Keywords
Rotifera, Fauna, Taxonomy, Turkish Thrace
Introduction
Rotifera has an important role in the continuity of matter and energy cycle in the aquatic ecosystem and abundance of rotifera spe-cies in an aquatic habitat give clues in determin-ing productivity level of this aquatic habitat (Demir et al., 2007). Besides, Rotifera is used as indicator for pollution and eutrophication because of their high reproduction rate and sensitivity to any ecological change in the waterbody (Lucinda et al., 2004).
Turkish Thrace which is located in Northwest of Turkey has borders to Balkan countries. Meriç and Tunca Rivers from Bulgaria and Arda River from Greece pour into Aegean Sea crossing the region. Besides, wetlands located in this region provide resting and staging area for birds that migrate from Europea to Asia or vice versa. Turkish Thrace is expected to have a high biodi-versity because it includes species from Balkan fauna because of its geological location.
In addition, agricultural activities are carried out by using modern technologies in Turkish Thrace, due to its vast lowlands and productive plains. Pollution caused by agriculture, industry and excessive population growth destroys a huge amount of freshwater ecosystems and causes more pollution at the wetlands in the region espe-cially by expanding via Meriç and Ergene rivers. Therefore many living organisms disappear or migrate to other regions.
There were limited number of researches done concerning Rotifera fauna of Turkish Thrace up to now and as a result of these studies, 82 species were identified in Turkish Thrace (Segers et al., 1992; Güher, 2003; Güher et al., 2004; Erdogan and Güher, 2005; Güher and Erdogan, 2008). But, when reasons pointed out above are consid-ered, there may be the possibility of new species arrivals or extinction, the studies performed seems insufficient. So, biological diversity have to be brought to light at once, in order to keep track of the possible alterations that might occur and take the necessary precautions in wetlands in Turkish Thrace.
Materials and Methods
This study was done between September 2007–April 2009, to determine freshwater rotif-era species of Turkish Thrace (Edirne, Tekirdag, Kirklareli). Samples were collected from 126 dif-ferent localities including all kinds of freshwater ecosystems like lakes, ponds, rivers and streams by using plankton net (55μm mesh size) (Figure 1). Not only horizontal sampling by using simple plankton net but also vertical sampling by using Hensen type plankton net were done in big and deep lakes like Altinyazi and Kadiköy dams. Furthermore, it was carried out by using small hand nets in shallow densely planted ecosystems. The names of the localities, sampling dates, co-ordinates and the numbers of the localities are given in Table 1.
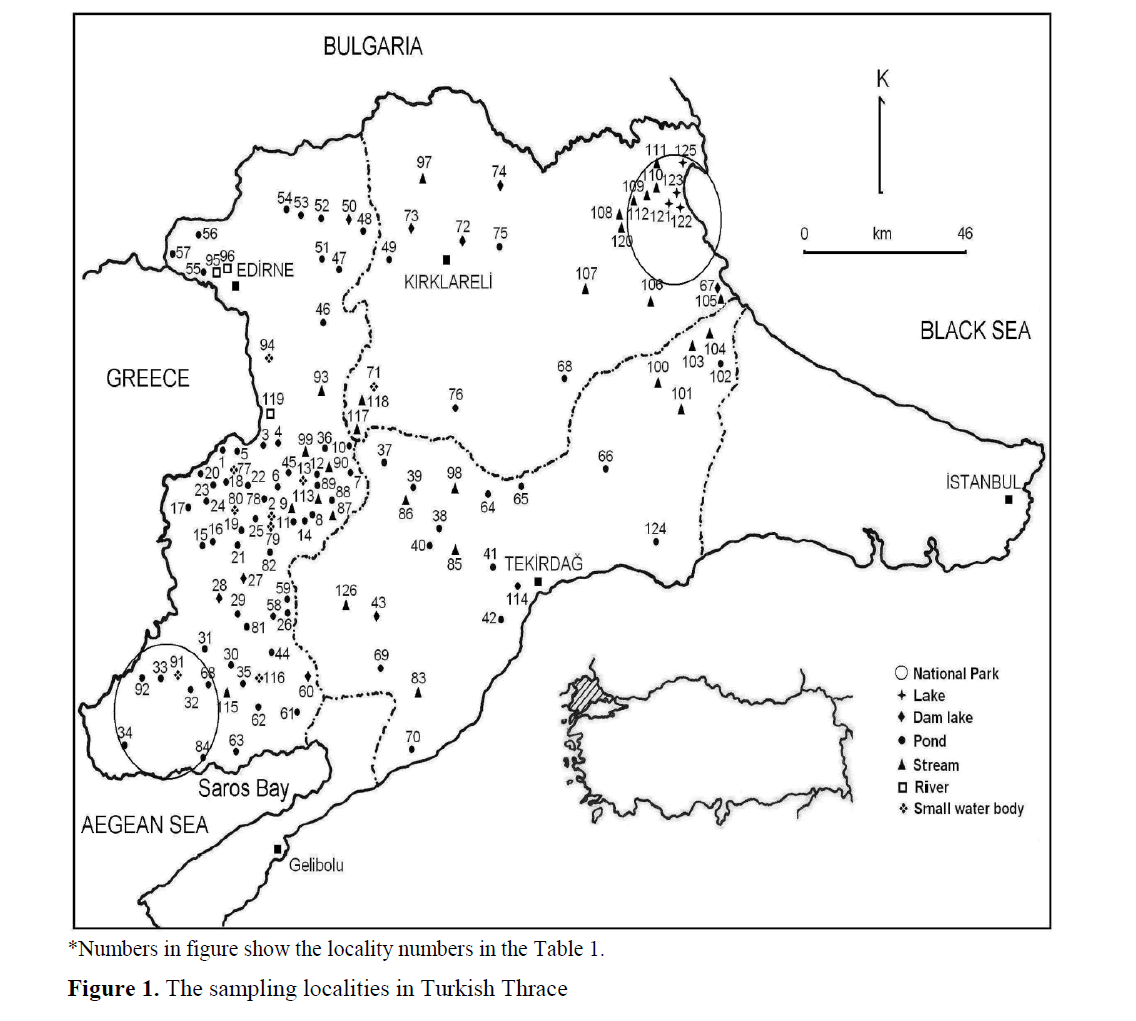
Figure 1. The sampling localities in Turkish Thrace

Table 1. Sampling localities and dates in Turkish Thrace
Samples were fixed in 4% formalin. Rotifera species were examined under the microscope of Olympus brand. By using diluted sodium hypo-chlorite, Trophi were isolated from some speci-men, and were prepared for light microscope. In identification of rotifer species, utilized by Kolisko (1974); Koste (1978a,b); Pontin (1978); Koste and Shiel (1989, 1990); Segers (1995); Jersabek et al. (2003).
