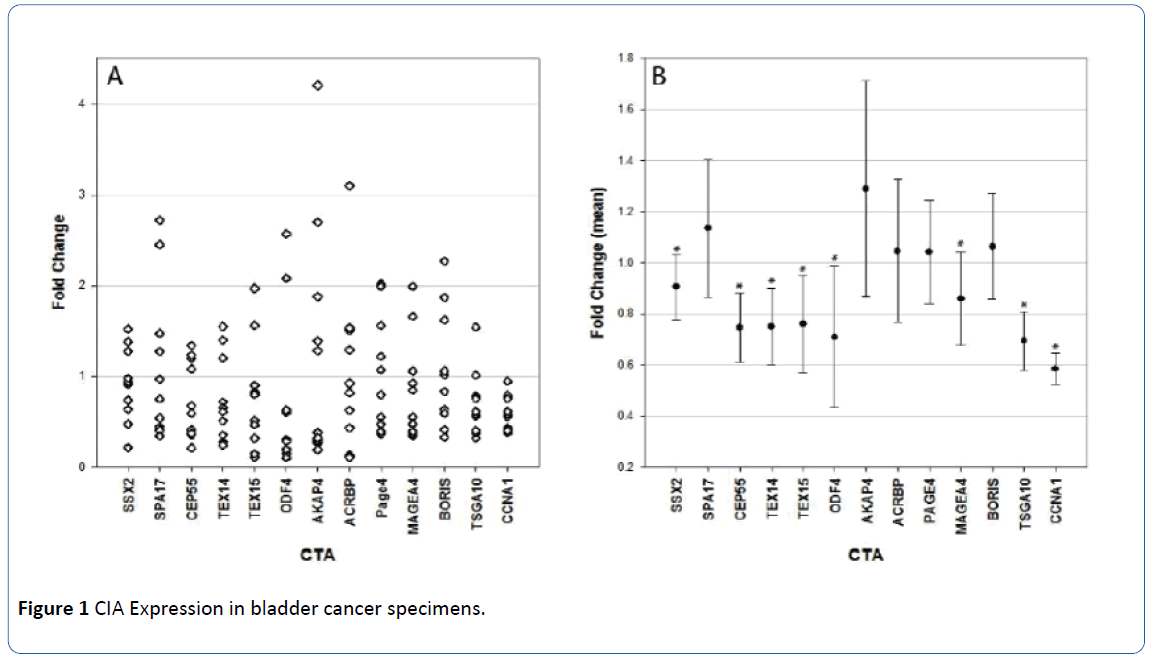
Figure 1 CIA Expression in bladder cancer specimens.
A. Values above 1 denote increased expression of the CTA in malignant tissue in comparison to normal. and values below 1 denote decreased expression of CTA in cancer tissue. Expression of individual CTA in bladder cancer specimens
B. Mean values of CTA in bladder cancer patients. As it can be seen. the expression of most CTA are decreased in the bladder cancer specimens in comparison to normal tissue. Statistically significant difference is denoted by *.
Expression of the same 13 CTA was studied in 10 RCC samples, (Figure 2). Five of the samples demonstrated clear cell type RCC, three papillary, one chromophobe, and one mixed papillary and chromophobe. One sample was classified as Fuhrman grade I, four as Fuhrman grade II, two as Fuhrman grade III and three as Fuhrman grade IV. Average comparative CTA expression increased with grade. The Fuhrman grade I sample had an average expression of 0.40. Samples of Fuhrman grades II and III had an average comparative CTA expression of 0.77. Fuhrman grade IV samples had an average comparative CTA expression of 2.12. Because of this difference in CTA expression, we combined kidney cancer samples with Fuhrman grade I to III together and compared CTA expression in these samples (total 7) to the Fuhrman grade IV samples (total 3). Expression of 11 of the 13 tested CTA (mean values) was increased in kidney cancer specimens with Fuhrman grade IV morphology (most aggressive morphological type). In specimens demonstrating Fuhrman grades I-III, the expression of all CTA was decreased, except for TEX14, which was mildly increased (Figure 2). Comparing the expression of all CTA tested in aggressive kidney cancer patients (Fuhrman grade IV) to corresponding normal kidney tissue, there was a statistically significant increase (P<0.001) in the expression of CTA in these tumors.
