Review Article - (2022) Volume 16, Issue 3
The Public Health and Clinical Importance of Amoebiasis
Rizwan Ullah*,
Mehreen Shafiq,
Mujaddad Ur Rehman,
Ibrar Khan,
Azam Hayat and
Iqra Jehangir
Department of Microbiology, Abbottabad University of Science and Technology, Pakistan
*Correspondence:
Rizwan Ullah, Department of Microbiology, Abbottabad University of Science and Technology,
Pakistan,
Tel: 03025516224,
Email:
Received: 16-Jan-2022, Manuscript No. Iphsj-22-12327;
Editor assigned: 18-Jan-2022, Pre QC No. Iphsj-22-12327 (PQ);
Reviewed: 28-Mar-2022, QC No. QC No. Iphsj-22-12327;
Revised: 02-Apr-2022, Manuscript No. Iphsj-22-12327(R);
Published:
11-Apr-2022, DOI: 10.36648/1791-809X.16.4.934
Abstract
Amoebiasis also called amoebic dysentery, first described by Fedor A. Lösch in 1875, caused by Entamoeba histolytic has great clinical impotence and is of public health significance. Histolytica has a simple life cycle involving the infective cyst that ingested through contaminated food and water and vegetative trophozoite. The pathogenesis of Entamoeba histolytica have different events like cell death, inflammation, and invasion which are performed with the help of different molecules like lectin, Amoeba pores and cysteine protease, etc. 80-90% of people infected with Entamoeba histolytica are asymptomatic (intraluminal amoebiasis) and remaining to develop manifestation like amoebic colitis, toxic megacolon, ulceration, ameboma and another extraintestinal amebiasis like amoebic liver abscess, pulmonary, cardiac and cerebral abscesses if trophozoite reaches haematogenous to these sites. The global burden caused by amebiasis is widespread. Worldwide 50 million people are affected by this disease and 100000 deaths are reported annually. The highest burden of amebiasis is in developing countries, particularly in the tropics and subtropics, where there is inadequate hygiene and access to sanitation. Microscopy, serological and molecular methods can use for diagnosis. Pharmacological therapy and surgical intervention are recommended. As there is no effective vaccine, prevention emphasizes on sanitation and access to clean drinking water.
Keywords
Amoebiasis; Dysentery; Entamoeba histolytica; Contaminated food; Water;
Sanitation
Introduction
A pseudopod-forming, non-flagellated protozoan parasite
Entamoeba histolytica causes an infection called amoebiasis or
amoebic dysentery that can be asymptomatic and self-limiting
(90%) and can also causes extensive mortality and morbidity
globally by the occurrence of diarrheal disease and abscess
formation in tissues of parenchyma such as liver(amoebic
liver abscess), lungs (Pulmonary amoebiasis), heart (cardiac
amoebiasis) and brain (cerebral amoebiasis) Amoebiasis was
first described by Fedor A. Lösch in 1875, in St. Petersburg Russia
Later, in 1903 Fritz Schaudinn has named Lösch's microorganism
causal of the dysentery, Entamoeba histolytica [1,2].
Amoebiasis is the second leading cause of death disease,
caused by parasite after malaria. In most areas of the world the
prevalence of this infection is not known because of difficulty
to identified E. histolytica and other amoebas with same
structures as Entamoeba dispar and Entamoeba moshkovskii [3].
Most diseases that show symptoms are caused by Entamoeba histolytica. Many years considered that Entamoeba dispar
was considered non-pathogenic but later it was reported
as pathogenic due to its presence in some cases of amoebic
colitis and amoebic liver abscesses [4]. Other four-nucleated
structurally similar organisms, Entamoeba moshkovskii, has been
seen in sewage as a free living but it is also enabled to colonize
the human intestine [5].
Both species Entamoeba histolytica and Entamoeba dispar are
found in two forms the hardy, infective cyst and the fragile,
potentially pathogenic trophozoite fecal oral route is the main
source of spread of these organisms. Some cases of spreading
through sexually has also been found. [6, 7] As natural hosts of this
disease are humans. When we ingest fecally-contaminated food
or water then amoebic infection occur. Mature cyst encystation
occurs in small intestine and trophozoite are released and move
to large intestine. The trophozoite increase by process of binary
fission and pass in feces and survive in external environment
for some days to weeks due to cyst wall. There ingestion causes
disease [6, 7].
The pathogenesis is mainly divided into three stages host cell
death, inflammation, and parasitic invasion by involvement of
different molecules like lectin, Amoeba pores, cysteine proteases
and EhMIF [8]. It causes the lysis of the tissues and proteolysis.
This disease is mainly found in developing countries like parts
of Central and South America, Africa, and Asia because of poor
sanitation, inadequate water treatment and low socio-economic
status. [9] Worldwide 50 million people are affected by this
disease and 100000 deaths reported annually [10]. Demographic,
behavioural, environmental and clinical characteristics that
linked with disease are counted among the risk factors in
developing countries [11]. The amoebiasis also occurs in people
in developed countries in travellers, immigrants and men who
have sex with men [2].