Also during field survey physicochemical pa-rameters like pH, dissolved oxygen, electric con-ductivity, and water temperature were measured at 65 localities in Turkish Thrace in order to indi-cate the general characteristics of three cities. These localities are given at Table 2.
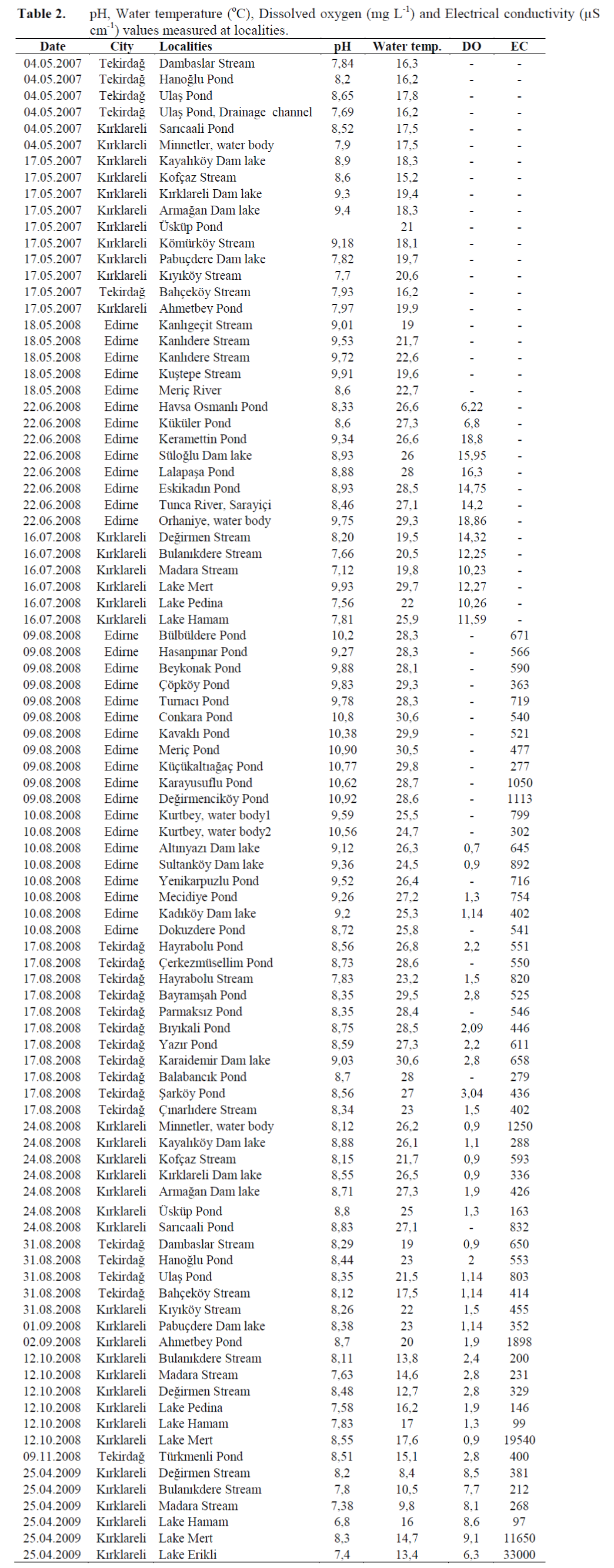
Table 2. pH, Water temperature (°C), Dissolved oxygen (mg L-1) and Electrical conductivity (μS cm-1) values measured at localities.
Results and Discussion
As a result of the examination of rotifer sam-ples taken from 126 localities in Turkish Thrace (Edirne, Tekirdag, Kirklareli) between September 2007-April 2009, a total of 115 Rotifera species belonging to 22 families have been identified. 47 of these species (referenced with stars) were new records for Turkish Thrace. Below is distribution according to localities and ecological properties determined for the species.
Philodina megalotrocha Ehrenberg, 1832: Locs. 1, 4, 9, 10, 14, 15, 20, 22, 26, 31, 35, 39, 41, 42, 43, 46, 47, 50, 54, 55, 57, 60, 62, 63, 65, 66, 67, 68, 69, 71, 75, 86, 94, 97, 98, 99, 101, 102, 104, 105, 107, 108, 110, 115, 116, 117, 118, 119, 121, 125.
It was found on Myriophylum sp., Thypa sp., Potamogeton sp. and Phragmites sp. pH: 7.2-9.9, Water temp.: 15-28 °C, DO: 1.3 mg L-1, EC: 206-754 μS cm-1.
*Rotaria neptunia (Ehrenberg, 1832): Locs. 23, 25, 38, 67, 68.
pH: 8.3; Water temp.: 29.5 °C; DO: 2.8 mg L-1; EC: 525 μS cm-1.
Dissotrocha aculeata (Ehrenberg, 1832): Locs. 4, 42, 71, 108.
It was found on Thypa sp. and Ceratophyllum sp. pH: 8.1-8.5; Water temp.: 26.2-27.3 °C; DO: 0.9-2.2 mg L-1; EC: 611-1250 μS cm-1.
*Adineta sp.: Loc. 71.
pH: 8.1; Water temp.: 26.2 °C; DO: 0.9 mg L-1; EC: 1250 μS cm-1.
Habrotrocha sp.: Locs. 4, 39, 55, 57, 66, 71, 97, 117, 122.
It was found on Potamogeton sp., Myriophyllum sp. and Phragmites sp. pH: 8.2; Water temp.: 19 °C; DO: 0.9 mg L-1; EC: 650 μS cm-1.
*Epiphanes macroura (Barrois & Daday, 1894): Locs. 12, 17, 25.
pH: 10.9; Water temp.: 30.5 °C; EC: 477 μS cm-1.
Proalides tentaculatus De Beuchamp, 1907: Locs. 2, 9, 17, 24, 26, 29, 44, 58.
*Proalides subtilis (Rodewald, 1940): Locs. 2, 6, 10, 14, 23, 29, 30, 31.
pH: 9.2; Water temp.: 28.3 °C; EC: 566 μS cm-1.
