Review Article - (2022) Volume 14, Issue 3
The importance of neopterin in COVID-19: the prognostic value and relation with the disease severity
Nadia Heidari,
Nadia Heidari,
Shima Hatamkhani,
Burcu Tekin,
Shahryar Alipour,
Roya Naderi,
Yeghaneh Farnamian and
Ilknur Akca
1Department of Bioengineering, Suleyman Demirel University, Isparta, Turkey
2Department of Biochemistry, Urmia University of Medical Sciences, Urmia, Iran
3Department of Biochemistry, Gorgan University of Medical Sciences, Urmia, Iran
4Cellular and Molecular Research Center, Urmia University of Medical Sciences, Urmia, Iran
5Department of Clinical Pharmacy, Urmia University of Medical Sciences, Iran
6Department of Biotechnology, Izmir Institute of Technology, Izmir, Turkey
7Department of Biochemistry and Applied Cell, Urmia University of Medical Sciences, Urmia, Iran
8Department of Physiology, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
9Student research Center, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
10Department of Biotechnology, Mersin University, Faculty of Sciences, Mersin, Turkey
*Correspondence:
Kevser Kubra Kırboga, Department of Bioengineering, Suleyman Demirel University, Isparta,
Turkey,
Email:
Received: 18-Feb-2022, Manuscript No. IPAOM-22-11550;
Editor assigned: 21-Feb-2022, Pre QC No. P-11550;
Reviewed: 07-Mar-2022, QC No. Q-11550;
Revised: 11-Mar-2022, Manuscript No. R-11550;
Published:
18-Mar-2022
Abstract
Coronavirus Disease 2019 [COVID-19], caused by severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], has rapidly evolved into a global health emergency. Neopterin [NPT], produced by macrophages when stimulated with interferon [IFN-] gamma, is an important cytokine in the antiviral immune response. NPT has been used as a marker for early assessment of disease severity in different of diseases. The main cause of NPT production is the proinflammatory cytokine IFN. Macrophage activation has also been revealed to be linked with disease severity in SARS-CoV-2 patients. We demonstrate the importance of NPT in the pathogenesis of SARSCoV- 2 and suggest that targeting of NPT in SARS-CoV-2 infection may be critical in the early prediction of disease progression and provision of timely management of infected individuals.
Keywords
SARS CoV-2; Neopterin; COVID-19; Prognosis
Introduction
Severe acute respiratory syndrome coronavirus-2
[SARS-CoV-2] infection has been spreading rapidly
around the world since it’s first appeared in China in
late 2019. The data show that approximately percent
80 of COVID-19 patients have mild disease percent 20
require hospitalization, and approximately percent 5
need intensive care admission [1]. COVID-19 has a poor
prognosis in elderly, male patients and, in patients with
comorbidities such as diabetes, cardiovascular disease, or
Chronic Obstructive Pulmonary Disease [COPD] [2-5]. In
patients infected with SARS-CoV-2, hyper-inflammation
and coagulopathy are known to be associated with disease
severity and death [6]. Elevated levels of inflammatory
markers, including C-reactive protein, ferritin, D-dimer,
inflammatory cytokines, and chemokines, and elevated
neutrophil to lymphocyte ratios are associated with disease
severity and mortality from COVID-19 [6]. High levels of
circulating cytokines, profound lymphopenia, substantial
mononuclear cell infiltration in the lungs and other organs
have been reported in severe cases compared to mild
COVID-19 cases [6]. Previous studies have shown that in
severe cases, the proportion of mononuclear phagocytes
increased and the composition of macrophages changed in
favor of monocyte-derived macrophages [6]. As a result,
high levels of cytokines linked to macrophage activation
including interferon- [IFN-], have been reported in SARSCoV-
2 patients [7]. NPT produced by macrophages
on stimulate with IFN-, a cytokine important in the
antiviral immune response. Serum NPT levels reflect the
activation phase of the cellular immune system, which is
important in the pathogenesis and progression of various
diseases [8]. Previous studies have shown an association
between serum NPT levels and prognosis in certain viral
infections, such as influenza, human immunodeficiency
viruses, hepatitis C virus, and dengue fever virus [9-11].
High levels of circulating cytokines have been reported in
patients with severe COVID-19. Therefore, targeting of
NPT in SARS-CoV-2 infection may be important for early
prognosis of disease progression and timely treatment of
infected patients. Serum NPT levels have been measured to assess the immune activation in several diseases, but only
a few studies have been conducted on individuals infected
with SARS-CoV-2. Therefore, this review is intended
to elucidate the importance of NPT as a diagnostic and
prognostic marker in COVID-19 patients.
Literature Review
Overview of Neopterin: biosynthesis, mechanisms of
tryptophan and oxidative
In the present pandemic, it is frequently observed
that cytokine storm occurs in patients with COVID-19
infection. When respiratory epithelial tissue is infected
by COVID-19 infection, inflammatory cytokines such
as IL-1, IL-6, IL-8, IL-12, TNF- and other chemokines
locally realized. subsequently, monocytes, macrophages,
neutrophils, DCs, and NK cells are recruited by cytokines
resulting in activation CD4+ and CD8+ T cells to synthesis
IFN- and TNF-, which induce lung injury. Furthermore,
high production IL-2, IFN-, GM-CSF, and TNF- leads to
anemia by macrophage activation and erythro-phagocytosis
[92,102]. IFN- is considered as a glycosylated protein of 25
kDa [103]. It is well established that IFNs are categorized into three categories; type I [IFN ], type II [IFN] and type
III [IFN][104].
IFN is produced mainly by natural killer [NK]
cells, natural killer T cells [NKT], activated lymphocytes
such as CD4 T helper type 1 [Th1] cells and CD8 cytotoxic
T cells, B cells, and professional antigen-presenting cells
[APCs] [105-110]. It is now apparent that Janus activated
kinases [JAKs] and STAT1 signal is trigger by binding IFN
to IFNAR1 and IFNAR2 receptors. Attaching of IFN to
IFNARs result in activation of tyrosine kinases JAK1 and
JAK2 phosphorylating the transcription factor STAT1
to form dimer then dimers translocate to the nucleus and
bind GAS to stimulate the transcription of these genes; for
example: IFN stimulate expression of immunoglobulin Fc
receptors on phagocytes and improve expression of MHC
antigens facilitating antigen presentation to T lymphocytes
[111,112]. TNF is classified as non-glycosylated protein
which has 157 residues [113] secreted by macrophages/
monocytes. TNF gene is located on chromosome 6
[50]. TNF plays a verity of roles in cell, for example; viral
replication, cell growth modulation, tumorigenesis, and
inflammation process. [114-117].
The expression of TNF gene is controlled by nuclear factor kappa b [NFB] and nuclear factor activated
T cells [NF-AT] [115,116] TNF signals through TNF
recep- tor 1 [TNFR1] and TNF receptor 2 [TNFR2]
[118]. Both pro-inflammatory and pro-apoptotic pathway
are triggered by the binding of the ligand soluble TNFand
transmembrane to the TNF receptor [TNFR1] and
TRAF2, respectively. TNFR1 stimulate NFB, MAPK and
Caspase-8 inducing inflammation, tissue degeneration,
apoptosis. On the other hand, TRAF2 can activate
MKLK leading to necroptosis [119,120]. Interleukin:
Interleukin [IL] refers to a class of cytokine prominently
secreted by leukocytes [121]. ILs regulates numerus
of function such as: stimulation and differentiation of
immune cells, proliferation, maturation. IL not only act
as pro-inflammatory agent but also have anti-inflammatory
properties [121]. Mature IL-6 has 185 amino acids. The
gene of IL-6 is located at chromosome 7 p21. This pleotropic
cytokine exerts numerous functions such as: inflammation,
immune response, and hematopoiesis which are produced
from T cells, macrophages, endothelial cells, fibroblasts and
monocytes [122]. Binding of IL-6 to its receptor initiates
cascades of signaling through JAK/STAT3 stimulating the
transcription of several factors such as: other cytokines and
adaptor proteins [123]. Taken together, Interleukins, TNF
and IFN play inseparable role in cytokine storm.
Association of Neopterin with the severity of
COVID-19: Statins, one of the best-selling prescription
drug class HMG-CoA reductase enzyme inhibitors
in the US, is known to have a favorable safety profile;
they contain the world's best selling prescription drug
atorvastatin. When looking at their biochemical effects,
the uncommon effects of statins that extend far beyond the
lipid profile and components such as LDL-C, HDL-C and
triglycerides ranging from nitric oxide and inflammatory
markers to polyunsaturated fatty acids [124]. Since statins
in the lipid-lowering drug class are inhibitors of 3-hydroxy-
3-methylglutaryl coenzyme A reductase [125], many at
risk [126] including young/old, male/female individuals.
It has well-documented benefits for moderate to high
cardiovascular disease [124].
To SARS-CoV-2 patients with a compromised
cardiovascular system and comorbidities including various cardiovascular diseases and hypertension may then become
inflicted with acute respiratory distress syndrome and
increased mortality. Statin can be given to the patient in
the manufacturing field and office, where AR is “little and
needs little in intensive care. In addition, in another study,
the continued use of statins in patients with COPD had to
be cautious about intubation. In addition to its benefits in
existing techniques in the technology used in practice, it can
also prove new roles that can benefit and can be achieved
from technologies derived from anti-inflammatory, antithrombodultic,
immuno-thrombodultic and immunothrombodulatory
and methods of sampling science.