Diagnosis can be done by direct microscopy of stools, body fluid or
tissue sample for cysts and trophozoite. However, this organism
is seen in only 30% of patient and is nonspecific for Entamoeba
histolytica. Other test like culturing and is enzyme analysis are
also used but these methods have limitations. These limitations
are overcome by use of antigen detection or molecular method
i.e., highly sensitive and important in differentiating Entamoeba
histolytica from Entamoeba dispar and Entamoeba moshkovskii.
(Tanyuksel and Petri, 2003). Symptomatic amoebiasis requires
hydration and use of drugs such as metronidazole, tinidazole
and diloxanide furoate etc. To prevent this disease avoid using
contaminated water and foods and maintain good environment
sanitation [11].
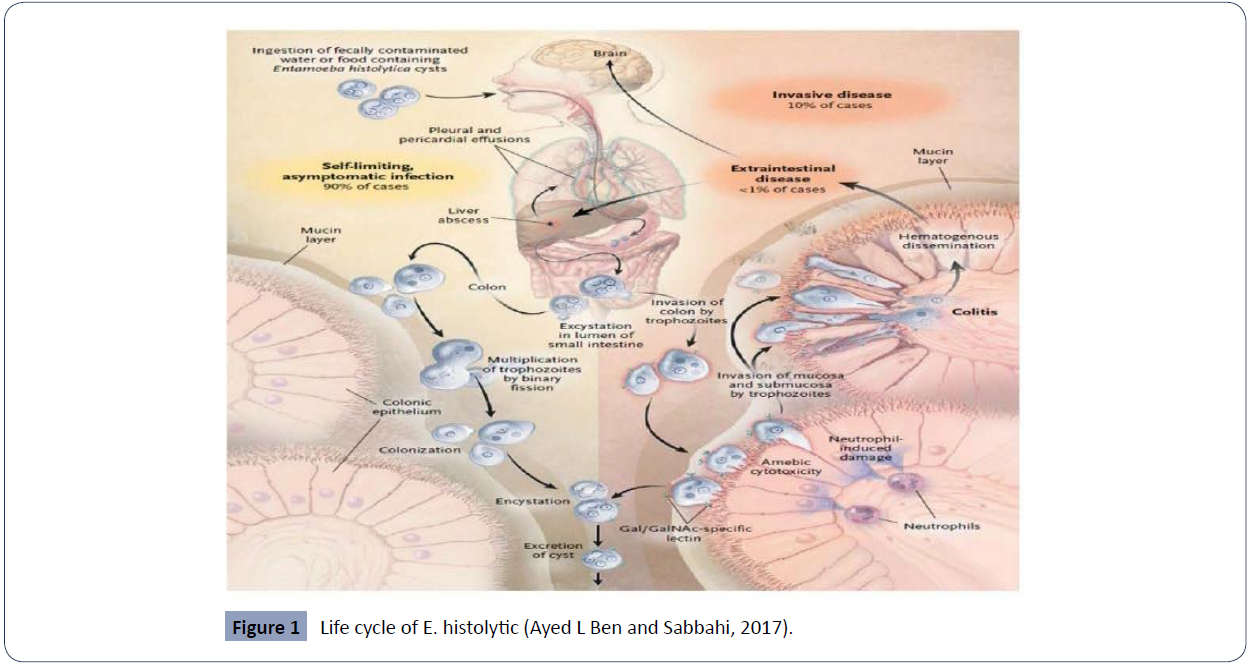
Life cycle
The life cycle of E. histolytica is simple and revolves around two
stages, either cyst that cause infection or invasive trophozoite
(Figure 1).
Figure 1 Life cycle of E. histolytic (Ayed L Ben and Sabbahi, 2017).
Infection begins when humans (natural host) ingest the cyst
found in food or water contaminated with feces. [12, 13] It
can also occur through person to person contact, swimming in
contaminated bodies of water and exposure in endemic areas
[13-16] where it can found on various material or on surfaces
including human hand that can be contaminated with feces
and lead to oral intake either directly or indirectly [12, 14 , 15]. Once the cyst is ingested, through gastrointestinal track
it reaches to small intestine and then to large intestine and
undergo encystation, which releases trophozoites of 10–20μm
in diameter in the lumen of the intestinal wall. The trophozoite
reproduces through binary fission then penetrates the colonic
mucosa, forming distinct flask-shaped ulcers. The trophozoites
can then gain access to the hepatic portal circulation and spread
to the liver, which produces an inflammatory reaction leading
to necrotic hepatocytes and subsequent abscess formation with
a characteristic ‘anchovy-paste’ exudate It also disseminates
to distant sites such as the brain and lungs hematogenously.
Symptoms may occur within weeks or years after ingestion. Cysts
and trophozoites are passed in stools. As cyst has protective all so
it can survive in environment from days to week and is responsible
for further transmission of the parasite. but trophozoite cannot
[17, 18].