*Anuraeopsis coelata (De Beuchamp, 1932): Locs. 16, 17, 24, 30.
Anuraeopsis fissa Gosse, 1851: Locs. 1, 6, 8, 9, 14, 16, 23, 25, 30, 37, 58, 67, 88, 94, 105, 121, 122.
It was found on Trapa natans. pH: 7.5-9.7; Water temp.: 16.2-29.3 °C; DO: 1.3-1.9 mg L-1; EC: 99-550 μS cm-1.
Anuraeopsis navicula Rousselet, 1911: Locs. 1, 2, 5, 6, 8, 9, 10, 14, 17, 22, 23, 24, 26, 29, 30, 31, 35, 36, 44, 45, 116, 122.
It was found on Potamogeton sp. pH: 7.8; Water temp.: 17°C; DO: 1.3 mg L-1; EC: 99 μS cm-1.
Brachionus angularis Gosse, 1851: Locs. 1, 2, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42, 43, 44, 45, 46, 48, 53, 55, 56, 57, 58, 60, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 76, 77, 78, 79, 80, 81, 82, 86, 88, 89, 91, 92, 94, 95, 96, 98, 114, 116, 119, 121, 122.
It was found on Myriophyllum sp. pH: 6,8-10,3; Water temp.: 16-29.9 °C; DO: 0.9-6.2 mg L-1; EC: 97-1250 μS cm-1.
Brachionus bidentatus (Anderson, 1889): Locs. 12, 15, 25, 55, 119.
pH: 8,6- 10,7; Water temp.: 22.7- 29.8 °C; EC: 277 μS cm-1.
Brachionus budapestinensis Daday, 1885: Locs. 2, 5, 9, 12, 23, 45, 48, 56, 94, 108
pH: 9.7, Water temp.: 29.3; °C; DO: 18.8 mg L-1.
Brachionus calyciflorus Pallas, 1766: Locs. 4, 5, 6, 7, 10, 11, 12, 13, 14, 16, 17, 19, 21, 23, 25, 26, 27, 28, 33, 34, 41, 43, 44, 45, 46, 49, 52, 53, 56, 65, 66, 68, 71, 72, 73, 74, 76, 77, 78, 79, 80, 82, 89, 92, 94, 96, 115, 116, 117, 119, 121.
pH: 8.6; Water temp.: 17.8 °C.
Brachionus diversicornis (Daday, 1883): Locs. 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 21, 24, 25, 26, 27, 29, 30, 31, 32, 36, 37, 41, 44, 46, 49, 54, 55, 56, 58, 60, 66, 76, 80, 88, 94, 109, 112.
pH: 8.3-10.9; Water temp.: 25.3-30.6 °C; DO: 0.7- 6.2 mg L-1; EC: 277-832 μS cm-1.
Brachionus falcatus Zacharias, 1898: Locs. 4, 7, 13, 17, 18, 20, 22, 40, 42, 46, 47, 55, 59, 64, 68, 70, 71, 105, 122.
pH: 6.8-10.6; Water temp.: 16-29.9 °C; DO: 0.9-6.8 mg L-1; EC: 97-1250 μS cm-1.
*Brachionus forficula Wierzejski, 1891: Locs. 1, 3, 5, 6, 7, 14, 15, 16, 17, 23, 24, 26, 46, 89, 94.
pH: 8.3-10.9; Water temp.: 26.6-30.5 °C; DO: 6.22 mg L-1; EC: 277-719 μS cm-1.
Brachionus leydigi Cohn, 1862: Locs. 1, 5, 12, 13, 14, 25, 28, 33, 37, 38, 41, 61, 68, 66, 93, 96, 98, 101, 112.
pH: 7.6-9.2; Water temp.: 16.2-28.3 °C; EC: 566 μS cm-1.
Brachionus patulus (O.F.Müller, 1786): Locs. 121 and 122
It was found on Myriophyllum sp., Ceratophyl-lum sp. and Trapa natans. pH: 7,5; Water temp.: 16.2 °C; DO: 1.9 mg L-1; EC: 146 μS cm-1.
Brachionus plicatilis O.F.Müller, 1786: Locs. 117, 123, 125.
pH: 7.4-9.7; Water temp.: 13.4-22.6 °C; DO: 6.3-9.1 mg L-1; EC: 11650 μS cm-1.
Brachionus quadridentatus Hermann, 1783: Locs. 2, 4, 9, 12, 22, 23, 25, 26, 29, 41, 42, 44, 45, 52, 53, 55, 56, 57, 60, 63, 68, 77, 80, 83, 89, 93, 96, 101, 112, 116, 117, 119, 121, 122, 123.
It was found on Thypa sp., Myriophyllum sp., Ceratophyllum sp., Phragmites sp., Potamogeton sp. and Trapa natans. pH: 6.8-10.5; Water temp.: 16-27.3 °C; DO: 0.9-2.2 mg L-1; EC: 97-611 μS cm-1.
Brachionus urceolaris (O.F.Müller, 1773): Locs. 1, 4, 5, 7, 9, 12, 14, 21, 25, 33, 37, 38, 41, 42, 43, 46, 48, 49, 53, 54, 55, 56, 59, 60, 66, 71, 73, 76, 77, 80, 83, 86, 89, 93, 94, 95, 96, 99, 114, 115, 116, 117, 119, 121, 122.
pH: 9.5-9.7; Water temp.: 21.7-22.6 °C.
Keratella cochlearis (Gosse, 1851): Locs. 1, 4, 5, 7, 8, 12, 14, 16, 17, 18, 19, 20, 21, 24, 25, 27, 28, 31, 33, 36, 37, 38, 39, 40, 41, 43, 44, 46, 47, 49, 50, 51, 52, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 81, 88, 89, 92, 95, 96, 101, 102, 105, 114, 119, 124.
pH: 7.7-10.6; Water temp.: 15.1-30.6 °C; DO: 0.9-6.8 mg L-1; EC: 163-1250 μS cm-1.
Keratella tecta (Lauterborn, 1900): Locs. 1, 2, 4, 5, 6, 7, 8, 9, 11, 13, 15, 16, 17, 18, 19, 21, 22, 23, 24, 26, 27, 28, 29, 31, 33, 36, 37, 38, 44, 45, 46, 48, 49, 50, 52, 53, 54, 55, 56, 58, 60, 63, 67, 68, 69, 76, 79, 81, 88, 92, 94, 105, 116, 119, 122, 124.
pH: 6.8-9.8; Water temp.: 16-29.8 °C; DO: 8.6 mg L-1; EC: 97-892 μS cm-1.
