Keywords
|
| Antimicrobial; Penicillin; Voltammetry; Graphite pencil; Ex-vivo |
Introduction
|
| in vivo infections, penicillin, and their antimicrobial detections are essential in medicinal therapy and microorganisms [1-3]. The structure of the b-lactamic ring and the carboxvlic group allows for much activity and is used in aerobic and anaerobic bacteria infections [4]. Here trace penicillin assay is usually performed in the fields of food [5], meat and milk [6], clinical allergic risks [7], blood [8], and urine [9]. Recently, various analytical techniques have been developed towards this end, such as liquid chromatography tandem mass spectrometry using electro spraying in the positive ionization mode (LC-ESI-MS/ MS) [10], capillary electrophoresis [11], capillary electrophoresis based on UV vis detection [12], capillary electrophoresis or reversedphase high-performance liquid chromatography (RP HPLC) with diode array absorbance detection [13], micro-emulsion electro kinetic chromatography [14], and liquid chromatography tandem mass spectrometry [15]. All these techniques are complicated and time-consuming, aside from requiring difficult processing skills and expensive and high-technology techniques. Moreover, urine, muscle, or tissue assays are required in the complicated separation [16], acceleration [17], and electrospray ionization [18] processes. However voltammetric methods are simple, fast, and inexpensive [19,20]. Recently few researches have been conducted on antimicrobial penicillin, such as using Fourier transformation continuous cyclic voltammetry (DL: 7.0 × 10-12 M) [21] or potentiometry with ion-selective electrodes [22]. Nonetheless, these rare techniques are used for specific properties and modified working systems that are still complicated and unusable for in vivo direct analysis. Moreover, they have been used only in laboratory conditions. In this study, a simpler system that employed handheld voltammetric circuits and an inexpensive PE sensor was used for raw urine analysis. In his system, the antimicrobial penicillin peak potential was determined using CV; and under this potential, the SW analytical parameters were optimized. The results approached a much lower detection limit (5.0 ng/L) than those of the existing common method (Detection limit: 0.002 ppm, 10 μg/L, 1.5 μg/L) [14,16,23]. Thus, this simpler system can be applied to low ranges of urine assay in rat fluid instead of in human samples. Moreover, while voltammetric, spectrometric, and other chromatographic sample preparations are complicated and time-consuming, these methods are performed directly in raw fluid and in non-treated conditions. The developed method is applicable to tissue monitoring, in vivo recognition, and other functions that require antimicrobial detection. |
Experimental Procedure
|