Pathogenesis
The pathogenies of E. histolytica can be classified as host cell death,
inflammation, and parasitic invasion. The different mechanism
like induction of programmed cell death, phagocytosis, and
trogocytosis are used by trophozoite to kill the host cell [19].
The pathogenesis by E. Histolytica begins when parasite adhere to
colonic mucosal layer through Gal / GalNAc lectin k by targeting the
O-linked polysaccharide side chains of mucin on colonic epithelial cells (Tsukui and Nozaki, 2016). The mucin secretagogue is produce
by E. Histolytica causes hyper secretion of mucus from goblet cell
for the diminution of mucin stores [20]. Several other molecules
involved in the pathogenesis of amebiasis, namely Amoeba pores
that insertion into the membrane of target cells causing their lysis
by forming ion channels or pores in it. The hydrolytic enzymes,
cysteine proteases cause hosts epithelial and inflammatory
cells destruction and subsequent invasion. During E. histolytica
infection, 90% of all cysteine protease activities is by CP-1, CP2
and CP-5. The CP-5 is the most predominant in degradation of
the epithelial mucus layer. The secretion of cysteine protease-5
induces the cleavage of C-terminus of the MUC2 protein, which
results in protective mucin barrier degradation, later invasion
and increased hyper permeability of the gut. E. Histolytica
can also invade the host defense by Peroxiredoxin, alcohol
dehydrogenase, and lipopeptidophosphoglycan [21]. For invasive
disease, the destruction of extracellular matrix is necessary. E.
histolytica produces E. histolytica migration inhibitory factor
(EhMIF), which causes mucosal inflammation and production of
matrix metalloproteinase (MMPs) [22]. MMPs break down the
extracellular matrix in the gut to promote cell migration and are
overexpressed, thus EhMIF further contributes to disseminate
disease by producing MMPs [23, 24] during the amoebic
pathogenesis, and trogocytosis and phagocytosis of cellular
debris are important processes. A trogocytosis is new mechanism
of pathogenicity, reported for E. histolytica trophozoites, which
consists of the ingestion of pieces of living cells It begins to occur
within one minute of contact with the host cell. Host cells are
still alive when the process starts, but eventually die due to
loss of membrane integrity. Trogocytosis of amoeba include
physiological temperature, rearrangement of amoeba actin,
Gal / GalNAc lectin, EhC2PK, and signaling-PI3K. Cell death after
amoeba trogocytosis can be caused by accumulated physical
damage to bitten cells (Ralston, 2015). According to the current
studies the interaction among the host’s intestinal flora and E.
histolytica can facilitate the pathogenic behavior, generating
more virulent and enter pathogenic bacteria cause increase in
Gal/GalNAc lectin expression in trophozoites of E. histolytica
which results in increased capacity of adhesion and cytopathic
effects. In the presence of certain gut bacteria, production of
proinflammatory cytokines was also increased, causing more
impairment of epithelium and facilitate trophozoite invasion [25, 26].
Clinical manifestation
In E. Histolytic infection, only 10%–20% people develop
symptomatic infection remaining 80%-90% are asymptomatic.
The reasons for this are poorly understood but result from an
interaction of several factors related to parasite, host, and
environment [27].
Intraluminal Amebiasis
Luminal amebiasis is an asymptomatic infection. In case of
personal history of travel to/arrival from an endemic area or a
history of this in either a household or sexual contact, Screening
for asymptomatic infection is recommended [28].
Amebic Colitis
Amebic Colitis is symptomatic intestinal infection with sub-acute
onset. The characteristic symptoms of amoebic colitis are watery
or bloody diarrhoea, with abdominal cramps, pain/tenderness,
and weight loss. Disease may be limited to the ascending colon
or cecum. If diagnosis and treatment is not timely, serious
complications such as fulminant infection which can result in
massive areas of colonic involvement with perforation and
peritonitis, toxic megacolon and fistulising perianal ulcerations
can occur. A risk factor for the development of fulminant
forms of amebic colitis is corticosteroid use diabetes mellitus,
alcoholism, malignancy/chemotherapy, and pregnancy. Patients
could be toxic in appearance, febrile, and hypotensive, with
profuse bloody diarrhoea, abdominal pain, distension, and signs
of peritonism. Toxic megacolon occurs in a patient with amebic
colitis and heavy use of the ant motility agent lope amide The
toxic megacolon development has linked to corticosteroid usage
and is impassive to anti amoebic therapy, requiring instant
surgery The most uncommon manifestation occur in amoebic
colitis is formation of ameboma. the development of tumor-like
granulation tissue in the colonic lumen, can mimic colonic cancer
It is characterized by pain and swelling in the right iliac fossa, or
with symptoms of bowel obstruction.