Still not explicitly applicable to the conversation, some
hospital statins are therefore included in education from
COVID-19 [2]. In addition, an analysis of in-hospital
deaths in 8910 COVID-19 patients from Asia, Europe,
and North America demonstrated a positive prognosis for
statin use. At the same time, statins positively affect the
endothelium under stress, since viral use increases the
endothelial damaging rendering thrombotic value [4].
If one person has something else across the U.S. due to
COVID-19 more than 10,000, from admission to use,
other prior use, comorbid conditions, hospital and bear
controlled more than 40% in the next and severely over
40. The result was associated with a greater than 25% risk
of developing severe symptoms. The observations and
teaching that statins were not considered as ingredients still
indicate preliminary information [125-127].
Some research results show that the statin effect is
not significant for COVID-19 when the comparative
data of users and non-users are examined. This is mainly
because statin users with COVID-19 disease are shown
to have a greater baseline risk driven by older age and
higher cardiovascular comorbidity burden. This could
in theory hide the potential protective effect of statins in
this particular subset of patients [128]. For this reason,
multivariate analyzes can give more positive results than
univariate analyzes. While it is known that there is a
30% reduction in fatal or severe COVID-19 infection
according to multivariate meta-analyses; does not confirm
a significant lack of protection in the data reported in the
univariate meta-analysis among statin users [128,129].
Low-Density Lipoprotein (LDL); ApoB is a large particulate molecule with a molecular weight of 2,000 kDa,
consisting of triacylglycerol, free cholesterol, cholesteryl
ester and phospholipid molecules. LDL, which contains
1600 cholesterol esters and 170 molecules of triglycerides
in its core, consists of 700 phospholipid molecules and
600 cholesterol molecules in the surrounding layer. In
its outer layer, there is apo B100 molecule. Half of LDL
fatty acids consist of Polyunsaturated Fatty Acids (PUFA).
PUFAs are protected from oxidation by antioxidants.
Oxidized LDL (ox-LDL) exposed to oxidative stress and
inflammation is seen in various diseases resulting in the
presence of oxidative stress and reactive oxygen derivatives.
High concentration of ox-LDL initiates cellular changes
resulting in cell death; ROS formation, caspase, protein
kinase activation, calcium homeostasis and proapoptotic/
antiapoptotic gene expression change [130]. NPT a
pyrazinopyrimidine compound, is used as a very popular
biomarker, especially in important pathologies in which
cellular immune mechanisms are activated [131]. Since high
NPT production is associated with increased production of
Reactive Oxygen Species (ROS) and low serum antioxidant
concentrations such as alpha-tocopherol, NPT can also be
considered as a marker of ROS generated by the active
cellular immune system. Therefore, NPT measurements
can predict not only the extent of cellular immune
activation, but also the extent of oxidative stress [132,168].
Hypochlorous acid (HOCl), an important inorganic
bactericidal compound of innate immunity, is effective
against a wide variety of microorganisms [133]. Stabilized
at pH 3.5–5.5, HOCl is a weak acid that interacts
with structural proteins or viral material to inactivate
microorganisms [134]. HOCl is known as the most
potent oxidant produced by neutrophils and is a potent microbicidal agent within these cells. Because of its
chemical nature, HOCl has never been used as a medicine
to treat infection. A remarkable feature of the immune
system is its ability to initiate an effective response against
invading pathogens by deploying a group of highly reactive
chemicals, including oxidized halogens, oxidizing radicals,
and single oxygen (Figures 1-3) [135]. HOCl is currently a
disinfectant approved under different brands by the US
Environmental Protection Agency for SARS-CoV-2.
HOCl, which interacts with structural proteins such as the
capsid or surface compounds of viruses, lipid envelope and
DNA/RNA materials, HOCl with concentrations as low
as 20 ppm has been found to be effective in disinfecting
surfaces including porous rayon. In addition, it is not toxic
to humans and has been found to be a disinfectant 80-200
times more effective than standard disinfection procedures
[134].
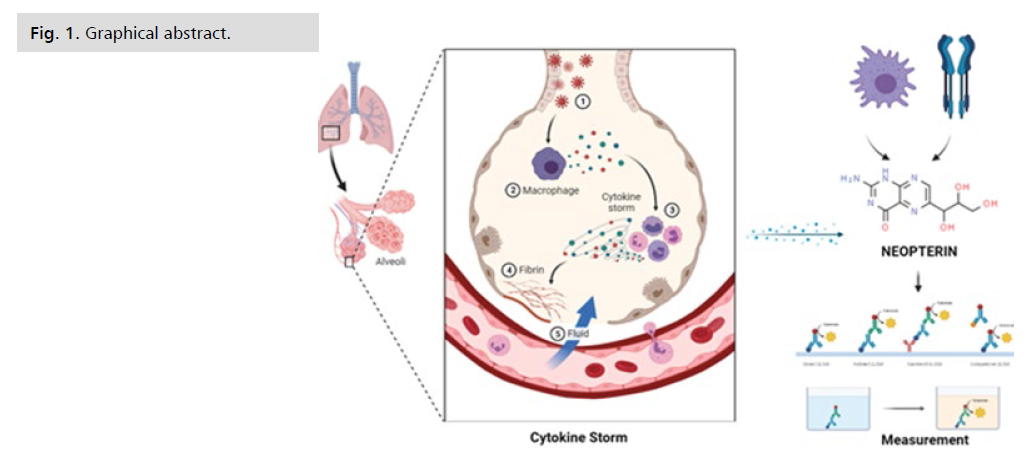
Figure 1: Graphical abstract.
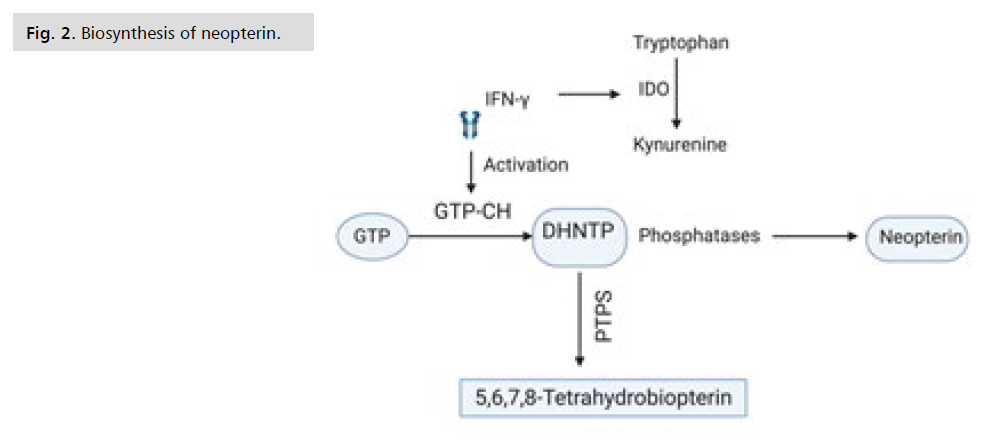
Figure 2: Biosynthesis of neopterin.
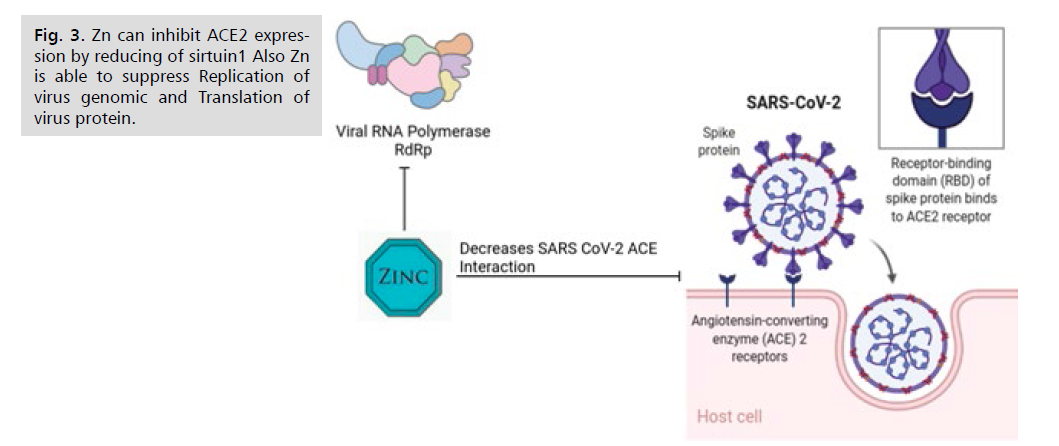
Figure 3: Zn can inhibit ACE2 expression by reducing of sirtuin1 Also Zn is able to suppress Replication of
virus genomic and Translation of virus protein.
The current data show that HOCl oxidizes NH2 to
form NPT. NH2 is described as an antioxidant and a
powerful radical scavenger. NPT remains stable at neutral
pH, while NH2 is oxidized to 7, 8-dihydroxyantopterine
in oxygen-saturated solution. Oxidizing agents in acidic
solution, for example MnO2 or I2, leave the NH2 side
chain unaffected and selectively oxidize the 7, 8-dihydro
structure of NH2 to form NPT.
The mechanism of oxidation remains unclear. The
reaction mixture not only has a balance of hypochlorite
and free acid, but also has oxidizing properties of dissolved
chlorine (Cl2) resulting from the decomposition of
HOCl. However, when HOCl content is quantified
spectrophotometrically, the oxidative potential of Cl is
not included. This may explain why the amount of NPT
formed exceeds the amount of HOCl administered. Since the quantification of NPT by fluorescence detection is
selective and sensitive in one study, the rate of formation of
NPT was monitored. NH2 is a non-fluorescent compound.