Keratella tropica (Apstein, 1907): Locs. 9, 13, 19, 20, 25, 26, 27, 28, 31, 33, 43, 44, 45, 46, 48, 58, 59, 63, 66, 68, 73, 91
It was found on Potamogeton sp.. pH: 9.3-10.6; Water temp.: 24.5-28.7 °C; DO: 0.9-18.8 mg L-1; EC: 799-1050 μS cm-1.
Keratella quadrata (O.F.Müller, 1786): Locs. 2, 4, 11, 13, 14, 25, 26, 27, 28, 33, 38, 40, 41, 43, 44, 46, 47, 49, 50, 51, 52, 57, 58, 59, 60, 61, 63, 64, 66, 67, 68, 71, 72, 73, 76, 92, 95, 96, 97, 98, 101, 115, 117, 122, 125, 126.
pH: 7.4-9.7; Water temp.: 13.4-27.3 °C; DO: 6.3 mg L-1; EC: 99-1250 μS cm-1.
*Kellicottia longispina (Kellicott, 1879): Locs. 47, 48, 50, 54, 73, 74, 75.
pH: 8.7-8.9; Water temp.: 18.3-27.3 °C; DO: 1.9 mg L-1; EC: 426 μS cm-1.
Notholca acuminata (Ehrenberg, 1832): Locs. 26, 33, 57, 66, 72, 73, 75, 86, 92, 93, 98, 99, 102, 123, 125.
pH: 8.3-9.3; Water temp.: 14.7-21 °C; DO: 9.1 mg L-1; EC : 11650 μS cm-1.
Notholca squamula (O.F.Müller, 1786): Locs. 1, 5, 9, 11, 12, 14, 23, 37, 42, 46, 55, 63, 64, 66, 67, 70, 71, 72, 75, 76, 83, 84, 89, 90, 94, 99, 100, 102, 108, 112, 117, 120, 126.
pH: 9.5; Water temp.: 19.4-22.6 °C.
Platyias quadricornis (Ehrenberg, 1832): Locs. 4, 71, 72, 117, 121.
It was found on Thypa sp.. Trapa natans and Ceratophyllum sp.. pH: 8.1-9.7; Water temp.: 21.7-26.2 °C; DO: 0.9 mg L-1; EC: 1250 μS cm-1.
Euchlanis dilatata Ehrenberg, 1832: Locs. 2, 4, 7, 9, 18, 20, 33, 39, 40, 41, 42, 43, 46, 47, 48, 50, 54, 55, 57, 63, 64, 65, 66, 67, 72, 73, 83, 86, 94, 98, 99, 100, 104, 105, 106, 107, 108, 110, 115, 117, 118, 119, 124, 126.
It was found on Thypa sp., Myriophyllum sp., Potamogeton sp., Ceratophyllum sp. and Phrag-mites sp..
pH: 7.7- 9.9; Water temp.: 17.5-28.5 °C; DO: 0.9-6.8 mg L-1; EC: 414-1113 μS cm-1.
Euchlanis meneta Myers, 1930: Locs. 4, 12, 39, 54, 65, 69, 71, 83, 117.
It was found on Thypa sp.. pH: 8.3-9.7; Water temp.: 21.7-28 °C; DO: 0.9-16.3 mg L-1; EC: 402-1250 μS cm-1.
*Euchlanis deflexa (Gosse, 1851): Locs. 9, 18, 21, 33, 39, 40, 41, 42, 47, 48, 55, 69, 70, 83, 86, 92, 93, 95, 96, 98, 99, 100, 101, 102, 104, 105, 106, 107, 108, 110, 115, 121, 122, 126.
It was found on Thypa sp. and Potamogeton sp.. pH: 6.8-8.5; Water temp.: 10.5-27.3 °C; DO: 1.1- 2.8 mgL-1; EC: 97-611 μS cm-1.
*Euchlanis incisa Carlin, 1939: Locs. 26, 104, 105, 110.
pH: 7.6-8.1; Water temp.: 14.6-17.5 °C; DO: 1.1-2.8 mg L-1; EC: 231-414 μS cm-1.
*Euchlanis lyra Hudson, 1886: Locs. 13, 16, 39, 41, 42, 63, 66, 72, 75, 83, 86, 93, 98, 100, 104, 106, 107, 120.
pH: 8.2-9.3; Water temp.: 8.4-19.4 °C; DO: 8.5 mg L-1; EC: 381 μS cm-1.
Mytilina mucronata (Müller, 1773): Loc. 121.
pH: 6.8; Water temp.: 16 °C; DO: 8.6 mg L-1; EC: 97 μS cm-1.
Mytilina ventralis (Ehrenberg, 1832): Locs. 47.
It was found on Potamogeton sp..
Lopocharis salpina (Ehrenberg, 1834): Locs. 6, 26, 38, 47, 48, 63, 71, 110, 117, 121, 122.
It was found on Trapa natans. pH: 7.6-9.7; Water temp.: 14.6-27.3 °C; DO: 0.9- 6.8 mg L-1; EC: 231-1250 μS cm-1.
Trichotria pocillum (O.F.Müller, 1776): Locs. 47, 71, 72, 73, 77, 82, 105, 117, 121.
It was found on Myriophyllum sp.. pH: 7.9-9.7; Water temp.: 17.5-22.6 °C.
Trichotria tetractis (Ehrenberg, 1830): Locs. 33, 39, 47, 57, 65, 72, 86, 117, 124.
It was found on Thypa sp. and Myriophyllum sp.. pH: 7.8-9.7; Water temp.: 15.1-27.3 °C; DO: 1.5-6.8 mg L-1; EC: 400-820 μS cm-1.
Colurella adriatica Ehrenberg, 1831: Locs. 26, 33, 39, 64, 66, 70, 83, 94, 102, 105, 107, 108, 110, 117, 121, 122, 123.
It was found on Thypa sp., Potamogeton sp., Myriophyllum sp., Ceratophyllum sp. and Trapa natans. pH: 7.8-9.7; Water temp.: 10.5-22.6 °C; DO: 7.7 mg L-1; EC: 212 μS cm-1.
Colurella uncinata (Müller, 1773): Locs. 39, 63, 66, 121, 122.
It was found on Myriophyllum sp., Potamogeton sp., Trapa natans, Ceratophyllum sp..