Disseminated Amebic Disease
The most common extraintestinal amoebiasis is amebic liver
abscess. Trophozoites enter to the liver by mean of hepatic
circulation, forming micro abscesses that finally combine to form a
well-circumscribed amebic liver abscess, generally in the posterior
right lobe [28]. The abscess contains inflammatory debris, dead
hepatocytes, and amoebic trophozoites surrounded by a rim of
connective tissue, with a characteristic chocolate-colored fluid
“anchovy paste” exudate [29] The two most common symptoms
of ALA are right hypochondriac pain or constant, aching right
upper quadrant pain , and fever (38.5 to 39.5°C), which generally
presents within 2-4 weeks in 50-80% of individuals(Kannathasan
et al., 2018; Bhatia and Sundaram, 2019; Patients may also suffer
with nausea, vomiting, weakness, weight loss, and referred pain
to the shoulder in some cases. Patients may or may not present
with jaundice [30]. It also has gastrointestinal symptoms typically
without concurrent dysentery. Hepatomegaly with point
tenderness over the liver can often be detected [31] Another
uncommon complain in ALA is cough and right sided pleural pain.
It is generally due to associated pleural effusion and compression
collapse of the underlying lung parenchyma (Sharma et al., 2010).
Leucocytosis, transaminitis, and elevated alkaline phosphatase
occur during ALA and it can use in laboratory evaluation. Imaging
reveals an abscess, typically on the right hepatic lobe [31, 32].
Anemia and hypoalbuminemia are quite common in amoebic
liver abbesses in comparison to pyogenic or bacterial abscesses
[33] the second most common extraintestinal organ affected
are lungs. Pulmonary amoebiasis generally occurs by direct
extension of an ALA or it also occur by direct haematogenous
spread from intestinal lesions or by lymphatic spread [34,35] The
most affected part of the lung is the right lower or middle lobe.
Patients present the symptoms that are fever, haemoptysis, right
upper quadrant pain, and referred pain to the right shoulder or intrascapular region. When a liver abscess ruptures into the
pleural space, pulmonary abscesses, broncho hepatic fistula and
empyema can occur. Patients usually present with brown colour
“anchovy sauce-like” pus or sputum [36].
Cardiac infection is another occasional complication in amoebiasis.
It occurs due to amoebic liver abscess rupture and spread to the
pericardium, which causes end pericardial rupture which leads
to cardiac tamponade, or slow onset of pericardial effusion. It
generally occurs when abscess is in the left lobe of the liver,
which is rare, as most cases posterior right lob is affected It can
present acutely with cardiac tamponade resulting from purulent
pericarditis, or with a slowly accumulating pericardial effusion.
Symptoms include severe chest pain, shortness of breath, and
edema from congestive heart failure or constrictive pericarditis
Inferior vena cava (IVC) thrombosis is another extremely rare
complication of ALA Mechanical compression of the IVC by a
large hepatic abscess or by erosion from a posterior liver abscess
can lead to embolism of the IVC and thromboembolic disease of
the lungs (Shamsuzzaman and Hashiguchi, 2002; McKenzie et al.,
2015). The exceedingly rare and lethal complication is cerebral
amoebiasis. Which occur by Haematogenous spread to the
brain? Worldwide, the frequency of cerebral infections caused
by E. histolytica is from 0.6% to 8.1% (Barrera et al., 2012; Cruz et
al., 2004). E. histolytica produces brain abscess, with a period of
incubation from a few days to several months
Epidemiology of amebiasis
E. Histolytica has miscellaneous distribution and the global
burden caused by amebiasis is widespread. The highest burden
of amebiasis in developing countries, particularly in the tropics
and subtropics, where there is inadequate hygiene practices and
access to sanitation [35]. Worldwide, 35–50 million symptomatic
cases occur annually, leading to approximately 55,000 deaths In
the developing parts of Central and South America, Africa, and
Asia, amebiasis is endemic. The incidence of amebiasis is low,
but amebiasis-related deaths still usually occur, accounting for
at least 5 deaths per year in the United States The travellers
and immigrants, who returns from endemic countries are
mostly affected from amebiasis in united states In developing
countries, the exact burden of amebiasis is difficult to measure.
The geographic region, study design, size of sample, incubation,
symptom and symptom, and the sensitivity of the diagnostic
modality used can affect the report. In addition, diagnostic
capacities and surveillance are often less in areas where E.
histolytica is endemic (Esquivel et al., 2015). The estimated
prevalence in Pakistan is 13.6% to 63.8%. Studies on amoebiasis
in Pakistan are based on microscopic examination of feces which
results in some misinterpretation, but still significant work has
done in Pakistan [35-38].
Diagnosis of Amoebiasis
In endemic areas, patients can be diagnosed for intestinal
amoebiasis by observing the clinical signs and symptoms like
gastrointestinal discomfort and watery or bloody diarrhoea [38]
The researchers had used several laboratories based for the
diagnosis of amoebiasis like microscopic examination, serological
methods including ELISA, indirect hem agglutination assay and latex agglutination assay [39] Antigen detection and molecular
tests. Often a combination of tests is required to diagnose the
amoebiasis.
Microscopy
Different microscopic techniques like wet preparation e.g direct
saline wet mount, concentration, and permanently stained
smears are employed in a diagnostic clinical laboratory for the
identification of E. histolytica/E. dispar/E. moshkovskii in feces
[40]. The stool sample should be examined within one hour of
collection for identification of motile trophozoite but it there is
any delay in examination then stool sample should be preserved
in polyvinyl alcohol (PVA), Schaudinn’s fixative or sodium
acetate-acetic acid-formalin (SAF) (Garcia and Shimizu, 1998).