| The electrochemical measurement system developed in this study was carried out using the second version of the new Bioelectronics-2 system from the authors’ institution. The developed circuitry was a computerized handheld voltammetric systems with a 3.0 V potential range, a 2 mA current range, a 10 pA measuring current, and a compact 3” × 2” × 1” size (as small as a cellular phone). It used a rechargeable battery with a USB power source and had a USB port data telecommunications system with a PC. It can be used for bioassays, microorganism detection, and sensor techniques for individual and laboratory applications. The PE working electrode was prepared using a 0.5 × 10 mm-diameter Hipolymer HB, and H- and 3H-grade pencil leads were used. The details of the PE had been described in previous papers [24]. Multi-walled carbon nanotubes (CNT: by catalytic CVD; outside diameter: 8 nm; inside diameter: 2-5 nm; length: 0.5-200 nm) were purified overnight prior to use via magnetic stirring in a 2 M nitric acid solution and washed using triple-distilled pure water. The mercury (HG)-immobilized CNT working electrode (HGCNT) was prepared using a paste composed of mixed CNT, metal mercury (triple-distilled C&S International. Merck), and reagent-grade (Sigma) mineral oil (4:4:2 weight ratio) [25]. The bismuth (BI)-immobilized CNT working electrode (BICNT) was prepared using the same method, and the mixed paste was inserted into a 2 mm-diameter needle-type plastic syringe, with a copper wire connected to the electric system. Ag/AgCl/ KCl was used as the reference electrode, and a platinum wire was used as the auxiliary electrode. The electrolyte solution used was 10 mL 0.1 M H3PO4, and the experiments were performed at room temperature without oxygen removal. The penicillin G (MW: 334.3901 g/mol) and all the reagents were used with standard-grade Sigma. Consequently, all the experiments were performed in an open circuit, and the SW stripping parameters were used at the optimum conditions. |
Results
|
|
Electrode potentials and square-wave stripping voltammograms
|
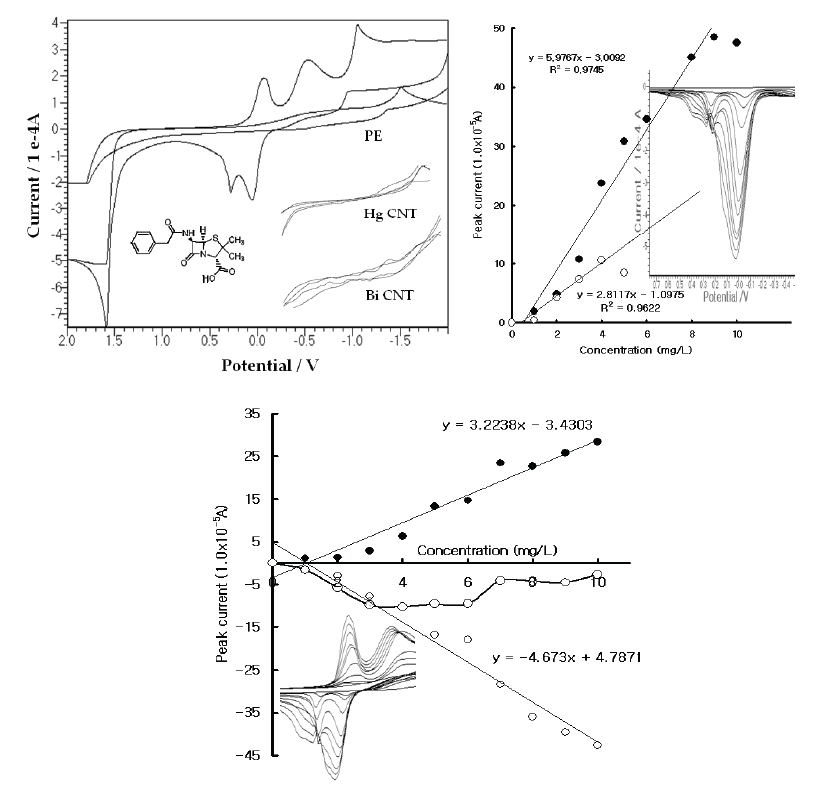
| In the wide potentials, the reaction potentials were examined in the 0.1 M H3PO4 electrolyte solution using the common and specially prepared handmade electrodes. Figure 1(A) shows the resulting CV voltammograms in the blank solution and the 30 mg/L Pen spike with the PE, HGCNT, and BICNT. In these voltammograms, both CNT sensors did not react at any potential and had very poor signals. The PE had a sensitive oxidation peak, however, and appeared at 0.0 V and 0.1 V. Moreover, -0.2 and -0.6 reduction peaks were obtained. As the cathodic can be used for voltammetric assays, more sensitive accumulation techniques were searched for. The SW stripping voltammetry was examined using the PE electrode. Figure 1(B) shows the results for the