Colurella colurus (Ehrenberg, 1830): Locs. 65, 72, 82, 97, 106, 107, 120.
pH: 8.2-9.1; Water temp.: 8.4-26.5 °C; DO: 8.5 mg L-1; EC: 336-381 μS cm-1.
Lepadella acuminata (Ehrenberg, 1834): Locs. 22, 57, 76, 121, 122.
It was found on Thypa sp., Potamogeton sp., Trapa natans and Myriophyllum sp.. pH: 8.83; Water temp.: 27.1 °C; EC: 832 μS cm-1.
Lepadella patella (O.F.Müller, 1786): Locs. 40, 46, 47, 65, 70, 105, 110.
It was found on Myriophyllum sp.. pH: 7.6-8.2; Water temp.: 14.6-22 °C; DO: 1.5-2.8 mg L-1; EC: 231-455 μS cm-1.
Lepadella ovalis (O.F.Müller, 1786): Locs. 12, 13, 14, 42, 47, 97, 100, 105, 110, 117, 121.
It was found on Potamogeton sp. and Myriophyllum sp.. pH: 7.3-9.7; Water temp.: 9.8-22.6 °C; DO: 1.5 mg L-1; EC: 268-455 μS cm-1.
*Lepadella ehrenbergi (Perty, 1850): Loc. 39.
It was found on Potamogeton sp.. pH: 8.5; Water temp.: 26.8 °C; DO: 2.2 mg L-1; EC: 551 μS cm-1.
Lecane bulla (Gosse, 1851): Locs. 4, 7, 10, 12, 14, 22, 26, 33, 39, 41, 42, 47, 48, 55, 63, 65, 66, 71, 98, 105, 117, 121, 122.
It was found on Thypa sp., Potamogeton sp., Phragmites sp., Myriophyllum sp., Trapa natans and Ceratophyllum sp.. pH: 7.5-9.7; Water temp.: 16.2-28.3 °C; DO: 0.9-18.8 mg L-1; EC: 146-1250 μS cm-1.
Lecane closterocerca (Schmarda, 1859): Locs. 39, 47, 57, 65, 66, 67, 72, 98, 99, 105, 110, 117, 121, 122, 123.
It was found on Potamogeton sp., Thypa sp., Trapa natans, Ceratophyllum sp. and Myriophyllum sp.. pH: 7.7-9.7; Water temp.: 19- 26.8 °C; DO: 0.9-2.2 mg L-1; EC: 336-803 μS cm-1.
Lecane hamata (Stokes, 1896): Locs. 4, 22, 25, 31, 39, 47, 46, 66, 94, 98, 121, 122.
It was found on Thypa sp., Potamogeton sp., Myriophyllum sp., Trapa natans and Cera-tophyllum sp..
pH: 8.2-9.7; Water temp.: 19-29.3 °C; DO: 0.9-18.8 mg L-1; EC: 650-799 μS cm-1.
*Lecane hastata (Murray, 1913): Locs. 5, 6, 9, 10, 12, 14, 25, 30, 31, 35, 38, 41, 61, 116.
pH: 8.3-9.5; Water temp.: 25.5-29.5 °C; DO: 2.8 mg L-1; EC: 525-799 μS cm-1.
Lecane inermis (Bryce, 1892): Locs. 4, 9 ve 53
It was found on Thypa sp.. pH: 8.5; Water temp.: 26.8 °C; DO: 2.2 mg L-1; EC: 551 μS cm-1.
Lecane furcata (Murray, 1913): Locs. 7, 9, 22, 39, 47, 58, 105, 121, 122.
It was found on Thypa sp., Nuphar lutea, Po-tamogeton sp., Trapa natans, Ceratophyllum sp. and Myriophyllum sp.. pH: 8.2-9.7; Water temp.: 22-28.3 °C; DO: 1.5 mg L-1; EC: 455-719 μS cm-1.
*Lecane flexilis (Gosse, 1886): Locs. 71, 105, 121.
It was found on Myriophyllum sp. pH: 8.2; Water temp.: 22 °C; DO: 1.5 mg L-1; EC: 455 μS cm-1.
*Lecane lamellata (Daday, 1893): Loc. 123.
pH: 8.3-8.5; Water temp.: 14.7-17.6 °C; DO: 9.1 mg L-1; EC: 11650-19540 μS cm-1.
Lecane lunaris (Ehrenberg, 1832): Locs. 13, 26, 33, 39, 40, 42, 47, 54, 55, 57, 65, 66, 67, 75, 79, 83, 105, 108, 110, 117, 121.
It was found on Thypa sp.,Potamogeton sp., Myriophyllum sp., Phragmites sp. and Cera-tophyllum sp.
pH: 7.7-9.7; Water temp.: 20.6-28 °C; DO: 2.2-14.75 mg L-1; EC: 551 μS cm-1.
Lecane luna (O.F.Müller, 1776): Locs. 6, 7, 10, 12, 14, 26, 33, 39, 41, 42, 47, 48, 55, 59, 63, 64, 65, 66, 67, 69, 71, 72, 73, 74, 75, 79, 96, 117, 121, 124.
It was found on Thypa sp., Potamogeton sp., Phragmites sp., Myriophyllum sp., Trapa natans and
Ceratophyllum sp.. pH: 7.6-10.8; Water temp.: 15.1-30.6 °C; DO: 0.9-14.2 mg L-1; EC: 163-1250 μS cm-1.
Lecane nana (Murray, 1913): Locs. 13, 105, 122.
pH: 8.2; Water temp.: 22 °C; DO: 1.5 mg L-1; EC: 455 μS cm-1.
Lecane quadridentata (Ehrenberg, 1832): Locs. 39, 42, 47.
It was found on Potamogeton sp. pH: 8.5; Water temp.: 26.8°C; DO: 2.2 mg L-1; EC: 551 μS cm-1.
Lecane stenroosi (Meissner, 1908): Locs. 5, 41, 45, 67, 68, 79.
pH: 8.3-8.7; Water temp.: 20-23 °C; DO: 1.1-1.9 mg L-1; EC: 352-1898 μS cm-1.
*Lecane pyriformis (Daday, 1905): Locs. 22, 39, 105, 122.
It was found on Thypa sp., Potamogeton sp. and Trapa natans. pH: 8.2; Water temp.: 22 °C; DO: 1.5 mg L-1; EC: 455 μS cm-1.
*Lecane stichaea Hauer, 1940: Locs. 105, 121.