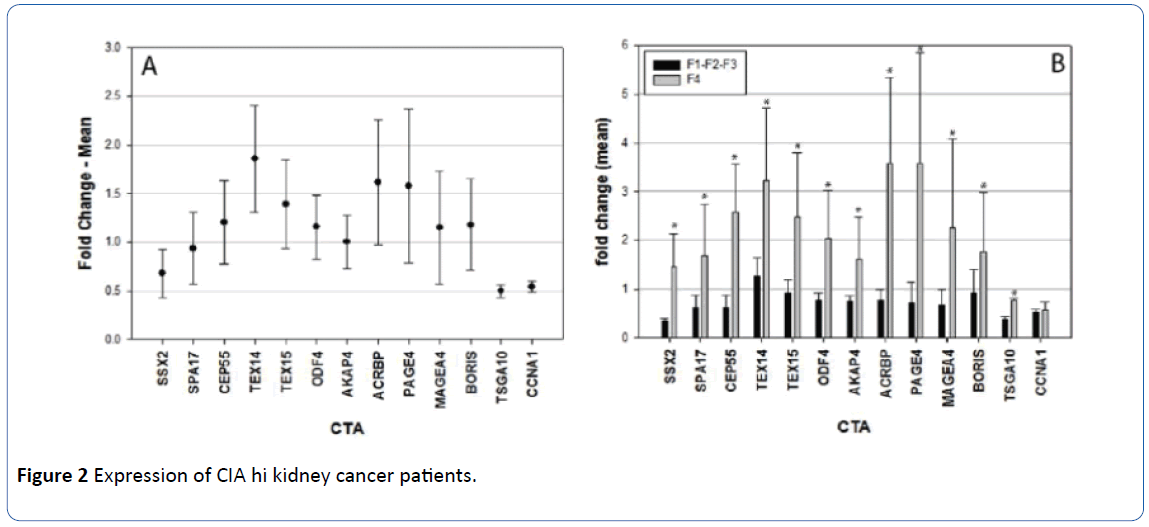
Figure 2 Expression of CIA hi kidney cancer patients.
Values above 1 denotes increased expression of the CIA in malignant tissue in comparison to nonnal. and values below 1 denotes decreased expression of CIA in cancer tissue.
A. Mean values of fold change of CTA in kidney cancer patients compared to nonnal tissue.
B. Expression of CTA in kidney cancer specimens according to Furhman's grade. F1-F2- F3 denote samples with the Furhman's grades1 to 3. and F4 denotes specimens with Furl man's grade 4. All differences are statistically significant (denoted by *) except in the case on CCNA1.
We also compared the expression of the separate CTA in kidney tumors with Fuhrman grades I-III to the kidney tumors with Fuhrman grade IV. There was a statistically significant increase in the expression of twelve CTA tested in kidney tumors with Fuhrman grade IV – SSX2 (P<0.001), SPA17 (P=0.024), CEP55 (P<0.001), TEX14 (P=0.008), TEX15 (P=0.009), ODF4 (P=0.008), AKAP4 (P=0.006), ACRBP P<0.001), PAGE4 (P<0.001), MAGEA4 (P=0.013), BORIS (P=0.024) and TSGA10 (P=0.021). The only CTA with no difference was CCNA1 (P=0.717).