milligram-range variations. In this voltammogram, two peak potentials were obtained; the 0.0 V voltammogram continued to increase while the 0.2 V peak disappeared; and the working curves linearly increased and appeared to have been sensitive. Moreover, the CV reactions were performed using the same cell systems. Figure 1(C) show the raw voltammograms for the reduction and oxidation, which appeared at 10 points. The oxidation also appeared with two peak potentials and yielded a sharp peak width, the CV and SW stripping working conditions of which are usable for high-range analytical applications. As the precision was sensitive, the SW sensor property was examined. |
|
PE sensor stabilization and analytical optimization
|
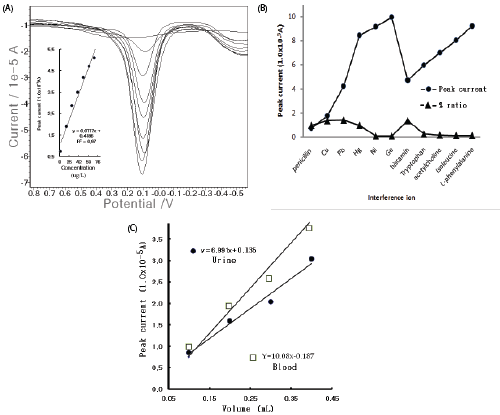
| In the optimum conditions, the electrode stability was examined using 15 repeat measurements in the electrolyte blank solutions and the 20 μg/L spike. Figure 2(A) illustrates the SW stripping peak current in the repeated conditions for the blank solutions and the 20 mg/L spike. The electrolyte blank appeared at 1.02 × 10-6 to 3.80 × 10-6 A, the standard deviation of which was 0.838. In the high 20 μg/L concentration, the first five points quickly increased and then stabilized. In this condition, the SW accumulation times and the SW amplitude variations were examined. In Figure 2(B), the calibration curve shows the accumulation time from the zero point of 0.599 × 10-3, which did not increase from 50 to 350 sec and was linear. The voltammogram in the inset shows the SW amplitude for eight points from 0.144 × 10-4 A to 1.91 × 1-4 A, which continually increased. Thus, at the 50- sec accumulation time, the 0.15 SW amplitude potential was fixed. Under this condition, other optimum values of the SW frequency, the SW incremental potential, the SW accumulation potential, and the electrolyte hydrogen ionic strength were examined (not shown here). The given standard was 10 mg/L. This behavior indicated that the optimum SW frequency of 50 Hz, the SW incremental potential of 1.0 mV, the accumulation potential of -1.7 V, and the final potential of 1.5 V were obtained, which results are not shown here. Under these conditions, the analytical working ranges were optimized. |
Discussion
|
|
Analytical linear ranges, interference, and the urine test
|
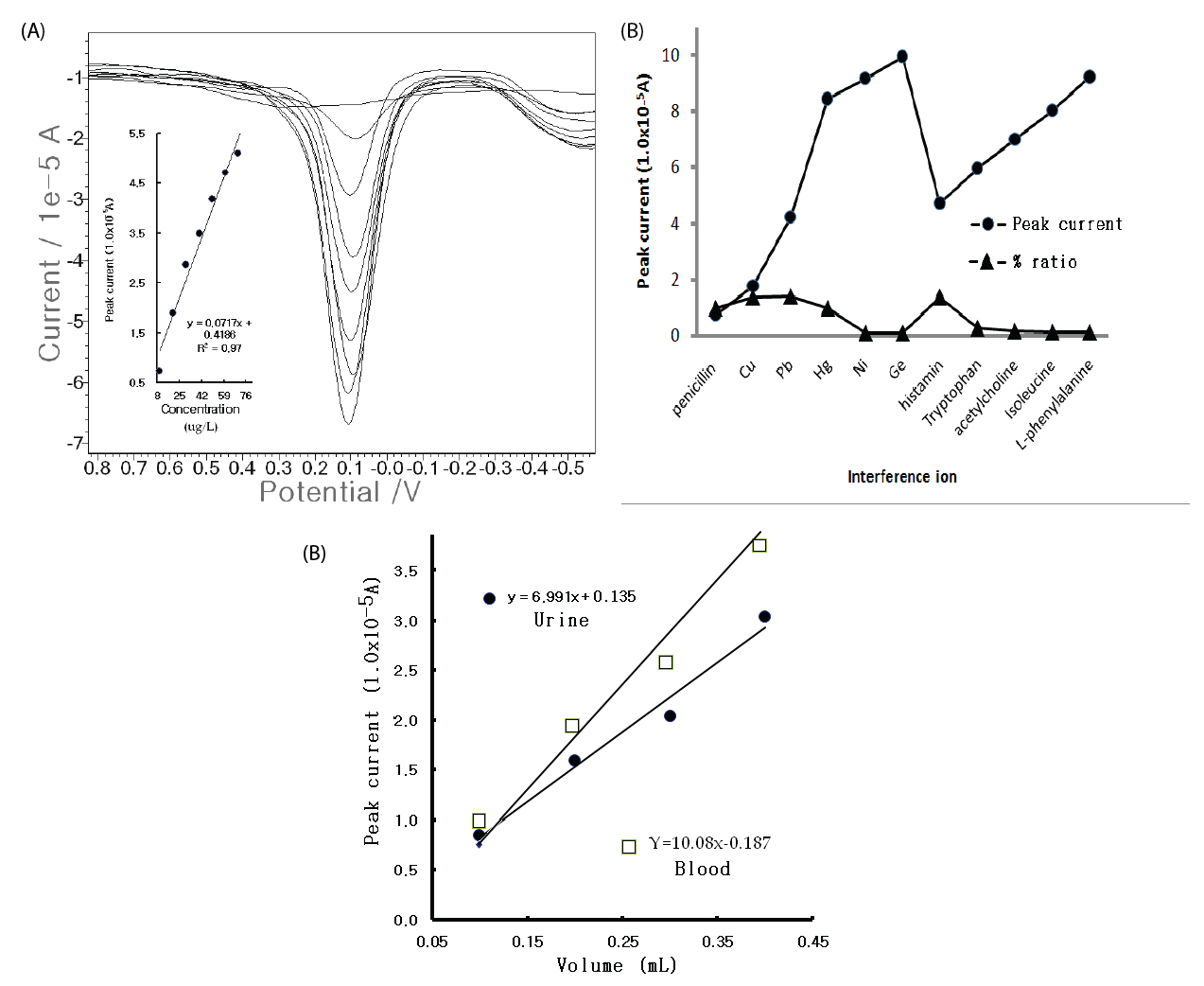
| For the optimum parameters, the usable working ranges were examined with CV and SW stripping voltammetry. Tenpoint microgram ranges were obtained from 0 to 100 μg/L, with y=0.025x+0.795 and R2=0.880, which are usable for food, blood, and clinical allergic risks, whereas the CV voltammogram was poor and had a high resolution, although the results are not shown here. Thus, more sensitive nano ranges were examined using SW, and the accumulation time was increased to 60 sec. The results