It was found on Ceratophyllum sp., Trapa natans and Myriophyllum sp. pH: 8.2; Water temp.: 22 °C; DO: 1.5 mg L-1; EC: 455 μS cm-1.
*Lecane ungulata Gosse, 1887: Locs. 39, 57, 66, 105, 121.
It was found on Thypa sp. pH: 8.5; Water temp.: 26.8 °C; DO: 2.2 mg L-1; EC: 551 μS cm-1.
*Proales fallaciosa Wulfert, 1937: Locs. 66, 82, 83, 85, 86, 87, 90, 93, 95, 96, 97, 98, 100, 103, 105, 106, 115, 117.
It was found on Potamogeton sp.
Cephalodella gibba (Ehrenberg, 1838): Locs. 2, 9, 12, 13, 17, 23, 35, 43, 45, 46, 47, 64, 65, 66, 67, 71, 72, 80, 83, 86, 87, 93, 94, 97, 98, 101, 102, 104, 105, 107, 108, 116, 117, 125.
It was found on Potamogeton sp. and Myriophyllum sp.. pH: 7.4-9.7; Water temp.: 13.4-22.6 °C; DO: 6.3 mg L-1; EC: 650-33000 μS cm-1.
*Cephalodella forficula Ehrenberg, 1830: Locs. 30, 55, 121, 122.
It was found on Phragmites sp., Ceratophyllum sp. and Myriophyllum sp.
*Cephalodella megalocephala (Glascott, 1893): Loc. 80.
Monommata sp.: Locs. 47, 121.
It was found on Potamogeton sp., Myriophyllum sp. and Trapa natans.
*Notommata glyphura Wulfert 1935: Locs. 9, 66, 97, 117, 121.
It was found on Ceratophyllum sp. and Myriophyllum sp. pH: 8.6-9.7; Water temp.: 15.2-22.6 °C.
*Notommata copeus Ehrenberg 1834: Locs. 117.
pH: 9.5-9.7; Water temp.: 21.7-22.6 °C.
Itura myersi Wulfert, 1935: Locs. 6, 10, 22, 66, 97, 115, 117, 121.
It was found on Thypa sp. and Ceratophyllum sp.. pH: 7.5-9.7; Water temp.: 15.2-22.6 °C; DO: 1.9 mg L-1; EC: 146 μS cm-1.
*Eospora ehrenbergi Weber, 1918: Locs. 42, 123.
pH: 8.5; Water temp.: 27.3 °C; DO: 2.2 mg L-1; EC: 611 μS cm-1.
*Pleurotrocha petromyzon Ehrenberg, 1830: Locs. 85, 90, 97, 115.
*Trichocerca capucina Wierzejski&Zacharias, 1893: Locs. 1, 4, 5, 7, 11, 14, 16, 19, 20, 21, 24, 27, 37, 38, 40, 41, 43, 48, 54, 57, 60, 65, 67, 68, 69, 74, 76, 78, 88, 89.
pH: 7.9-10.9; Water temp.: 17.5- 30.6 °C; DO: 0.7-16 mg L-1; EC: 352-1113 μS cm-1.
Trichocerca elongata (Gosse, 1886): Locs. 1, 2, 5, 6, 9, 10, 14, 17, 22, 23, 25, 26, 44, 94.
pH: 9.75; Water temp.: 29.3 °C; DO: 18.8 mg L-1.
*Trichocerca cylindrica (Imhof, 1891): Locs. 16, 37, 41, 46, 48, 56, 57, 60, 67, 105, 122.
pH: 7.7-8.3; Water temp.: 20.6-23 °C; DO: 1.1 mg L-1; EC: 352 μS cm-1.
*Trichocerca iernis (Gosse 1887): Locs. 121, 122.
It was found on Trapa natans. pH: 7.81; Water temp.: 25 °C; DO: 11.5 mg L-1.
*Trichocerca longiseta (Schrank, 1802): Loc. 121.
*Trichocerca porcellus (Gosse, 1886): Locs. 13, 25, 26, 39, 47, 65, 71, 121.
Trichocerca pusilla (Jennings, 1903): Locs. 2, 14, 17, 31, 39, 43, 47, 48, 53, 58, 61, 71, 75, 122.
pH: 7.8-10.9; Water temp.: 17-30.5 °C; DO: 1.3-18.8 mg L-1; EC: 99-477 μS cm-1.
Trichocerca rattus (O.F.Müller, 1776): Locs. 13, 47, 109, 121, 122.
It was found on Potamogeton sp.
*Trichocerca similis (Wierzejski, 1893): Locs. 48, 50, 71, 75, 122.
pH: 6.8-9.3; Water temp.: 16-26.6 °C; DO: 8.6 mg L-1; EC: 97-1250 μS cm-1.
*Trichocerca tenuior (Gosse, 1886): Locs. 121.
It was found on Trapa natans. pH: 7.56; Water temp.: 22 °C; DO: 10.2 mg L-1.
*Trichocerca tigris (Müller, 1786): Locs. 121, 122.
*Trichocerca insignis (Herrick, 1885): Locs. 122.
pH: 7.81; Water temp.: 25.9 °C; DO: 11.59 mg L-1.
Synchaeta pectinata (Ehrenberg, 1832): Locs. 1, 3, 4, 5, 7, 11, 12, 14, 16, 17, 18, 20, 21, 22, 24, 26, 29, 30, 31, 33, 36, 37, 38, 39, 40, 41, 42, 44, 45, 46, 47, 49, 50, 54, 55, 56, 57, 58, 60, 62, 63, 64, 65, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 81, 82, 86, 89, 92, 96, 98, 101, 102, 105, 114, 121, 124, 125.
pH: 7.5-10.6; Water temp.: 15.1-28.7 °C; DO: 0.9-16.3 mg L-1; EC: 146-1250 μS cm-1.
Synchaeta oblonga Ehrenberg, 1832: Locs. 1, 2, 7, 11, 12, 16, 18, 20, 23, 26, 28, 29, 33, 37, 38, 39, 41, 42, 47, 50, 54, 58, 60, 61, 62, 63, 64, 66, 67, 72, 73, 74, 75, 82, 88, 92, 93, 94, 102, 105, 110, 119, 122, 123, 124, 125.
*Synchaeta stylata (Wierzejski, 1893): Loc. 72
*Synchaeta sp.: Loc. 123.