Discussion
CTA have been shown to play physiologic roles during spermatogenesis in transcription regulation, meiosis, germ cell apoptosis and self-renewal of spermatogonia. While the exact physiologic roles of most CTA have not been fully uncovered, the known physiologic importance of CTA in cell cycle regulation during spermatogenesis has fueled much speculation surrounding the role of CTA in tumorigenesis.6
CTA have been shown to be variably expressed in different cancers [19]. There is much research showing that progression of tumorigenesis is often associated with increased expression of CTA [20-24]. However, certain CTA appear to demonstrate decreasing expression with disease progression and increased expression of certain CTA may actually be associated with improved prognosis [25,26]. Given the apparent variable expression of CTA, we aimed to investigate whether there might exist a subpopulation of CTA which are down regulated in renal and bladder tumors. To this aim, we chose to study expression of 13 CTA which we have previously observed to be down regulated in prostate cancer tissues (data not shown).
In our renal cell carcinoma tissue samples, average CTA expression was 0.40 for Fuhrman grade I samples and 0.77 for Fuhrman grades II and III. This suggests that, for lower grade tumors, there was actually decreased expression of the chosen CTA. However, average expression of Fuhrman grade IV samples was 2.12 which was significantly greater than the expression in samples with Fuhrman grades I-III or that observed in normal renal tissues. In fact, expression of virtually all tested antigens (except TEX14) was decreased overall in kidney cancer specimens with Fuhrman grades I-III, while almost all CTA (except TSGA10 and CCN1) demonstrated increased expression in the Fuhrman grade IV samples. These findings are consistent with a recent study which showed a correlation between increased expression of the CTA MAGEA3 and advanced renal cell carcinoma [15].
While the overexpression of certain CTA may confer a growth advantage in certain cases, [27,28] overall increased expression of CTA in advanced kidney cancer tissue might be one of the reasons for the success of immunotherapy in kidney cancer patients since many CTA are highly immunogenic. Further studies are needed to clarify this point.
Regarding bladder cancer, certain studies have shown a similar association of upregulation of CTA with disease progression. One study using qPCR to investigate expression of CEP55 in urothelial cell carcinoma found significantly greater gene expression in muscle-invasive tumors compared to nonmuscle invasive cancers [17]. Similar differential mRNA and protein expression was seen with the CTA PBK/TOPK [16].
Other studies have shown that increased expression of CTA can actually be associated with improved prognosis [29]. Kawai et al. looked at 84 bladder specimens from patients who had undergone radical cystectomy and found that five-year cancerspecific survival was improved in those patients with higher expression of heat shock protein 105 compared to those with lower expression of this CTA25 suggesting that there may exist an antibody-mediated anti-tumor response that has not yet been entirely elucidated. These results are perhaps not surprising considering that CTA have been shown to be highly immunogenic and capable of stimulating robust T and B-cell responses [19].
In our series of advanced muscle-invasive bladder cancer specimens, the expression of the examined CTA was decreased overall compared to matched benign controls. Especially significant was the decrease in the expression of CCNA1 gene, which plays a pivotal role in cell cycle regulation. Is it possible that decreased expression of this gene allowed high grade urothelial carcinoma cells to have uncontrolled growth?
Overall decrease in the expression of these antigens could possibly decrease tumor recognition by the host immune system. Studies showing high expression of certain CTA in cases of advanced disease are promising, [30] as is researchers’ ability to achieve positive immunological response with CD8+ T cells with specificity for certain tumorassociated antigens. Unfortunately, these findings have not been translated into clinical success [8]. Could downregulation of certain CTA in advanced disease be one reason for this lack of success? Further research is needed to determine more broadly which CTA might be downregulated and how this might aid in tumor avoidance of the host immune system.
Increased expression of several CTA has been shown to be associated with demethylation. Exposure to hypo-methylating agents such as 5-azacitidine can result in increased expression of these genes. Conversely, methylation has been shown to cause gene repression. In the MAGE genes, the CG-rich promoters are highly methylated in somatic tissues where gene expression is absent but unmethylated in germ cells. These data suggest that methylation of these promoters is a silencing mechanism in many CTA to prevent expression in somatic tissue [5].