The UV/Vis signal is an overlap of NH2 and NPT, and
apart from electrochemical detection, no suitable HPLC
method is known to separate these two compounds. It
was also concluded that alternative quantification of NH2
after iodine oxidation after HOCl oxidation is impossible
due to additional products formed. NPT is thought to act
instead as a pro-oxidant, depending on conditions such as
the nature of the oxidant, pH, or absence/presence of iron
ions. Evidence is provided for the first time that reactive
species can act on the NPT/NH2 ratio independently,
thereby altering the redox modulatory properties of these
pteridines. A dynamic ratio has been noted in conformity
with the idea that pteridines are redox modulators rather
than a static model of stable NPT/NH2 excretion. HOCl
has been shown to increase the oxidative potential in the
local microenvironment by increasing the NPT-mediated
prooxidative potency and decreasing the antioxidative
capacity of NH2. A conversion of the NPT/NH2 ratio was
also found, with NPT concentrations sometimes found
higher than NH2 concentrations compared to NH2 alone
[135].
In recent years, COPD has been defined as an
inflammatory disease with systemic consequences. COPD
may also predispose individuals to the presence of other
comorbidities, such as arterial hypertension, diabetes, and
cardiovascular disease, which can potentially affect the
outcome and severity of COPD. Therefore, the coexistence
of associated diseases is common and may affect COPD
disease progression and prognosis. According to the stated
experience, higher NPT levels have been found in patients
with cardiac and renal diseases, and these can be expressed
as reflecting attacks of viral etiology [136]. It is supported
by data that mortality and NPT after pneumonia are risk
factors for respiratory tract infection and cardiovascular
events. The first line of these observations may have
clinical implications when assessing COPD severity and
exacerbation [137].
NPT can be potentially expressed as a promising
inflammatory mediator. It has been reported to act as a
mediator of cell immunity against intracellular pathogens
such as viruses, parasites and intracellular bacteria. It is
widely accepted that COPD is associated with an increased
systemic inflammatory response compared to controls, and
this inflammatory response is reported in patients with
stable COPD at higher levels of NPT when compared to
control groups [138]. NPT is released from monocytic cells
after stimulation with interferon gamma (IFN-γ) as a wellestablished
biomarker of cellular activation. IFN-γ also
promotes the degradation of tryptophan in the kynurenine
pathway, which produces several neuroreactive metabolites,
including quinolic acid, which may contribute towards
neurological disorders [139]. Briefly, NPT is an oxidized
form of dihydroneopterin during antioxidant reactions in
which high levels of NPT in serum and other biological
fluids are associated with high production of ROS and induction of oxidative stress (OS) during intense activation
of cellular immunity [73]. High concentrations of NPT
have been reported to be detected in every neuro-COVID
patient studied according to studies [140]. NPT was
elevated in cerebrospinal fluid samples of patients with
COVID-19 and neurological abnormalities [139]. Also,
the serum level of NPT can distinguish viral infection of
the lower respiratory tract from a bacterial one, it can be
noted a twofold higher increase in its viral state compared to
bacterial infection. In brain damage caused by COVID-19,
NPT levels were found to be high in cerebrospinal fluid
(CSF) from patients [73].
It is accepted that uveitis patients with co-morbidities
such as diabetes mellitus, hypertension and cardiovascular
disease are at higher risk if they develop COVID-19.
Asymptomatic retinal complications of SARS-CoV-2
infection have also been reported, but the prevalence is
unknown. Research currently points to studies describing
uveitis, retinitis, retinal vasculitis, and optic disc
involvement in animals after coronavirus infection [141].
No reports of COVID-19-associated uveitis have been
published to date, but thin retinal microvascular pathology
and small lesions have been described in the ganglion cell
and inner plexiform layers [142].
NPT plasma level has been measured in many
autoimmune diseases. Due to the overstimulation of
monocyte/macrophage cells by T lymphocytes in patients
with RA, NPT may be an indicator of both cellular and
innate immune activity in these patients. Higher NPT
concentrations have been shown to be associated with
increased cardiovascular risk in the general population.
As is known, cardiovascular disorders are one of the most
important causes of mortality in patients with RA. Studies
have shown that NPT levels increase with age in both
RA and control groups; In addition, it was found that
RA patients increased with disease onset age and disease
duration. The reason for higher NPT levels in male RA
patients is not clear, but it can be stated here that higher
anti-CCP antibody contributes to increased inflammation
and NPT levels in these patients [143,144].
Association of Neopterin with symptoms in
COVID-19 patients: NPT is an independent prognostic
factor for COVID-19 sever ty [56]. It appears in the blood
before the onset of clinical symptoms, rises in acute stages
of viral infection, and is linked to severe dyspnea, a more
extended hospitalization period, and other complications
[73]. While Covid19 is generally identified as pulmonary
infection, it brings disturbances to various organ systems
in the body with their related symptoms. The association
of non-pulmonary clinical signs and symptoms with NPT
in patients with COVID-19 has not been thoroughly
investigated. Some scarce studies reported the probable
association of NPT in body fluids with gastrointestinal,
neurologic, and renal signs and symptoms [73].
The pooled prevalence of gastrointestinal (GI) symptoms
(including nausea, vomiting, diarrhea, abdominal pain,
and anorexia) in patients with confirmed COVID-19 was
18% with diarrhea being the most significant [145] Some patients show gastrointestinal symptoms (e.g., nausea and
diarrhea) as an initial manifestation of the disease [146] and
patients with a severe form were more likely to experience
GI symptoms.
Fecal NPT is assumed as a surrogate of cellular viral
immune response and may be an indicator of intestinal
inflammation in COVID-19 patients [10,147]. SARSCoV-
2 can impose injuries to the gut mucosa by its ability to
infect and replicate in the enterocytes. Intestinal epithelial
cells express two critical proteins for SARS-CoV-2 cell
entry, simultaneously: ACE2, and transmembrane serine
protease [148], make the oral-fecal route a potential route
for infection [147].
In a study on 37 hospitalized COVID-19 patients
(Non-ICU setting) with a median age of 62 years and
a high level of C-reactive protein (evidence for systemic
inflammation), fecal NPT values were elevated (more than
614.7 ng/g) in comparison with control healthy subjects.
Seventeen patients who had GI symptoms (diarrhea and/
or nausea and/or vomiting) demonstrated even higher
NPT values in the stool. This subgroup of patients was
also found to have elevated serum C-reactive protein
concentration and body temperature on the day of stool
sampling compared with the low NPT group, suggesting
the presence of systemic inflammation. The fecal NPT did
not significantly differ according to the type of the GI sign
or symptoms. The infected cells (including enterocytes)
release selected cytokines and chemokines that induce
intestinal inflammation and underlie GI symptoms [147].
Considering that the results of this study are based on a
limited sample size, and SARS-COV-2 RNA was confirmed
in only 35% of the patients, we should sound a note of
caution about such findings. As SARS-CoV-2 infection
is closely related to previous SARS in several aspects, it
is assumed that SARS-CoV-2 RNA may have an ability
to spread into the CNS via the membrane-bound ACE2,
resulting in clinical neurological signs and symptoms [141].
NPT is an informative biomarker of central nervous
system immune activation in various viral infectious
settings, including HIV-1 infection and influenza [148,152]. NPT level in the serum and cerebrospinal fluid (CSF)
increased in 6 patients with moderate to severe COVID-19
infection who also presented neurological disorders.
Neurologic symptoms were encephalopathy, extreme
fatigue, memory loss, personality changes, moderate
neck stiffness, photophobia, somnolence, dysgeusia,
disorientation [141]. High CSF NPT may be inspired by a
forceful systemic inflammatory response induced by SARSCoV-
2 infection [149]. This observation may outline the
COVID-19-induced CSF inflammation and brain injury
[73,141]. There is still considerable ambiguity about the
pathophysiological basis of profound elev ated CSF NPT
in COVID-19 infection and its use as a prognostic factor
for neurological symptom development [149].
All we know about the role of NPT in COVID-19-
induced acute kidney injury are in the light of studies
evaluating NPT in severe COVID-19 setting. The severe form may be accompanied by acute kidney injury in about
half of the cases [150]. Even though it is reported that
elevated serum creatinine and blood urea concentrations are
associated with high serum NPT, some other studies failed
to provide a meaningful correlation in severe COVID-19
cases [76]. Many studies believed that high serum NPT
concentrations related to the severity of the infection,
deteriorated renal function, and higher temperature upon
hospital admission [56]. Therefore, future studies on the
current topic are required to elucidate the exact role of
NPT in the clinical symptoms of patients with COVID-19
infection.
Measurement of Neopterin in COVID-19: NPT is
one of the measurable prognostic substances which are
produced by the immune system in the human. Recently,
due to its cost- effective and easily detectable features, NPT
has become a very important marker for usage in the clinic
to predict disease progression. Because the high amount
of NPT mirrors extremely activated cellular immunity,
It has been used for the diagnosis of several diseases and
their treatment selections [10,151]. Since the 19thcentury
[14], NPT levels were detected and often used as a
progression prediction marker for the diseases. Especially,
In infectious diseases like bacterial parasitic and viral,
detection of NPT levels became highly useful in terms of
the monitoring activation of cellular immunity [153,154].