*Polyarthra euryptera (Wierzejski, 1893): Loc. 27
Polyarthra vulgaris (Carlin, 1943): Locs. 1, 2, 4, 5, 6, 7, 8, 11, 14, 15, 16, 17, 18, 19, 20, 21, 27, 28, 29, 34, 37, 38, 39, 40, 41, 42, 43, 46, 47, 48, 50, 52, 53, 54, 55, 57, 59, 60, 61, 63, 64, 65, 67, 69, 72, 73, 74, 75, 76, 78, 81, 88, 94, 105, 109, 122.
Polyarthra dolichoptera (Idelson, 1925): Locs. 1, 2, 6, 7, 10, 12, 14, 17, 20, 22, 23, 24, 26, 29, 31, 33, 37, 38, 41, 42, 46, 47, 54, 55, 58, 63, 64, 71, 76, 79, 81, 92, 95, 105, 122.
Polyarthra remata (Skorikov, 1896): Locs. 1, 2, 3, 4, 5, 6, 7, 8, 10, 11, 14, 17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 41, 42, 44, 45, 46, 47, 48, 50, 54, 56, 57, 58, 60, 63, 64, 66, 67, 68, 69, 71,73, 74, 75, 79, 80, 81, 82, 87, 88, 89, 95, 96, 98, 99, 105, 114, 116, 121, 122, 124.
*Asplanchna girodi (De Guerne, 1888): Loc. 36.
Asplanchna priodonta Gosse, 1850: Locs. 1, 3, 4, 5, 6, 7, 8, 11, 12, 14, 16, 17, 18, 19, 20, 21, 23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 62, 63, 64, 65, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 81, 89, 94, 102, 105, 114, 121, 122, 125.
Asplanchna sieboldi (Leydig, 1854): Locs. 4, 5, 8, 9, 13, 14, 15, 17, 18, 19, 20, 21, 25, 26, 27, 28, 29, 30, 32, 33, 35, 36, 37, 38, 39, 40, 41, 43, 44, 45, 46, 48, 49, 53, 55, 58, 59, 60, 62, 63, 67, 68, 74, 75, 76, 78, 79, 88, 89, 116.
Dicranophorus grandis (Ehrenberg, 1832): Locs. 4, 9, 39, 41, 46, 47, 71, 72, 110, 115, 117.
*Dicranophoroides caudatus (Ehrenberg, 1834): Loc. 121.
Paradicranophorus hudsoni Glascott, 1893: Locs. 86, 99, 115, 117, 118, 126.
Encentrum saundersiae (Hudson, 1885): Loc. 122.
Floscularia sp.: Locs. 4, 42, 46, 47, 55, 65, 66, 71, 77, 105, 121, 122.
Testudinella patina (Hermann, 1783): Locs. 41, 42, 50, 62, 63, 66, 70, 73, 80, 86, 100, 104, 121, 123, 125.
*Testudinella emarginula (Stenroos, 1898): Locs. 39, 105, 121.
*Testudinella mucronata (Gosse, 1886): Loc. 105.
*Testudinella parva (Ternetz, 1892): Loc. 39.
Pompholyx sulcata (Hudson, 1885): Locs. 1, 4, 8, 11, 14, 17, 18, 20, 25, 26, 27, 28, 30, 32, 33, 37, 38, 41, 43, 46, 48, 49, 50, 51, 52, 53, 54, 55, 58, 60, 61, 63, 64, 66, 67, 68, 69, 70, 73, 75, 76, 81, 86, 105, 122.
Filinia longiseta (Ehrenberg, 1834): Locs. 2, 5, 6, 12, 14, 15, 17, 25, 26, 28, 29, 33, 35, 37, 38, 39, 41, 42, 43, 45, 46, 55, 56, 57, 60, 66, 67, 68, 70, 74, 77, 78, 80, 91, 105, 116, 121, 122.
*Filinia longiseta saltator Gosse, 1886: Locs. 31, 79, 89, 94.
*Filinia opoliensis (Zacharias, 1898): Locs. 4, 7, 11, 13, 18, 20, 25, 28, 33, 39, 40, 42, 43, 45, 52, 55, 59, 64, 65, 67, 70, 73, 109.
Filinia terminalis (Plate, 1886): Locs. 1, 4, 5, 7, 11, 12, 14, 16, 17, 18, 23, 26, 25, 29, 30, 32, 33, 37, 39, 40, 41, 43, 44, 46, 48, 54, 55, 57, 58, 60, 66, 68, 70, 71, 73, 74, 75, 76, 80, 86, 89, 92, 95, 98, 105, 116, 122, 125.
*Conochilus dossuarius Hudson, 1875: Locs. 1, 3, 4, 5, 6, 7, 8, 10, 11, 13, 15, 16, 17, 18, 20, 21, 24, 25, 26, 29, 30, 32, 33, 37, 38, 39, 40, 41, 42, 43, 46, 47, 48, 49, 50, 54, 55, 60, 61, 63, 64, 67, 68, 69, 70, 72, 73, 74, 75, 76, 78, 88, 89.
*Conochilus unicornis Rousselet, 1892: Locs. 40, 121.
Hexarthra fennica (Levander, 1892): Locs. 28, 37, 41, 45, 48.
*Hexarthra mira Hudson, 1871: Locs. 33, 65, 73.
*Collotheca ornata (Ehrenberg, 1832): Locs. 1, 3, 5, 6, 7, 9, 13, 14, 15, 20, 21, 26, 29, 35, 39, 54, 60, 61, 63, 64, 66, 70, 72, 74, 75, 77.
At 65 of 126 localities physicochemical pa-rameters such as water temperature, pH, conduc-tivity, and dissolved oxygen were also measured during the field study to show the overall char-acteristics of Turkish Thrace and the three prov-inces (Table 2).
When the distribution of species identified in Turkish Thrace is examined, it is seen that Bra-chionus angularis, Asplanchna priodonta, Poly-arthra remata, Synchaeta pectinata and Keratella cochlearis are the most common species in the region. These are cosmopolite species with wide distribution. They can tolerate low and high ranges of temperature and salinity and are seen in waterbody throughout the year. So, they can be found in every kind of aquatic ecosystem (Koste, 1978a; De Manuel Barrabin, 2000; Fontenato et al., 2008). Two rare species in the region, Myti-lina ventralis and Mytilina mucronata, inhabit in littoral and benthic region and rarely in plankton. Lecane lamellata prefers saline habitats (Segers et al., 1992; Fontenato et al., 2008). So, it was found only in lake Mert which is a lagoon in this study. The distribution of T.bicristata, T.longiseta, T.tenuior and T.insignis is limited with lakes Hamam and Pedina. These species are periphytic (Pejler and Berzins, 1993a). These species are found less in number in the region because sampling was done specifically for planktonic species.