The trophozoites are more likely to be observed in loose stools
which contain mucous, pus and occult blood. Whereas cyst can
be observed in both formed and loose stool. Stool specimens
can be observed either unstained or stained with Lugol's or
D'Antoni's iodine which makes the nucleus perfectly visible By
using stains like methylene blue, Giemsa, Wright’s and iodinetrichrome,
the morphology, size and number of nuclei can be
clearly observed. The modified iron haematoxylin and Wheatley’s
trichrome stains are recommended for routine use Presence
of ingested red blood cell in cytoplasm (erythrophagocytosis)
is considered as diagnostics for dysenteric patients. This can
also use to distinguish the E. histolytica and E. dispar In case of
ALA, microscopic examination of the thick brown pus (anchovy
paste) contains dead and deformed hepatocytes, red blood
cells and some polymorphs. The common staining techniques
hematoxylins and eosin (H&E), periodic-acid Schiff (PAS)and
immune staining are used to visualize the morphological changes
in the liver tissue and differentiates the amoebas against the
surrounding cells (Bancroft and Gamble, 2008). Advantage of
microscopy is that it is widely available and require Minimal
equipment and reagents but it is time-consuming, has poor
sensitivity and specificity, multiple stools need to be submitted,
cannot differentiate from other Entamoeba spp and skilled
observer required because inadequate training and diagnostic
testing may lead to misdiagnosis.
Culture method
From more than 80 years, culture techniques are being used
for isolation of Entamoeba species by using xenic and axenic
cultural media. The xenic cultivation was introduce by Boeck
and Dr bohlav in 1925. The xenic diphasic media include egg
slant medium now modified and known as Locke-egg (Clark
and Dimond, 2002) still in use today particularly in research
studies. The axenic cultivation of E. Histolytica was introduced by
Diamond in 1961 Fecal specimens, rectal biopsy specimens, and
liver abscess aspirates can be used for culturing of E. Histolytica
(Blessmann, 2002). The cultivation of E. Histolytica in a clinical
diagnostic laboratory is not feasible as a standard procedure.
It is difficult, expensive, labour-intensive and. less sensitive
than microscopy. It is not recommended as routine diagnostic
procedure for the detection of Entamoeba species because of
overgrowth of bacteria fungi and other protozoans [40].
Isoenzyme analysis
Iso enzyme analysis of cultured amebae by means of zymodeme
enzymes assist in the differentiation of Entamoeba species A
zymodeme is a group of amoeba strains that have the same
electrophoretic pattern and mobilities for numerous enzymes
such as malic enzyme, hexokinase, glucose phosphate isomerase,
and phosphoglucomutase isoenzyme Out of 24 different
zymodemes 21 are from human isolates (nine E. histolytica and
twelve E. dispar) and three from experimentally cultured amoeba
strains. To differentiate the two Entamoeba species, band are
counted e.g. there are three zymodeme bands for E. histolytica
(II, XIV, and XIX) and one for E. dispar (The isoenzyme analysis is
difficult and time consuming It requires four to ten days to grow
significant number of trophozoite and is not always successful
The overgrowth of bacteria, fungi and protozoan is another
major problem during isoenzyme analysis. It sometime also gives
false negative results of some microscopy positive stool sample
[34, 36]. Because of its less sensitivity it is not recommended for
routine use and is replaced by molecular diagnosis
Antigen Detection
The limitations regarding diagnosis of amoebiasis through
methods like stool microscopy is overcome by antigen detection
test that is easy to use but particularly in in low endemic areas,
it has variable sensitivity and specificity For antigen detection
in fecal samples, ELISA is developed by investigators. It has
advantages than other methods being used for diagnosis of
amoebiasis like It can differentiate E. histolytica from E. dispar,
It has excellent sensitivity and specificity and Non experience
laboratory personnel can also use this test E. Histolytica specific
antigen-based ELISA kits use monoclonal antibodies for detection
of antigen like Tech Lab E. Histolytica I and Entamoeba CELISAPATH
which detect Gal/GalNAc lectin, Optimum S kit which
detect Serine-rich antigen and ProSpecT E. Histolytica micro
plate assay which detect EHSA antigen (Tanyuksel and Recently,
an E. Histolytica QUIK CHEK immunochromatographic (IC) assay,
was approved by the US Food and Drug Administration (FDA). It is
simple to perform and has a quick turnaround time [26] Antigen
detection test may have high sensitivity in endemic areas but
reduced sensitivity in nonendemic areas. It is simple to perform,
have rapid turnaround time, and is commercially available
but disadvantage is that it has poor sensitivity for amebic liver
abscess. It requires fresh, not fixative preserved stool for analysis.