Currently, we are facing the COVID-19 viral infection and
useful information about the disease and its progression
has become very important. Several articles showed that
measuring NPT levels can guide to observing the infection
degree of COVID-19 prognosis.
One of the first studies for detecting the usefulness
of NPT levels in COVID-19, Ozer et al. showed that
the NPT levels found 46 nmol/L in patients who have
coronavirus and this result pointed to the fact that the
patients have COVID-19 NPT levels much higher than
the people have no viral infection or have mild COVID-19
[56]. Additionally, other studies support these findings
with similar results. For instance, Bellmann-Weiler and
her colleagues used 115 patients’ serum samples to measure
NPT levels and they found that the NPT levels were similar
to the first study which is above 40 nmol/L. Moreover,
they concluded that the high amount of NPT [45 nmol/L]
can be useful for the early prediction of high- risk group
COVID-19 patients [77]. In clinic usage, NPT has mostly
been detected in serum and urine [155]. Also, shown that
it could be measured in the cerebrospinal fluid [156] and
saliva [157] as well and besides this, some studies detected
NPT in the synovial fluid [158] and pancreatic secretion
[159-169]. Measurement of NPT is immensely simple in
body samples and it can be made with several techniques. Tables 1, 2 represents all studies that were measure and point
to the importance of NPT levels in the COVID-19.
According to the table, ELISA has been the first choice
for measuring the levels of NPT. ELISA is one of the
labeled immunoassays and simply this technique uses the
antibody-antigen interactions as an immunocomplex to
detect the desired molecule in the sample.
| Year |
Number of pateints |
Finding |
| 2021 |
214 |
Taking of Zn did not significantly affect the duration of symptoms versus control group. |
| 2020 |
191 Zinc group 1 [n=96]
Without zinc group [n=95] |
Zinc supplements did not improve the clinical efficacy of hydroxychloroquine. |
| 2001 |
91 |
Zinc treatment did not attenuate total symptom score. |
| 2004 |
153 |
Zinc supplementation significantly reduces duration of fever and very ill status in boys, but not in girls. |
| 2016 |
53 |
Zinc treatment was able to increase the number of functional T. |
| 2008 |
50 |
Zinc supplementation decreased both the production of inflammatory cytokines and oxidative stress marker: |
| 2010 |
108 |
Zinc, selenium and vitamin C treatment may alleviate symptoms in COPD. |
| 2014 |
301 |
Zinc amino acid chelate had a better effect in acute respiratory |
| 2019 |
64 |
Zinc supplementation reduced the number of days of ALRI in Thai children, as well as their stay in hospital |
Table 1: Some studies evaluate the effect of Zn supplementation on people in different condition.
| Sample Size |
Sample |
Neopterin Level (Mean) |
P-Value |
Measurement Technique |
| 103 patients |
Serum |
46 nmol/L |
p<0.001 |
ELISA |
| 34 patients |
Serum |
42 nmol/L |
p<0.0001 |
ELISA and HPLC |
| 115 patients |
Serum |
56.6 nmol/L |
p<0.001 |
ELISA |
| 37 patients |
Fecal Sample (Stool) |
>614.7 ng/g |
- |
ELISA |
| 45 patients |
Serum |
44.90 nmol/L |
p<0.05 |
ELISA |
Table 2: Neopterin detection techniques and levels in COVID-19.
Generally, a particular molecule binds to its antibody
that contains special binding sites for its specific antigen.
Also, the antibodies can be detected with the ELISA tests
[170]. For the detection and measurement of NPT, the
ELISA test contains rabbit-anti-NPT that is antibody
binding sites for both sample NPT and enzyme-attached
antigen. These antigen-antibody complexes then bind to
the specific surface of the test for detection. Due to its
flexibility, the ELISA test can apply and design for various
diseases. The test uses up to 96 well plates and this allows
one to do look at the multiple samples at the same time
[162]. Thus a lot of samples can observe and the results can
be obtained in a little time. Also, its usage is very simple
then doesn’t need special learning. The other advantageous
usage of ELISA is its sensitivity and specificity [163].
With a small sample size, desired substances can be
detected through specific antigen-antibody interactions.
Additionally, the test has some drawbacks. While applying
fluid samples it doesn’t need pretreatment but non- fluid
samples like stool need be pretreatment for the test. One of
the studies represent in the Table 4 used the stool sample
to measure NPT levels. They made some dilution processes
before the applying test on the samples. Then they used
the supernatant of the samples for test respectively [147].
There are other potential methods that measure and detect
NPT. Lately, their usage did not present in COVID-19
infection but they used it for measuring NPT levels in
several diseases and infections. The technique RIA is one
of the labeled immunoassays [163]. Different from the
ELISA, the RIA technique uses radioactive isotopes rather
than enzymes as a label. Although the procedure is similar
to ELISA, RIA has some differences and drawbacks [160]
such as, in the RIA test due to its labeled radioactive
isotopes, the trained people need for the preparation
and does the experiment. Also, the storage and disposal
of radioactive substances require special procedures that
must do carefully. Most importantly, if these isotopes are
not disposed of in the right way, they can cause radiation
hazardous. Notwithstanding it has some difficulties, The
RIA test is successful for the detection of biomarkers.
S´anchez-Regan˜a et al. concluded that the determination
of NPT could be done with the RIA and results showed
that the RIA is highly accurate [161].
Another potentially used technique for the measurement
of NPT is HPLC that is uses the liquid sample mixture,
several pumps, and columns to specify biological substances.
Additionally, the detector of the HPLC system is enabled
to determine substances quantitatively [163]. Although the
use of High-Pressure Liquid Chromatography [HPLC] for
detecting fluorimetric signals has been widely used, many
of the procedures described have practical limitations. This
is mainly due to the difficulty in detecting contaminant
peaks in blood samples. Carru et al. used the longer
column in their experiment. In these conditions, the NPT
concentration achieved with phosphate buffer was totally
resolved from impurities. The concentration achieved with
water as eluents were also decreased by about 20% wing
to several features of NPT, the measurement techniques
should choose carefully. The specificity of ELISA enables it to detect NPT not only in serum samples but also in urine
and other body materials. Conversely to ELISA, the HPLC
method should be used to detect NPT, mostly in urine
samples in terms of being NPT levels very low in the serum
rather than urine [164]. Also, the high fluorescent feature
makes NPT easily detectable in the urine samples by HPLC.
Furthermore found that the NPT detection with RIA was
uncertain in the urine samples [18]. Therefore they conclude
that the RIA should just be used for the measurement of
NPT in serum samples. Recently, biosensors have become
promising devices for the measurement of NPT. Also, with
the fastly growing human population, the demand for fast
and accurate point of care biosensor devices increased.
Biosensors are highly useful analytic devices with several
good features such as portability, simplicity, and the bestdesired
feature its specificity [164]. As a chemosensor,
Sharma et al. used the molecular imprinting method that
generates an artificially synthesized receptor polymer for
the NPT. They showed that their molecularly imprinted
film was sensitive and also give a chance to differentiate
NPT analogs from samples [165,173]. The specification
of progression of COVID-19 patients is highly possible
through the measurement of NPT levels. Therefore, the
demand for improved measurement techniques for NPT
has become important for the characterization of the
disease severity and infectious degrees of patients who have
the SARS COV-2 virus.
Prognostic value of Neopterin in COVID-19: There
is incomplete evidence about prognostic biomarkers that
could ad- vantage physicians to categorize COVID-19 cases
that are likely to improve a poor outcome. In COVID-19
patients, it is recognized that overexcited inflammation and
coagulopathy are related with death in patients and disease
severity. The results showed that compared with mild
cases of COVID-19, high levels of circulating cytokines,
profound lymphopenia, and significant infiltration of
mononuclear cells into the lungs and other organs occur in
very severe inflammatory conditions [166]. High rates of
circulating biomarker such as cytokines were described in
severe COVID-19 patients. The serum rates of the immune
motivation NPT have revealed to be of predictive value
in patients with SARS-CoV-1 [15,164] and Early studies
suggest that serum NPT may be useful in classifying SARSCoV-
2 patients [56,76]. It has also been shown that high
levels of cytokines associated with macrophage activation,
including IFN-, are present in patients with SARS-CoV-2
[7]. Also proved based on different reports that raised
kynurenine/ tryptophan ratio are usual in COVID-19 and
associate narrowly with elevated NPT levels, and like NPT
also kynurenine/ tryptophan is related with undesirable
results [76]. The same observations have been made in
other infections, such as HIV-1 [14]. Overall, the IFNinduced
immune response to viral in- fections may lead
to increased NPT concentrations as well as increased
tryptophan degradation and increased kynurenine to
tryptophan ratio [13]. According to the different results of
the studies, various scenarios have been presented about
the role of neoprene and how it increases in people with
COVID-19. Most are related to the role of antioxidants in controlling ROS [14,167]. These include a variety of
vitamins, especially vitamin B, so that a decrease in vitamin
B levels is associated with an increase in homocysteine and
NPT levels in people with the disease [164,161,168].