are shown in Figure 3(A). Concentration increments of 0-80 μg/L and a sharp, linear and very sensitive peak width were obtained. Under these conditions, the analytical detection limit was examined using blank solutions based on the peak current from the noise characteristics of the data for S/ N=3, and 5.0 ng/L was attained. While the results are satisfactory for ex vivo and in vivo applications, various interference effects remained in the biological assay. Thus, the metal and organic ions were examined with 10 counter-ions in the 1 mg/L Pen spike. Figure 3(B) shows the results. Mercury and histamine had strong effects, but the other ions were weak and had no effect. The developed technique is applicable in any field for medicinal diagnosis and allergic risk analysis. Figure 3(C) shows the results of the tests wherein rat urine and blood samples were used instead of human fluid. Using the standard addition methods, 1 mL of the standard 1,000 mg/L Pen was injected in the rat muscle; and 20 mins after, one drop of urine and one drop of blood was extracted from the rat bladder and tail with a needle-type catheter suction. The fluid was examined using SW stripping. |
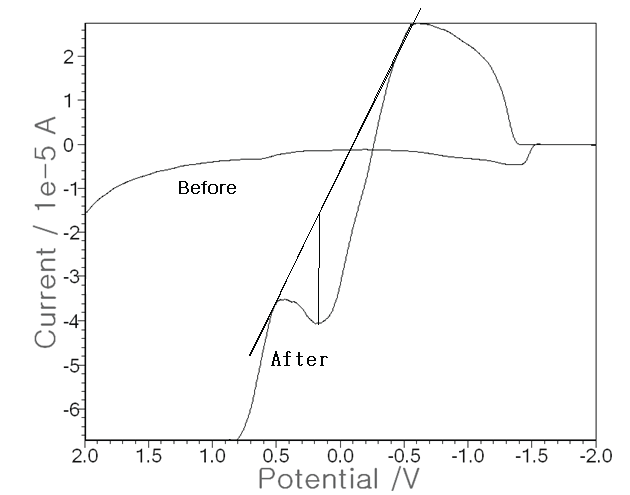
| Using the optimum conditions, more advanced techniques were attempted with the non-treated raw urine as the electrolyte solution instead of a 0.1 M NH4H2PO4 buffer. In 0.5 mL of rat urine, three electrode systems were put and SW stripping voltammetry was performed. Figure 4 shows the results. The base voltammogram (before) is that of raw urine, for which no peak current was attained and in which Pen was not injected. Then the increased voltammogram (after) is that of the 1,000 ppm Pen injected in the muscle, for which a peak of 2.30 × 10-5 A was obtained. Thus, the developed technique can be applied directly to raw urine instead of to an electrolyte buffer solution. These results are applicable to real-time diagnosis of living tissue or to fluid assays. |
Conclusion
|
| A newly fabricated handheld voltammetric system was used for antimicrobial penicillin analysis. In this system, a fast recognition time was used with only a 60 sec SW accumulation time. Moreover, simple and inexpensive PE-type working sensors were used for analytical optimization, which achieved an SW frequency of 50 Hz, an SW incremental potential of 1.0 mV, an accumulation potential of -1.7 V, and a final potential of 1.5 V. Under these conditions, sensitive working ranges and low analytical detection limits were attained. The developed system can be applied to ex vivo assays and non-treated direct urine analysis. It can also be used for in vivo and real-time assays; on nontreated pharmaceuticals, food, and drugs; and in other fields that require real-time monitoring. |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|