Antibody Detection test
For the detection of anti-amoebic antibodies several assays are
commonly used such as ELISA, indirect hem agglutination (IHA),
indirect immunofluorescence assay (IFA), latex agglutination,
immune electrophoresis, counter immune electrophoresis (CIE),
amoebic gel diffusion test, immunodiffusion and complement
fixation test [26, 28] The most common technique that has
been used to investigate the epidemiology of symptomatic
amoebiasis due to its reliability and ease of performance is
ELISA [40] Some commercially available antibody detection
assays for extraintestinal amoebiasis are IHA Cellognost-
Amoebiasis, Novagnost Entamoeba IgG, Bichro-Latex Amibe,
I.H.A. Amoebiasis, Amoebiasis Serology microwell II EIA and RIDASCREEN IgG Entamoeba. Antibody detection method has
high sensitivity and specificity but serology remains positive for
years after resolution of infection, so less helpful in endemic
areas and more useful for travelers. Antibody response is often
detectable by the time of presentation but may need to be
repeated in 7–10 days if initially negative (Shirley et al., 2018).
Molecular Biology-Based Diagnostic Tests and
PCR
To solves the problems of microscopic or culture-based diagnosis
and take advantage of the sensitivity, specificity, and simplicity
of newer techniques, molecular biology-based technology has
become commonly used For differentiation and detection of the
Entamoeba species in stools, tissues and liver lesions aspirates,
different variants of DNA amplification techniques are used
like conventional PCR, nested PCR, real-time PCR, multiplex
PCR and loop-mediated isothermal amplification (LAMP) For
recognition and discrimination of the three Entamoeba species,
(E. Histolytica/E. dispar/E. moshkovskii) many genes are targeted
like small subunit rRNA, gene encoding a 30-kDa protein, DNA
highly repetitive sequences, haemolysin gene (HLY6), cysteine
proteinase, gene encoding serine-rich E. histolytica SREHP protein,
actin gene and tandem repeats in extra chromosomal circular
DNA (Freitas et al., 2004; Vermeil et al., 2004) some examples
include like Conventional PCR target Extra-chromosomal circular
DNA of E. histolytica, 30-kDa antigen gene of pathogenic E.
histolytica, HLY6 gene, Nested PCR target 16S-like RNA, Realtime
PCR target 18S rRNA, small subunit rRNA gene 16 s rRNA
The restriction site polymorphism analysis method involving
amplification followed by restriction fragment length
polymorphism analyses of the small- and large-subunit rDNA,
is a remarkably effective tool to evaluate different Entamoeba
species Molecular methods have high sensitivity and specificity
but expense and requirement for technical expertise may limit
use in resource-limited settings.
Rapid diagnostic test
The preferred diagnostic tool in developing countries is rapid
diagnostic tool that avoids the need of expensive equipment
(Peeling and Mabey, 2010; Chin et al., 2013). Some rapid
diagnostic tests for intestinal amoebiasis are RIDA®QUICK
Cryptosporidium/Giardia/Entamoeba Combi, RIDA Quick
Entamoeba test, E. histolytica Quik Chek, Triage Micro Parasite
Panel and Prototype of lateral flow dipstick test (Saidin et al.,
2019). The Triage Micro Parasite Panel are rapid (less than 15
min) and results can be easily read and interpreted on the test
device. Furthermore, minimal training on the assay is required.
Additionally, there is no cross-reactivity with the Triage Micro
Parasite Panel with other intestinal parasites (A. lumbricoides,
E. coli) that identified in stool samples (Dimond and Clark,
1993). The TPP kit has limitation that it cannot differentiate E.
histolytica, E. dispar and E. moshkovskii and can only use for fresh
or fresh-frozen non-preserved stools. In case of ALA, there is no
rapid diagnostic test. However, two tests that are lateral flow
dipstick test and immunochromatographic test seemed to have
good potential for rapid diagnosis of ALA.
Imaging
To detect liver abscess ultrasonography (cystic intrahepatic
hypoechoic lesion), abdominal computed tomography (nonenhancing
centre surrounded by a rim of inflammation), and
magnetic resonance imaging are good modalities [36, 37].
Treatment
Treatment of amoebiasis includes pharmacological therapy,
surgical intervention, and preventives measures. Metronidazole
and imidazole are drug of choice for treatment for amebic colitis
and amebic liver disease. For an uncomplicated amoebic liver
abscess surgical drainage is unnecessary and should avoided. If
metronidazole is not effective after 72 hours of treatment the
abscess should be drained in this case. Aspiration is largely being
replaced by percutaneous catheter drainage [37]. In patients
unsuitable for percutaneous drainage (elderly, frail, septic shock,
multilocular cysts) laparoscopy is the preferred option.
Amoebiasis can be prevented by following rudimentary hygienic
habits like proper washing of hands with soap after using of
toilet and before handling any food product, proper cleaning of
bathrooms and toilets, use of purified drinking water either by
boiling, filtration or chlorination [40].