It is also one of the possible mechanisms related to iron
levels. If the amount of inflammatory factors in the blood
increases, iron may be more likely to be stored [ferritin],
which the results of some studies prove the same, and the
amount of NPT is associated with an increase in ferritin
[162,163, 166,170]. NPT formed by macrophage cells on
motivation with IFN-, which is a main factor in the antiviral
immune reaction, therefore it can be used to forecast the
severity of disease in COVID-19 cases [56]. In patients
with infection diseases, improved NPT levels were known
in body fluid samples like saliva, urine, blood and CSF,
e.g., during cytokine therapies but also in diseases that are
related with stimulation of the T-helper-1 immune cells
such as autoimmune pathologies, mycobacterial infections
and numerous types of tumor cells and with viral infections
including SARS-CoV-1 and HIV-1 and recently also in
COVID-19 with SARS-CoV-2 [15,62,78,165,172]. The
results of studies have shown that serum levels of NPT
are closely related to the severity of COVID-19, with
levels starting to rise from the 3-4 day of SARS-CoV-2
infection, being correlated with severe dyspnea, Take a
long stay hospital and other complications [73]. The other
measurement of NPT levels is able to prepare valuable data
in patients with current COVID-19 disease. Higher NPT
levels emerge to describe to an epidemic and widespread
infection and thus to an improved infectious condition,
whereas a normal or very low NPT is revealing for quiet
infection lacking or existence of fewer active infection [76].
Nevertheless, further studies are needed to confirm this
conclusion, which is still in the early stages.
Challenges and future perspectives: COVID-19
is considered as a cytokine storm and affects multiorgan
inflammatory infection. The mortality rate of this
pulmonary and systemic injury is ascertained by the
severity of inflammation and coagulation. Due to this,
prevention, early diagnosis in corporation with effective
therapeutic interventions is urgently needed for saving
lives. NPT is an early critical marker for the progression
and severity of immune disease or may be useful together
with the several inflammatory markers to suggest a
diagnosis of SARS-CoV-2. It is also postulated as a
sensitive marker of oxidative stress which could decrease
inflammation through suppressing NF-B signaling and
NLRP3 inflammasomes. Other studies indicated that, NPT contribute with high produc- tion of ROS and NFB,
which lead to pro-inflammatory gene rise including
inducible nitric oxide synthase [iNOS] and trigger
inflammatory processes. Furthermore, we need to consider
that the elevated level of NPT may be related to other
pathological conditions or inflammation- related diseases
not only confined to COVID-19, such as RA, nephropathy,
neuropsychiatric anomalies or cancers. Within this view,
further studies are required to address the exact role of
NPT in the clinical symptoms of patients with COVID-19
infection. Also, It is important to highlight that among the
knowledge gaps of COVID-19 there are diagnostic errors
with laboratory testing as well as their interpretation in
patient management. While, NPT appears in the blood
before the onset of clinical symptoms; it considered as an
independent prognostic factor for COVID-19 severity.
However, what appears to issue from this evaluation is
that NPT values have significantly enhanced in individuals
with severe SARS-CoV-2 infection in comparison with
those with milder forms of the disease. Therefore, it could
be logical to assume immediate measurement of cellular
immune activation marker namely NPT in patients and
subsequently a longitudinal monitoring, so as to identify
a subgroup of patients with progressive inflammatory
situation.
Conclusion
In conclusion, NPT level have a significant correlation
with the severity of COVID-19 and can be considered as
a macrophage activation and sensitive indicator to predict
disease risk. Further studies should also be planned to clarify
whether targeting of NPT in SARS-CoV-2 infection may
be critical in the early assessment of disease progression and
prognosis of infected patients.
Declaration of Competing Interest
The authors declare that they have no known competing
financial interests or personal relationships that could have
appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from
funding agencies in the public, commercial, or not-forprofit
sectors.
REFERENCES
- Zhao Q, Meng M, Kumar R (2020) Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int J Infect Dis 96:131–135
[Crossref], [Google Scholar], [Indexed at]
- Zheng Z, Peng F, Xu B (2020) Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 81:e16–e25
[Crossref], [Google Scholar], [Indexed at]
- Yang J, Zheng Y, Gou X (2020) Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94:91–95
[Crossref], [Google Scholar], [Indexed at]
- Bentivegna E, Luciani M, Spuntarelli V (2020) Extremely Severe Case of COVID-19 Pneumonia Recovered Despite Bad Prognostic Indicators: a Didactic Report. SN Compr. Clin Med 2:1204–1207
[Crossref], [Google Scholar], [Indexed at]
- Kaur N, Gupta I, Singh H (2020) Epidemiological and Clinical Characteristics of 6635 COVID-19 Patients: a Pooled Analysis. SN Compr Clin Med 2:1048–1052
[Crossref], [Google Scholar], [Indexed at]
- Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20:355–362
[Crossref], [Google Scholar], [Indexed at]
- Jungraithmayr TC, Reschke M, Grebe SO, Lange H, Radsak K, et al. (2001) Assessment of cytomegalovirus infections using neopterin and a new immunoblot. Clin Chim Acta 310:63-69
[Crossref], [Google Scholar]
- Michalak Ł, Bulska M, Strząbała K, Szcześniak P (2017) Neopterin as a marker of cellular immunological response. Hig Med Dosw 71:727-736
[Crossref ] [Google Scholar]
- Pizzini A, Kurz K, Santifaller J (2019) Assessment of neopterin and indoleamine 2,3dioxygenase activity in patients with seasonal influenza: A pilot study. Influenza Other Respir Viruses 13: 603–609
[Crossref], [Google Scholar], [Indexed at]
- Eisenhut M (2013) Neopterin in Diagnosis and Monitoring of Infectious Diseases. J Biomark 1–10
[Crossref], [Google Scholar], [Indexed at]
- Chan CPY, Choi JWY, Cao K-Y (2006) Detection of serum neopterin for early assessment of dengue virus infection. J Infect 53: 152–158
[Crossref], [Google Scholar], [Indexed at]
- Müller MM, Curtius HC, Herold M, Huber CH (1991) Neopterin in clinical practice. Clin Chim Acta 201:1-16
[Crossref], [Google Scholar]
- Murr C, Widner B, Wirleitner B, Fuchs D (2002) Neopterin as a marker for immune system activation. Curr Drug Metab 3:175-187
[Crossref], [Google Scholar]
- Fuchs D, Weiss G, Wachter H (1993) Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int Arch Allergy Immunol 101:1-6
[Google Scholar]
- Hailemichael W, Kiros M, Akelew Y, Getu S, Andualem H, et al. (2021) Neopterin: A Promising Candidate Biomarker for Severe COVID-19. J Inflamm Res 14:245–251
[Crossref], [Google Scholar], [Indexed at]
- Wirleitner B, Reider D, Ebner S, Böck G, Widner B, et al. (2002).Monocyte-derived dendritic cells release neopterin. J Leukoc Biol 72:1148-1153
[Crossref], [Google Scholar], [Indexed at]
- Sghiri R, Feinberg J, Thabet F (2005) Gamma Interferon Is Dispensable for Neopterin Production In Vivo. Clin Vaccine Immunol 12: 1437–1441
[Crossref], [Google Scholar], [Indexed at]
- Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, et al. (1989) Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta 1012:140-147
[Crossref], [Google Scholar], [Indexed at]
- Terness P, Bauer TM, Röse L, Dufter C, Watzlik A (2002) Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. Exp Med 196:447-457
[Crossref], [Google Scholar], [Indexed at]
- Hol JW, Stolker RJ, Klimek M, Stronks DL, Fekkes D, et al. (2014) The tryptophan kynurenine pathway, neopterin and IL-6 during vulvectomy and abdominal hysterectomy. J Biomed Sci 21:1-11
[Crossref], [Google Scholar], [Indexed at]
- Widner B, Leblhuber F, Fuchs D (2002) Increased neopterin production and tryptophan degradation in advanced Parkinson's disease. J Neural Transm Suppl 109:181-189
[Crossref], [Google Scholar], [Indexed at]
- Fuchs D, Möller AA, Reibnegger G, Werner ER, Werner-Felmayer G, et al. (1991) Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett 28: 207-211
[Crossref], [Google Scholar], [Indexed at]
- Toygar M, Aydin I, Agilli M (2015) The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Hum Exp Toxicol 34: 198–204
[Crossref], [Google Scholar], [Indexed at]
- Schobersberger W, Hoffmann G, Grote J, Wachter H, Fuchs D, et al. (1995) Induction of inducible nitric oxide synthase expression by neopterin in vascular smooth muscle cells. FEBS Lett 377:461–464