The expression of antibodies to certain CTA has been shown to improve patient survival and response to chemotherapy raising the possibility that hypo-methylating agents such as 5- azacitidine might – if given in low doses aimed at upregulating CTA expression – improve response to chemotherapy [27,29,31]. Therapeutic induction of CTA has been demonstrated in both multiple myeloma [32] and in acute myelogenous leukemia [33,34]. On the other hand, considering that full-dose chemotherapy in general suppresses immune response, it might be more beneficial to combine a hypomethylating agent to increase CTA expression with immunomodulatory agents, such as lenalidomide, [32,35] or immune check point inhibitors like nivolumab. We are currently using a mouse model to study the effectiveness and antitumor mechanisms of these combinations.
The results found here raises several questions. For one, it would be interesting to look at expression in these tissues across a wider variety of CTA to see whether there might be particular patterns in certain populations of CTA. It would also be interesting to compare expression of CTA in muscle-invasive versus noninvasive bladder cancer tissues to see whether expression of certain CTA decreases with tumor stage. Of particular interest would be any CTA that has shown promise in previous vaccine trials. It would also be interesting to see whether CTA which have been shown to have increased expression in muscle-invasive disease also demonstrated this increased expression in our tumor samples.
Conclusion
The utility of CTA is potentially multifold. CTA could become targets for future immunotherapies. Considering that most of the specimens examined in this study demonstrated decreased expression of the CTA, the use of hypo-methylating agents – known to increase the expression of CTA – may improve the recognition of tumor by the host immune system and improve efficacy of other immunotherapeutic treatments. A second potential use for CTA would be to discover improved biomarkers to better predict presence and aggressiveness of disease.
9132
References
- Fratta E, Coral S, Covre A, Parisi G, Colizzi F, et al. (2011) The biology of cancer testis antigens: putative function, regulation and therapeutic potential. MolOncol 5: 164-182.
- Mkrtichyan M, Ghochikyan A, Davtyan H, Movseyan N, Loukinov D, et al. (2011)Cancer-testis antigen, BORIS based vaccine delivered by dendritic cells is extremely effective against a very aggressive and highly metastatic mouse mammary carcinoma. Cell Immunology 270: 188-197.
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, et al. (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254: 1643-1647.
- Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, et al. (2005) The DNA sequence of the human X chromosome. Nature 434: 325-337.
- Simpson A J, Caballero O L, Jungbluth A, Chen YT, Old LJ, et al. (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615-625.
- Cheng, YH, Wong EW, Cheng CY (2011) Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis 1: 209-220.
- Bart J, Groen HJ, van der Graaf WT, Hollema H, Hendrikse NH, et al. (2002) An oncological view on the blood-testis barrier. Lancet Oncol 3: 357-363.
- Kulkarni TSP, RajagopalanK, Kim R, MooneySM, GetzenbergRH(2012) Cancer/testis antigens and urological malignancies. Nature Reviews Urology 9: 386-396.
- Chen Y T, Scanlan M J, Sahin U, Türeci O, Gure AO, et al. (1997) A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. ProcNatlAcadSci94: 1914-1918.
- Achim A, Jungbluth K J B, Denise Kolb, Kristin Iversen, KerenCoplan, et al. (2000): Expression of Mage-Antigens in Normal Tissues and Cancer. International Journal of Cancer85: 460.
- Scanlan MJ, Simpson AJ, Old LJ (2004) The cancer/testis genes: review, standardization, and commentary. Cancer Immun4: 1.
- Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT (2002) Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunological Reviews 188: 22-32.
- Yamanaka K, Miyake H, Hara I, Gohji K, Arakawa S, et al. (1998) Expression of MAGE genes in renal cell carcinoma. Int J Mol Med 2: 57-60.
- Garg M, Kanojia D, Khosla A, Dudha N, Sati S, et al. (2008) Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res68: 8240-8248.
- Yin B, Zeng Y, Wang X, Liu G, Zhang M, et al. (2014) Expression and clinical significance of cancer-testis genes in clear cell renal cell carcinoma.Int J ClinExpPathol 7: 4112-4119.
- Singh PK, Srivastava AK, Dalela D, RathSK, Goel MM, et al. (2014) Expression of PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) in human urinary bladder transitional cell carcinoma. Immunobiology219:469-474.