Conclusion
Amoebiasis still remains problem for public health and of great
clinical importance especially in developing countries. Advances
in molecular epidemiology and pathogenesis have advanced
our understanding of amoebiasis causative agent E. histolytic,
but as most patients are asymptomatic (80-90%), diagnosis and
treatment can be challenging for clinicians, potentially leading
to continuous spread of the disease. Still there is no vaccine so
preventive measures like good sanitation and hygienic lifestyle
should be followed.
REFERENCES
- Ackers J P, Mirelman D (2006) Progress in research on Entamoeba histolytica pathogenesis. Curr Opin Microbiol 9: 367-373.
Indexed at, Google scholar, Crossref
- Alkofer B, Dufay C, Parienti J J, Lepennec V, Dargere S, et al. (2012)Are pyogenic liver abscesses still a surgical concern? A Western experience. HPB Surg.
Indexed at, Google Scholar, Crossref
- Alvarado-Esquivel C, Hernandez-Tinoco J, Sanchez-Anguiano LF (2015) Seroepidemiology of Entamoeba histolytic infection in general population in rural Durango, Mexico. J Clin Med Res 7: 435-9.
Indexed at, Google Scholar, Cross Ref
- Anwar A, Khan N A, Siddiqui R (2018) Combating Acanthamoeba spp. cysts what are the options? Parasites vectors 11: 1-6.
Indexed at, Google Scholar, Crossref
- Aydin C, Piskin T, Sumer F, Barut B, Kayaalp C (2010) Laparoscopic drainage of pyogenic liver abscess. J Soc Laparoendosc Surg 14: 418.
Indexed at, Google Scholar, Crossref
- Ayed L Ben, Sabbahi S (2017) Part three Specific excreted pathogens: Environmental and epidemiology aspects. Histolytica Glob Water Pathog Proj.
Indexed at, Google scholar
- Bansal A, Bansal A K, Bansal V, Kumar A (2016) Liver abscess: catheter drainage v/s needle aspiration. Inter Sur J 2:20-25.
Indexed at, Google scholar, Crossref
- Barkhurdar M, Jan S, Kakar N H, Shaheen B, Farooq M S (2019) Prevalence of Entameoba Hsitolyica in Stool Samples of Diarrheal Patients. Ann Punjab Med Coll 13: 251-254.
Indexed at, Google Scholar
- Begum S, Quach J, Chadee K (2015) Immune evasion mechanisms of Entamoeba histolytica: progression to disease. Front Micro 6: 1394.
Indexed at, Google scholar, Crossref
- Bhatia S J, Sundaram S (2019) Amoebic liver abscess with synchronous colitis: lessons learnt in recent times. J Assoc Physicians India 67: 11.
Indexed at, Google scholar
- Blessmann J, Van Linh P, Nu P A T, Thi H D, Muller-Myhsok B (2002) Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. AJTHAB 66: 578-583.
Indexed at, Google Scholar, Crossref
- Caballero-Salcedo A, Viveros-Rogel M, Salvatierra B, Tapia-Conyer R, Sepulveda-Amor J (1994) Seroepidemiology of amebiasis in Mexico. AJTHAB 50: 412-419.
Indexed at, Google Scholar, Crossref
- Castillo de la Cruz M, Luis J, Barredo G, Mendizabal guerra R, Felix I et al. (2004) Absceso Cerebral multicentrico causado por Entamoeba histolytica. Arch Neurocienc 9: 59-62.
Indexed at, Google scholar
- Chadee K, Meerovitch E (1985) Entamoeba histolytica: early progressive pathology in the cecum of the gerbil (Meriones unguiculatus) AJTHAB 34: 283-291.
Indexed at, Google scholar, Crossref
- Chin C D, Chin S Y, Laksanasopin T, Sia S K (2013) Low-cost micro devices for point-of-care testing. In Point-of-care Diagnostics on a chip Berlin Heidelberg 3-21.
Indexed at, Google Scholar, Crossref
- Chin Y T, Lim Y A L, Chong C W, Teh C S J, Yap I K S et al. (2016) Prevalence and risk factors of intestinal parasitism among two indigenous sub-ethnic groups in Peninsular Malaysia. Infect Dis Poverty 5: 77.
Indexed at, Google Scholar, Crossref
- Clark C G (1993) PCR detection of pathogenic Entamoeba histolytica and differentiation from other intestinal protozoa by riboprinting. Diagnostic molecular microbiology. Principles and applications. American Society for Microbiology, Washington DC 468-474.
Indexed at, Google scholar, Cross Ref
- Clark C G, Diamond L S (1991) Ribosomal RNA genes of ‘pathogenic and nonpathogenic’ Entamoeba histolytica is distinct. Mol Biochem Parasitol 49: 297-302.
Indexed at, Google Scholar, Cross Ref
- Clark C G, Diamond L S (2002) Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 15: 329-341.
Indexed at, Google scholar, Crossref
- Cordel H, Prendki V, Madec Y, Houze S, Paris L et al. (2013) Imported amoebic liver abscess in France. PLoS Negl Trop Dis 7: e2333.