[Crossref], [Google Scholar], [Indexed at]
- Berdowska A, Zwirska-Korczala K (2001) Neopterin measurement in clinical diagnosis. J Clin Pharm Ther 26:319–329
[Crossref], [Google Scholar], [Indexed at]
- Svoboda P, Ko SH, Cho B (2008) Neopterin, a marker of immune response, and 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress, correlate at high age as determined by automated simultaneous high-performance liquid chromatography analysis of human urine. Anal Biochem 383: 236-242
[Crossref], [Google Scholar], [Indexed at]
- Wirleitner B, Schroecksnadel K, Winkler C, Fuchs D (2005) Neopterin in HIV-1 infection. Mol Immunol 42: 183-194
[Crossref], [Google Scholar], [Indexed at]
- El-Lebedy D, Hussein J, Ashmawy I, Mohammed AM (2017) Serum level of neopterin is not a marker of disease activity in treated rheumatoid arthritis patients. Clin Rheumatol 36: 1975–1979
[Crossref], [Google Scholar], [Indexed at]
- Asci A, Baydar T, Cetinkaya R, Dolgun A, Sahin G (2010) Evaluation of neopterin levels in patients undergoing hemodialysis. Hemodial Int. 14: 240–246
[Crossref], [Google Scholar], [Indexed at]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D (2002) Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun 16: 590-595
[Crossref], [Google Scholar], [Indexed at]
- Widner B, Leblhuber F, Fuchs D (2002) Increased neopterin production and tryptophan degradation in advanced Parkinson's disease. J Neural Transm 109: 181–189
[Crossref], [Google Scholar], [Indexed at]
- Peng Q ‐L, Zhang Y ‐M, Liang L (2020) A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin Exp Immunol 199:314–325
[Crossref], [Google Scholar], [Indexed at]
- Svingen G, Pedersen E, Seifert R (2018) Fibrinogen and Neopterin Is Associated with Future Myocardial Infarction and Total Mortality in Patients with Stable Coronary Artery Disease. Thromb Haemost 47:778–790
[Crossref], [Google Scholar], [Indexed at]
- Pichler R, Fritz J, Heidegger I (2017) Predictive and prognostic role of serum neopterin and tryptophan breakdown in prostate cancer. Cancer Sci 108: 663–670
[Crossref], [Google Scholar], [Indexed at]
- Yalcin S, Demir ME, Ozturk R, Kılınç AŞ, Suer H, et al. (2020) Prognostic effects of SuPAR and Neopterin Levels on Patients with Lung Cancer. Pteridines. 31:136–141
[Google Scholar]
- Akyurek F, Tuncez Akyurek F (2020) Investigation of pregnancy associated plasma protein‐A and neopterin levels in Behçet's patients. Dermatol Ther 33:e13443
[Crossref], [Google Scholar], [Indexed at]
- Kemeriz F, Gönül M, Cengiz FP, Emiroğlu N, Cemil BÇ, et al. (2019) Evaluation of Neopterin Level and Disease Severity in Patients with Psoriasis Vulgaris Treated with Narrowband UVB. Indian J Dermatol 64:447-450
[Crossref], [Google Scholar], [Indexed at]
- Peng Q‐L, Zhang Y ‐M, Liang L (2020) A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin Exp Immunol 199: 314–325
[Crossref], [Google Scholar], [Indexed at]
- Akbulut HH, Celik I, Akbulut A, Yuce P, Kiliç SS (2005) Serum neopterin levels in patients with brucellosis. J Infect 51: 281-286
[Crossref], [Google Scholar], [Indexed at]
- Barutcuoglu B, Bozdemir AE, Dereli D, Parildar Z, Mutaf MI, et al. (2006) Increased serum neopterin levels in women with polycystic ovary syndrome. Ann Clin Lab Sci 36:267-272
- Yildirim Y, Gunel N, Coskun U, Pasaoglu H, Aslan S,et al. (2008) Serum neopterin levels in patients with breast cancer. Med. Oncol. 25: 403–407
- Wagner R, Hayatghebi S, Rosenkranz M, Reinwein D (1993) Increased serum neopterin levels in patients with Graves' disease. Exp Clin Endocrinol 101:249-254
[Crossref], [Google Scholar], [Indexed at]
- Aysun; Baba H (2018) Neopterin: a possible biomarker in gastrointestinal cancer. Ankara Univ Eczacilik Fak Derg. 42: 32–41
- Kondera-Anasz Z, Mertas A (1999) Level of Serum Neopterin and Interleukin-6 In Patients with Thyroid Diseases. Pteridines. 10:197–201
[Google Scholar]
- Höbarth K, Szabo N, Hallas A, Aulitzky W, Marberger M, et al. (1994) Serum neopterin as a parameter for monitoring the course of renal cell carcinoma during interferon-gamma therapy. J Clin Immunol 70: 241-244
[Crossref], [Google Scholar], [Indexed at]
- Nancey S, Boschetti G, Moussata D (2013) Neopterin Is a Novel Reliable Fecal Marker as Accurate as Calprotectin for Predicting Endoscopic Disease Activity in Patients with Inflammatory Bowel Diseases. Inflamm Bowel Dis 19:1043–1052
- El-Lebedy D, Hussein J, Ashmawy I, Mohammed AM (2017) Serum level of neopterin is not a marker of disease activity in treated rheumatoid arthritis patients. Clin Rheumatol 36:1975–1979
[Crossref], [Google Scholar], [Indexed at]
- Bonagura VR (2020) Rosenthal DW Infections that cause secondary immune deficiency. Stiehm's Immune Deficiencies 1035–1058
[Crossref], [Google Scholar]
- Lopes M, Marques P, Silva B (2021) Guillain-Barré syndrome as the first presentation of human immunodeficiency virus infection. BMC Neurol 21:321
- Purrmann R (1941) Konstitution und Synthese dessogenanntenAnhydroleukopterins. Liebigs Ann 284–292
- Rembold H, Buschmann L (1963) Struktur und Synthese desNeopterins. Chem Ber 1406–1410
- Biron CA (1994) Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol 6:530-538
[Google Scholar]
- Ding X, Li S, Zhu L (2021) Potential effects of HMGB1 on viral replication and virus infection-induced inflammatory responses: A promising therapeutic target for virus infection-induced inflammatory diseases. Cytokine Growth Factor Rev
[Crossref], [Google Scholar], [Indexed at]
- Sakurai A, Goto M (1967) Neopterin: isolation from human urine. J Biochem 61:142-145
[Crossref], [Google Scholar], [Indexed at]
- Ozger HS, Dizbay M, Corbacioglu SK (2021) the prognostic role of neopterin in COVID‐19 patients. J Med Virol. 93:1520–1525
[Crossref], [Google Scholar], [Indexed at]
- Nathan CF (1987) Secretory products of macrophages. J Clin 79:319–326
[Crossref], [Google Scholar], [Indexed at]
- Hausen A, Fuchs D, Grünewald K, Huber H, König K (1981) Urinary neopterine as marker for haematological neoplasias. Clin Chim Acta 117: 297-305
[Crossref], [Google Scholar], [Indexed at]
- Zheng B, Cao K-Y, Chan CPY (2005) Serum neopterin for early assessment of severity of severe acute respiratory syndrome. J Clin Immunol 116:18–26
- Petrova VN, Russell CA (2018) The evolution of seasonal influenza viruses. Nat Rev Microbiol 16: 47–60
- Chan CPY, Choi JWY, Cao K-Y (2006) Detection of serum neopterin for early assessment of dengue virus infection. J Infect 53: 152–158
- Reibnegger G, Auhuber I, Fuchs D (1988) Urinary neopterin levels in acute viral hepatitis Hepatology 8: 771–774
- Reibnegger G, Fuchs D, Hausen A, Werner ER, Werner-Felmayer, et al. (1989).Neopterin and viral infections: diagnostic potential in virally induced liver disease. Biomed Pharmacother 43: 287-293
[Crossref], [Google Scholar], [Indexed at]
- Nübling CM, Chudy M, Volkers P, Löwer J (2006) Neopterin levels during the early phase of human immunodeficiency virus, hepatitis C virus, or hepatitis B virus infection. Transfusion 46:1886–1891
[Crossref], [Google Scholar], [Indexed at]
- Mildvan D, Spritzler J, Grossberg SE (2005) Serum Neopterin, an Immune Activation Marker, Independently Predicts Disease Progression in Advanced HIV-1 Infection. Clin Infect Dis 40: 853–858
[Google Scholar], [Indexed at]
- Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y (2020) How COVID-19 induces cytokine storm with high mortality. Inflammation and regeneration 40:1-7
[Crossref], [Google Scholar], [Indexed at]
- Robba C, Battaglini D, Pelosi P, Rocco PRM (2020) Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med 14: 865–868
[Crossref], [Google Scholar], [Indexed at]
- Abdelmoaty MM, Yeapuri P, Machhi J (2021) Defining the Immune Responses for SARS-CoV-2-Human Macrophage Interactions. Front immunol 12
[Crossref], [Google Scholar], [Indexed at]
- Kandasamy M (2021) NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch Pharmacol 394: 561–567
[Crossref], [Google Scholar], [Indexed at]
- Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M (2021) The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology 29: 91–100
- Freeman TL, Swartz TH, (2020) Targeting the NLRP3 Inflammasome in Severe COVID-19. Front Immunol 11:1518
- De Paula, Martins R, Ghisoni K, Lim CK, Aguiar AS Jr, et al. (2018) Neopterin preconditioning prevents inflammasome activation in mammalian astrocytes. Free Radic Biol Med 115:371-382
- Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE-S, et al. (2021) The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem 476:4161–4166
[Crossref], [Google Scholar], [Indexed at]
- Zeng F, Huang Y, Guo Y (2020) Association of inflammatory markers with the severity of COVID-19: A meta-analysis. J Infect Dis 96:467–474
[Crossref], [Google Scholar], [Indexed at]