- Singh PK, Srivastava AK, Rath SK, Dalela D, Goel MM, et al. (2015) Expression and clinical significance of Centrosomal protein 55 (CEP55) in human urinary bladder transitional cell carcinoma. Immunobiology 220: 103-108.
- Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med10: 909.
- Krishnadas DK, Bai F, Lucas KG (2013) Cancer testis antigens and immunotherapy. ImmunoTargets and Therapy 2: 11-19.
- Xu X, Tang X, Lu M, Tang Q, Zhang H, et al. (2014) Overexpression of MAGE-A9 predicts unfavorable outcome in breast cancer. ExpMolPathol97: 579-584.
- Cuffel C, Rivals J P, Zaugg Y, Salvi S, Seelentag W, et al. (2011) Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer 128: 2625-2634.
- Suyama T, Shiraishi T, Zeng Y, Yu W, Parekh N, et al. (2010) Expression of Cancer/Testis Antigens in Prostate Cancer Is Associated With Disease Progression. Prostate 70: 1778-1787.
- Hudolin T, Juretic A, Spagnoli G C, Pasini J, Bandic D, et al. (2006)Immunohistochemical Expression of Tumor Antigens MAGE-A1, MAGE-A3/4, and NY-ESO-1 in Cancerous and Benign Prostatic Tissue. The Prostate 66: 13-18.
- von Boehmer L, Keller L, Mortezavi A, Provenzano M, Sais G, et al. (2011) MAGE-C2/CT10 protein expression is an independent predictor of recurrence in prostate cancer. PLoS One 6: e21366.
- Kawai T, Enomoto Y, Morikawa T, Matsushita H, Kume H, et al. (2014) High expression of heat shock protein 105 predicts a favorable prognosis for patients with urinary bladder cancer treated with radical cystectomy. MolClinOncol 2: 38-42.
- Sampson N, Ruiz C, Zenzmaier C,Bubendorf L, Berger P (2012) PAGE4 Positivity Is Associated with Attenuated AR Signaling and Predicts Patient Survival in Hormone-Naive Prostate Cancer. American Journal of Pathology 181: 1443-1454.
- Gupta A, Nuber N, Esslinger C, Wittenbrink M, Treder M, et al. (2013) A novel human-derived antibody against NY-ESO-1 improves the efficacy of chemotherapy. Cancer Immun 13: 3.
- Whitehurst AW, Xie Y, Purinton SC, Cappell KM, Swanik JT, et al. (2010) Tumor antigen acrosin binding protein normalizes mitotic spindle function to promote cancer cell proliferation. Cancer Res 70: 7652-7661.
- Ohue Y, Kurose K, Mizote Y, Matsumoto H, Nishio Y, et al. (2014) Prolongation of overall survival in advanced lung adenocarcinoma patients with the XAGE1 (GAGED2a) antibody. Clin Cancer Res 20: 5052-5063.
- Ademuyiwa FO, Bshara W, Attwood K, Morrison C, Edge SB, et al. (2012) NY-ESO-1 cancer testis antigen demonstrates high immunogenicity in triple negative breast cancer. PLoS One 7:e38783.
- Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, et al. (2011) Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. ProcNatlAcadSci 108: 16723-16728.
- Toor AA, Payne KK, Chung HM, Sabo RT, Hazlett AF, et al. (2012) Epigenetic induction of adaptive immune response in multiple myeloma: sequential azacitidine and lenalidomide generate cancer testis antigen-specific cellular immunity. Br J Haematol158: 700-711.
- Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, et al. (2010) Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 116: 1908-1918.
- Atanackovic D, Luetkens T, Kloth B, Fuchs G, Cao Y, et al. (2011) Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol 86: 918-1022.
- Wei A, Tan P, Perruzza S, Govindaraj C, Fleming S, et al. (2015) Maintenance lenalidomide in combination with 5-azacitidine as post-remission therapy for acute myeloid leukaemia. Br J Haematol 169: 199-210.