Indexed at, Google scholar, Crossref
- Cornick S, Chadee K (2017) Entamoeba histolytica: host parasite interactions at the colonic epithelium. Tissue Barriers 5:e1283386.
Indexed at, Google scholar, Crossref
- Diamond L S (1961) Axenic cultivation of Entamoeba histolytica. Sci 134: 336-337.
Indexed at, Google Scholar, Crossref
- Diamond L S, Clark C G (1993) A Redescription of Entamoeba Histolytica Schaudinn, 1903 (Emended Walker, 1911) Separating It from Entamoeba Dispar Brumpt 1925. J Eukaryot Microbiol 40:340-344.
Indexed at, Google Scholar, Crossref
- Dolabella S S, Serrano-Luna J, Navarro-García F, Cerritos R, Ximénez C et al. (2012) Amoebic liver abscess production by Entamoeba dispar. Ann Hepatol 11: 107-117.
Indexed at, Google scholar, Crossref
- El-Dib N A (2017) Entamoeba histolytica: an overview. Curr Trop Med Rep 4: 11-20.
Indexed at, Google Scholar
- Fleming R, Cooper C J, Ramirez-Vega R, Huerta-Alardin A, Boman D et al. (2015) Clinical manifestations and endoscopic findings of amebic colitis in a United States-Mexico border city: a case series. BMC research notes 8: 781.
Indexed at, Google Scholar, Crossref
- Freitas M A R, Vianna E N, Martins A S, Silva E F, Pesquero J L et al. (2004) A single step duplex PCR to distinguish Entamoeba histolytica from Entamoeba dispar. Parasitol 128:625-628.
Indexed at, Google Scholar, Crossref
- Furst C, Gomes M, Tafuri W, Silva E (2002) Biological aspects of a Brazilian strain of Entamoeba dispar. Pathologica 94: 22-27.
Indexed at, Google scholar
- Galván-Moroyoqui J M, Del Carmen Dominguez-Robles M, Franco E, Meza I (2008) The interplay between Entamoeba and enteropathogenic bacteria modulates epithelial cell damage. PLoS Negl Trop Dis 2: e266.
Indexed at, Google scholar, Crossref
- Garcia L S (2001) Diagnostic medical parasitology. Manual of Commercial Methods in Clinical Microbiology 274-305.
Indexed at, Google Scholar, Crossref
- Garcia L S, Shimizu R Y (1998) Evaluation of intestinal protozoan morphology in human fecal specimens preserved in Eco Fix: comparison of Wheatley’s trichrome stain and Eco Stain. J Clin Microbiol 36: 1974-1976.
Indexed at, Google Scholar, Crossref
- Gardiner B J, Simpson I, Woolley I J (2015) Caught in the act a case of fulminant amoebic colitis. JMM Case Rep 2: e000081.
Indexed at, Google scholar, Crossref
- Geurts N, Opdenakker G, Van den Steen P E (2012) Matrix metalloproteinases as therapeutic targets in protozoan parasitic infections. Pharmacol Ther 133: 257-279.
Indexed at, Google scholar, Crossref
- Ghosh S, Padalia J, Moonah S (2019) Tissue destruction caused by Entamoeba histolytica parasite: cell death, inflammation, invasion, and the gut microbiome. Curr Clin Microbiol Rep 6: 51-57.
Indexed at, Google scholar, Crossref
- Gonzalez-Ruiz A, Haque R, Aguirre A, Castanon G, Hall A et al. (1994) Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J Clin Pathol 47: 236-239.
Indexed at, Google Scholar, Crossref
- Gonzalez-Ruiz A, Haque R, Rehman T, Aguirre A, Hall A et al. (1994) Diagnosis of amebic dysentery by detection of Entamoeba histolytica fecal antigen by an invasive strain-specific, monoclonal antibody-based enzyme-linked immunosorbent assay. J Clin Micro 32: 964-970.
Indexed at, Google Scholar, Crossref
- Graffeo R, Archibusacci C M, Soldini S, Romano L, Masucci L (2016) Entamoeba dispar: a rare case of enteritis in a patient living in a nonendemic area. Case Rep Gastro Med.
Indexed at, Google Scholar, Crossref
- Gunther J, Shafir S, Bristow B, Sorvillo F (2011) Amebiasis-related mortality among United States residents, 1990–2007. AJTMH 85: 1038-1040.
Indexed at, Google Scholar, Crossref
- Haider S S, Baqai R, Qureshi F M, Boorom K (2012) Blastocystis Cryptosporidium spp., and Entamoeba histolytica exhibit similar symptomatic and epidemiological patterns in healthcare-seeking patients in Karachi. Paras res 111: 1357-1368.
Indexed at, Google Scholar, Crossref
- Haque R, Huston C D, Hughes M, Houpt E, Petri Jr W A (2003) Amebiasis. New England J Med 348: 1565-1573.
Indexed at, Google scholar, Crossref
Citation: Ullah R, Shafiq M, Rehman MU, Khan I, Hayat A, et al. (2022) The Public Health and Clinical Importance of Amoebiasis. Health Sci J. Vol. 16 No. 3: 934.