- Palabiyik SS, Girgin G, Tutkun E, Yilmaz ÖH, Baydar T, et al. (2013) Immunomodulation and Oxidative Stress in Denim Sandblasting Workers: Changes Caused by Silica Exposure. Arh Hig Rada Toksikol. 64:431–437
- Robertson J, Gostner JM, Nilsson S, Andersson L-M, Fuchs D, et al. (2020) Serum neopterin levels in relation to mild and severe COVID-19. BMC Infect Dis 20: 942
- Bellmann-Weiler R, Lanser L, Burkert F, Seiwald S, Fritsche G, et al. (2021) Neopterin Predicts Disease Severity in Hospitalized Patients With COVID-19. Open Forum Infect Dis 8:521
[Crossref], [Google Scholar], [Indexed at]
- Edén A, Kanberg N, Gostner J (2020) CSF biomarkers in patients with COVID-19 and neurological symptoms. Neurology 96:e294-300
[Crossref], [Google Scholar], [Indexed at]
- Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: An integrative review. J Res Med Sci 18: 144-157
[Google Scholar], [Indexed at]
- Razzaque MS (2021) COVID-19 pandemic: Can zinc supplementation provide an additional shield against the infection? Comput Struct Biotechnol J 19:1371-1378
[Crossref], [Google Scholar], [Indexed at]
- Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, et al. (2004) International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:99-203
[Crossref], [Google Scholar], [Indexed at]
- Samad N, Sodunke TE, Abubakar AR (2021) The Implications of Zinc Therapy in Combating the COVID-19 Global Pandemic J Inflamm Res 14:527–550
[Crossref], [Google Scholar], [Indexed at]
- Overbeck S, Rink L, Haase H (2008) Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp 56:15–30
[Crossref], [Google Scholar], [Indexed at]
- Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci 12: 88-93
[Google Scholar], [Indexed at]
- Allen JI, Perri RT, McClain CJ, Kay NE (1983) Alterations in human natural killer cell activity and monocyte cytotoxicity induced by zinc deficiency. J Lab Clin Med 102:577-589
[Google Scholar], [Indexed at]
- Murgia C, Lang CJ, Truong-Tran AQ, Grosser D, Jayaram L, et al. (2006) Zinc and its specific transporters as potential targets in airway disease. Curr Drug Targets 7: 607-627
[Crossref], [Google Scholar], [Indexed at]
- Te Velthuis AJW, Van Den Worm SHE, Sims AC, Baric RS, Snijder EJ, et al. (2010) Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog 6: e1001176
- Shang J, Wan Y, Luo C (2020) Cell entry mechanisms of SARS-CoV-2. Proc. Natl Acad Sci USA 117:11727–11734
[Crossref], [Google Scholar], [Indexed at]
- Patel VB, Zhong J-C, Grant MB, Oudit GY (2016) Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure. Circ Res 118:1313–1326
- Thomas S, Patel D, Bittel B (2021) Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection. JAMA Netw Open 4: e210369
[Crossref], [Google Scholar], [Indexed at]
- Abd-Elsalam S, Soliman S, Esmail ES (2020) Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: a Randomized, Multicenter. Trial Biol Trace Elem Res 199:3642-3646
[Google Scholar], [Indexed at]
- Ronald (2001) Ineffectiveness of Intranasal Zinc Gluconate for Prevention of Experimental Rhinovirus Colds. Clin Infect Dis 33: 1865–1870
[Crossref], [Google Scholar], [Indexed at]
- Mahalanabis D, Lahiri M, Paul D (2004) Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am J Clin Nutr 79:430–436
[Crossref], [Google Scholar], [Indexed at]
- Barnett JB, Dao MC, Hamer DH (2016) Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr Nutrition 103: 942–951
[Crossref], [Google Scholar], [Indexed at]
- Ananda, Frances, Bao B, Snell D, James (2008) Duration and Severity of Symptoms and Levels of Plasma Interleukin-1 Receptor Antagonist, Soluble Tumor Necrosis Factor Receptor, and Adhesion Molecules in Patients with Common Cold Treated with Zinc Acetate. J Infect Dis 197: 795–802
[Crossref], [Google Scholar], [Indexed at]
- Isbaniah F, Wiyono WH, Yunus F, Setiawati A, Totzke U, et al (2011) Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: results from a randomized controlled trial. J Clin Pharm Ther 36:568–576
[Crossref], [Google Scholar], [Indexed at]
- Sánchez J, Villada OA, Rojas ML (2013) Efecto del zinc aminoquelado y el sulfato de zinc en la incidencia de la infección respiratoria y la diarrea en niños preescolares de centros infantiles. Biomédica 34: 79-91
[Crossref], [Google Scholar]
- Rerksuppaphol S, Rerksuppaphol L (2019) A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatr Rep 11:15-20
[Crossref], [Google Scholar], [Indexed at]
- Al-Samkari H, Berliner N (2018) Hemophagocytic Lymphohistiocytosis. Annu Rev Pathol 13:27-49
[Crossref], [Google Scholar], [Indexed at]
- Suntharalingam G, Perry MR, Ward S (2006) Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. N Engl J Med 355: 1018–1028
[Crossref], [Google Scholar], [Indexed at]
- Fam CM, Eisenberg SP, Carlson SJ, Chlipala EA, Cox GN, et al. (2014) PEGylation Improves the Pharmacokinetic Properties and Ability of Interferon Gamma to Inhibit Growth of a Human Tumor Xenograft in Athymic Mice. J Interferon Cytokine Res. 34:759–768
[Crossref], [Google Scholar], [Indexed at]
- Rabaan AA, Al-Ahmed SH, Muhammad J (2021) Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 9: 436
[Crossref], [Google Scholar], [Indexed at]
- Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ (1983) Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol 130: 1784-1789
[Google Scholar], [Indexed at]
- Matsushita H, Hosoi A, Ueha S (2015) Cytotoxic T Lymphocytes Block Tumor Growth Both by Lytic Activity and IFNγ-Dependent Cell-Cycle Arrest. Cancer Immunol Res 3:26–36
[Crossref], [Google Scholar], [Indexed at]
- Girardi M, Glusac E, Filler RB (2003) The distinct contributions of murine T cell receptor (TCR)gammadelta+ and TCRalphabeta+ T cells to different stages of chemically induced skin cancer. Exp Med 198:747-755
[Crossref], [Google Scholar], [Indexed at]
- Ribot JC, Debarros A, Pang DJ (2009) CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17–producing γδ T cell subsets. Nat Immunol 10: 427–436
[Crossref], [Google Scholar], [Indexed at]
- Yu J, Wei M, Becknell B (2006) Pro- and Antiinflammatory Cytokine Signaling: Reciprocal Antagonism Regulates Interferon-gamma Production by Human Natural Killer Cells. Immunity 24:575–590
[Crossref], [Google Scholar], [Indexed at]
- Bao Y, Liu X, Han C (2014) Identification of IFN-γ-producing innate B cells. Cell Res 24:161–176
[Crossref], [Google Scholar], [Indexed at]
- Griffin DE (2002) Cytokines and Chemokines. Encyclopedia Virol 620-624
- Lau JF, Curt MH (2002) Mechanisms of Type I interferon cell signaling and STAT-mediated transcriptional responses. Mt Sinai J Med 69:156-168
[Google Scholar], [Indexed at]
- Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5: 375–386
[Crossref], [Google Scholar], [Indexed at]
- Itai T, Tanaka M, Nagata S (2001) Processing of tumor necrosis factor by the membrane-bound TNF-α-converting enzyme, but not its truncated soluble form. Eur j biochem 268: 2074–2082
[Crossref], [Google Scholar], [Indexed at]
- Spriggs DR, Deutsch S, Kufe DW (1992) Genomic structure, induction, and production of TNF-alpha. Immunol Ser 56:3-34
[Google Scholar], [Indexed at]
- Aggarwal BB, Gupta SC, Kim JH (2012) Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood J American Society Hematol 119:651–665
[Crossref], [Google Scholar], [Indexed at]
- Faustman DL, Davis M (2013) TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front immunol 4:478
[Crossref], [Google Scholar], [Indexed at]
- Vilcek J, Lee TH (1991) Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem 266:7313-7316
[Google Scholar], [Indexed at]
- Banner DW, D'Arcy A, Janes W (1993) Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 73: 431-445
[Google Scholar ] [Indexed at]
- Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF Receptor Superfamilies. Cell 104:487–501
[Crossref], [Google Scholar]
- Kalliolias GD, Ivashkiv LB (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12: 49–62
[Crossref], [Google Scholar], [Indexed at]
- Justiz Vaillant AA, Qurie A (2021) Interleukin. StatPearls Publishing, Treasure Island
[Indexed at]
- Narazaki M, Kishimoto T (2018) The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int J Mol Sci 19:3528
[Crossref], [Google Scholar], [Indexed at]
- Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV (2019) The Role of Interleukin 6 During Viral Infections. Front Microbiol 10:1057
- Golomb BA, Evans MA (2008) Statin Adverse Effects. Am J Cardiovasc Drugs 8: 373–418
- Fan Y, Guo T, Yan F, Gong M, Zhang X A, et al. (2020) Association of Statin Use With the In-Hospital Outcomes of 2019-Coronavirus Disease Patients: A Retrospective Study. Front Med 7:835
[Crossref], [Google Scholar], [Indexed at]
- Chow R, Im J, Chiu N (2021) The protective association between statins use and adverse outcomes among COVID-19 patients: A systematic review and meta-analysis. PLoS One16: e0253576
[Crossref], [Google Scholar], [Indexed at]
- Vuorio A, Kovanen PT (2021) statins as Adjuvant Therapy for COVID-19 to Calm the Stormy Immunothrombosis and Beyond. Front Pharmacol 11:579-548
[Crossref], [Google Scholar], [Indexed at]
- Daniels LB, Ren J, Kumar K (2021) Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. PLoS One. 16: e0254635
[Crossref], [Google Scholar], [Indexed at]
- Aparisi A, Amat-Santos IJ, Lopez OD, Marcos-Mangas M, Gonzalez-Juanatey JR, et al. (2021) Impact of statins in patients with COVID-19. Rev Esp Cardiol 637-640
- Scheen AJ (2020) Statins and clinical outcomes with COVID-19: Meta-analyses of observational studies. Diabetes Metab 47: 101-220
[Crossref], [Google Scholar], [Indexed at]
- Can U (2016) Okside-LDL ve Reseptörü Lektin Benzeri Ox-LDL Reseptör-1. Genel tıp derg 26
[Google Scholar]
- Baydar T, Palabıyık S, Sahin G (2009) Neopterin: Günümüzün Popüler Biyogöstergesi mi? Turkiye Klinikleri J Med Sci 29:1280-1291
- Murr C, Widner B, Wirleitner B, Fuchs D (2002) Neopterin as a marker for immune system activation. Curr Drug Metab 3:175-187
- Wang L, Bassiri M, Najafi R, Najafi K, Yang J, et al. (2007) Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds
- Nguyen K, Bui D, Hashemi M (2021) The Potential Use of Hypochlorous Acid and a Smart Prefabricated Sanitising Chamber to Reduce Occupation-Related COVID-19 Exposure. Risk Manag Healthc Policy 14:247–252
[Crossref], [Google Scholar], [Indexed at]
- Widner B, Mayr C, Wirleitner B, Fuchs D (2000) Oxidation of 7,8-dihydroneopterin by hypochlorous acid yields neopterin. Biochem Biophys Res Commun 275:307-311
[Crossref], [Google Scholar], [Indexed at]
- Dominguez J, Lacoma A, Prat C (2011) Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 6:157
[Crossref], [Google Scholar], [Indexed at]
- Garrod R, Marshall J, Barley E, Fredericks S, Hagan G (2007) The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD) Prim Care Respir J 16:236–240
- Lacoma A, Prat C, Andreo F, Dominguez J (2009) Biomarkers in the management of COPD. Eur Respir Rev 18: 96–104
[Crossref], [Google Scholar]
- Achar A, Ghosh C (2020) COVID-19-Associated Neurological Disorders: The Potential Route of CNS Invasion and Blood-Brain Barrier Relevance. Cells 9: 2360
- Edén A, Simrén J, Price RW, Zetterberg H, Gisslén M,et al. (2021).Neurochemical biomarkers to study CNS effects of COVID‐19: A narrative review and synthesis. J Neurochem 159: 61–77
[Crossref], [Google Scholar], [Indexed at]
- Zierhut M, De Smet MD, Gupta V (2020) Evolving Consensus Experience of the IUSG-IOIS-FOIS with Uveitis in the Time of COVID-19 Infection. Ocul Immunol Inflamm 28:709–713
[Crossref], [Google Scholar], [Indexed at]
- Smith JR, Lai TYY (2020) Managing Uveitis during the COVID-19 Pandemic. Ophthalmology 127: e65–e67
[Crossref], [Google Scholar], [Indexed at]
- Arshadi D, Nikbin B, Shakiba Y, Kiani A, Jamshidi AR, et al. (2013) Plasma level of neopterin as a marker of disease activity in treated rheumatoid arthritis patients: association with gender, disease activity and anti-CCP antibody. Int Immunopharmacol 17:763-767
[Crossref], [Google Scholar], [Indexed at]
- Cheung KS, Hung IFN, Chan PPY (2020) Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 159: 81–95
[Crossref], [Google Scholar], [Indexed at]
- Zhang J, Garrett S, Sun J (2021) Gastrointestinal symptoms, pathophysiology, and treatment in COVID-19. Genes Dis 8:385-400
[Crossref], [Google Scholar], [Indexed at]
- Grabherr F, Effenberger M, Pedrini A, Mayr L, Schwärzler J, et al. (2021) Increased Fecal Neopterin Parallels Gastrointestinal Symptoms in COVID-19. Clin Transl Gastroenterol 12
[Crossref], [Google Scholar], [Indexed at]
- Macdonald-Laurs E, Koirala A, Britton PN (2019) CSF neopterin, a useful biomarker in children presenting with influenza associated encephalopathy?. Eur J Paediatr Neurol 23: 204–213
[Crossref], [Google Scholar], [Indexed at]
- Hirsch JS, Ng JH, Ross DW (2020) Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218
[Crossref], [Google Scholar], [Indexed at]
- Wachter H, Fuchs D, Hausen A, Reibnegger G, Werner ER (1989) Neopterin as marker for activation of cellular immunity: immunologic basis and clinical application. Adv Clin Chem 27: 81-141
[Crossref], [Google Scholar], [Indexed at]
- Denz H, Fuchs D, Hausen A (1990) Value of urinary neopterin in the differential diagnosis of bacterial and viral infections. Wien Klin Wochenschr 68: 218–222
[Crossref], [Google Scholar], [Indexed at]
- Cesur S, Aslan T, Hoca NT (2014) Clinical importance of serum neopterin level in patients with pulmonary tuberculosis. Int J Mycobacteriol 3: 5-8
[Crossref], [Google Scholar], [Indexed at]
- Werner ER, Bichler A, Daxenbichler G, Fuchs D, Fuith LC, et al. (1987) Determination of neopterin in serum and urine. Clin Chem 33:62–66
[Crossref], [Google Scholar], [Indexed at]
- Hagberg L, Cinque P, Gisslen M (2010) Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 7:1-12
[Crossref], [Google Scholar], [Indexed at]
- Mahendra L, Mahendra J, Borra SK, Nagarajan A (2014) Estimation of salivary neopterin in chronic periodontitis. Indian J Dent Res 25: 794-796
[Crossref], [Google Scholar], [Indexed at]
- Zhou S-J, Sun Z-X, Liu J (2013) Neopterin concentrations in synovial fluid may reflect disease severity in patients with osteoarthritis. Scand J Clin Lab Invest 73: 344–348
[Crossref], [Google Scholar], [Indexed at]
- Tilg H, Königsrainer A, Krausler R, et al. (1992) Urinary and pancreatic juice neopterin excretion after combined pancreas-kidney transplantation. Transplant 53:804-808
[Crossref], [Google Scholar], [Indexed at]
- Ma LN, Zhang J, Chen HT, Zhou JH, Ding YZ (2011) An overview on ELISA techniques for FMD. Virol J 8:1-9
[Crossref], [Google Scholar], [Indexed at]
- Centi S, Tombelli S, Puntoni M, Domenici C, Franek M,et al (2015) Palchetti I. Detection of biomarkers for inflammatory diseases by an electrochemical immunoassay: the case of neopterin. Talanta 134:48-53
[Crossref], [Google Scholar], [Indexed at]
- Clark MF, Lister RM, Bar-Joseph (1986) M ELISA techniques. Meth Enzymol 118:742-766
[Crossref]
- Miles LE, Hales CN (1968) Labelled antibodies and immunological assay systems. Nature 219:186-189
[Crossref], [Google Scholar], [Indexed at]
- Yalow RS (1982) The limitations of radioimmunoassay (RIA). Trends Anal Chem 1:128-131
[Crossref], [Google Scholar]
- Sánchez-Regaña MM, (2000) Serum Neopterin as an Objective Marker of Psoriatic Disease Activity: Clinical Report Acta Derm. Venereol 80:185–187
[Google Scholar]
- Dutov AA, Nikitin DA, Rinchinov ZT, Tereshkov PP, Tsydendambaev PP, et al. (2007) HPLC determination of neopterin in biological liquids for clinical purposes. Russ J Phys Chem A 81:421–423
[Google Scholar]
- Carru C, Zinellu A, Sotgia S (2004) A new HPLC method for serum neopterin measurement and relationships with plasma thiols levels in healthy subjects. Biomed Chromatogr 18: 360–366
[Crossref], [Google Scholar], [Indexed at]
- Turner APF (2013) Biosensors: sense and sensibility. Chem Soc Rev 42:3184
- Sharma PS, Wojnarowicz A, Sosnowska M, Benincori T, Noworyta K, et al. (2016) Potentiometric chemosensor for neopterin, a cancer biomarker, using an electrochemically synthesized molecularly imprinted polymer as the recognition unit. Biosens Bioelectron 77:565-572
[Crossref], [Google Scholar], [Indexed at]
- Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20:355–362
[Crossref], [Google Scholar], [Indexed at]
- Zheng B, Cao KY, Chan CPY (2005) Serum neopterin for early assessment of severity of severe acute respiratory syndrome. Clin Immunol 116:18–26
- Gostner JM, Becker K, Ueberall F, Fuchs D (2015) The good and bad of antioxidant foods: An immunological perspective. Food Chem Toxicol 80:72-79
[Crossref], [Google Scholar], [Indexed at]
- Ponti G, Roli L, Oliva G (2021) Homocysteine (Hcy) assessment to predict outcomes of hospitalized Covid-19 patients: a multicenter study on 313 Covid-19 patients. Clin Chem Lab Med 59:e354–e357
[Crossref], [Google Scholar], [Indexed at]
- Vargas-Vargas M, Cortés-Rojo C (2020) Ferritin levels and COVID-19. Rev Panam Salud Publica 44:1
- Lin Z, Long F, Yang Y, Chen X, Xu L, et al. (2020) Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect 814:647–679
- Rainer TH, Chan CP, Leung MF, Leung W, Ip M, et al. (2009) Diagnostic utility of CRP to neopterin ratio in patients with acute respiratory tract infections. J Infect 58:123-130
[Crossref], [Google Scholar], [Indexed at]
- Koç DÖ, Sipahi H, Sürmeli CD, Çalık M, Bireroğlu N, et al. (2020) T. Serum Neopterin Levels and the Clinical Presentation of COVID-19. Pteridines 31:185-192
[Crossref], [Google Scholar]