When the distribution of 115 species which were identified according to provinces is exam-ined, it is seen that Kirklareli has the highest number in species richness with 99. Edirne is the second with 97 species and Tekirdag is the third with 72 species. 82 species have been recorded in Edirne as result of former studies done in Turkish Thrace, (DSI., 1986; Segers et al., 1992; Erdogan and Güher, 2005; Güher and Erdogan, 2008). 65 of these species are found in this study as well, 17 of them could not be found and 32 new spe-cies discovered in this study. Although, some ro-tifer species exist throughout the year, some exist in spesific seasons of the year or disappear for a long time and appear again (Kolisko, 1974; Pen-nak, 1989). It is thought that this is the reason why 17 species identified in the former studies could not be identified in this study. 72 species which were identified in this study are evaluated as first records for Tekirdag because there weren’t any former studies concerning Rotifera in Tekirdag. In Kirklareli, Güher (2003) had ex-amined zooplanktonic organisms of Mert, Erikli, Hamam and Pedina lakes and had recorded 17 genuses belonging to Rotifera. One of these, ge-nus Ascomorpha, could not be found in this study. 99 species in this study are evaluated as first records for Kirklareli, because there is no record on rotifera in species level in the region.
Edirne is rich in wetlands, especially with ponds, and has the highest number of sampling locality (78 localities). Edirne is expected to have much more species because rotifera species are more common in shallow waters. In Tekirdag and Kirklareli, number of localities are equal where samplings are done (24 localities). But when the number of species identified is considered, it is seen that Kirklareli is superior to other provinces in terms of species richness. Kirklareli and espe-cially Igneada is a region that hosts specific hab-itats such as lagoons, containing high diversity of species. Besides, there are streams sourcing from Istranca Mountains feeding lakes and ponds in Kirklareli. Tekirdag has wide and flat agricultural lands and a limited number of water reservoirs which are built for agricultural irrigation. In ad-dition, pollution caused by intensive industrial areas limit species diversity in Tekirdag.
The physicochemical parameters measured in Turkish Thrace vary as follows: pH 6.8-10.9, Conductivity 97-33000 μS cm-1 and Dissolved oxygen 0.7-18.8 mg L-1 (Tablo 2).
When measured pH values are considered, it is seen that freshwaters in Turkish Thrace are al-kaline. pH is higher in Edirne than other prov-inces. This situation is supported by Brachionus, Filinia and Polyarthra species which are charac-teristic for the alkaline waters and which widely distribute in Edirne (Emir, 1989; Koste, 1978a; Kaya and Altindag, 2007a).
Although electrical conductivity is lower in Tekirdag, it was found quite high in Mert and Erikli lagoons located in Igneada region of Kirklareli. The extensive existance of Bra-chionus plicatilis, Synchaeta sp. and Hexartra species in these lakes, which are usually common in saline water, supports this view (Altindag and Sözen, 1996).
When measured dissolved oxygen values are examined it is seen that, the amount of dissolved oxygen in ponds and small lakes, is related to the increase in phytoplankton density due to the in-crease in water temperature in spring.
Polyarthra vulgaris and Keratella quadrata are found in oxygen-rich waters (Koste, 1978a; Emir and Demirsoy, 1996; Pennak, 1989). These species are found commonly in ponds and small lakes in this study, too. The distribution of Rotif-era species is directly proportional to water tem-perature. Branco et al. (2002) stated that the ex-istence of E.dilatata. and B.calyciflorus is closely related to the increase in water temperature. Most of the species identified in the region, appear due to the increase in water temperature, especially in spring and summer.
Of these species identified in Turkish Thrace, Rotaria neptunia, Anuraeopsis coelata, A.fissa, Brachionus angularis, B.calyciflorus, B.leydigi, B.plicatilis, Keratella quadrata, K. tecta, Eu-chlanis dilatata, Mytilina mucronata, Trichotria pocillum, Lecane lunaris, Pleurotrocha petromyzon, Trichocerca capucina, T.cylindrica, T.pusilla, T.porcellus, Polyarthra dolichoptera, P.vulgaris, P.euryptera, Synchaeta pectinata, S.oblonga, Asplanchna girodi, Pompholyx sul-cata, Filinia longiseta and F.terminalis are indi-cators of eutrophycation (Kolisko, 1974; Koste, 1978a; Berzins and Pejler, 1989; Pejler and Ber-zins, 1993a; Altindag and Sözen 1996; Michaloudi et al.,1997; Altindag and Özkurt, 1998; Bekleyen, 2001, 2003; De Manuel Barra-bin, 2000; Koste and Terlutter, 2001; Bekleyen and Tas, 2008; Kehayias et al., 2008). In addi-tion, Lopocharis salpina is indicator of dystro-phy, Kellicottia longispina, Keratella cochlearis, Asplanchna priodonta, Conochilus unicornis are indicators of oligotrophy (Kolisko, 1974; Koste, 1978a; Emir and Demirsoy, 1996; De Manuel Barrabin, 2000).
Whereas the species which are indicator of eutrophy distribute widespreadly, species which are indicator of oligotrophy have limited distri-bution. This situation shows that most of the wetlands in the region are eutrophic. Besides, distribution of rotifer species identified gives in-formation about pollution degree of wetlands in Turkish Thrace.
Asplanchna priodonta, Keratella cochlearis, Trichocerca bicristata and T.porcellus are indi-cator of Oligosaprobi, Asplanchna girodi, A.sieboldi, Philodina megalotrocha, Dissotrocha aculeata, Trichotria pocillum, T.tetractis, Myti-lina ventralis, Lepadella patella, Lecane luna, L.closterocerca, L.lunaris, L.bulla, Platyias quadricornis, Colurella adriatica, Euchlanis di-latata, E.incisa, Notommata copeus, Ceph-alodella gibba, Trichocerca rattus, T.bicristata, T.longiseta and Polyarthra vulgaris are indicator of Oligo-Beta saprobi, Testudinella patina, Pom-pholyx sulcata and Filinia longiseta are indicator of Beta mesosaprobi, Brachionus angularis, B.calyciflorus and Dicranophoroides caudatus is indicator of Alfa-Beta mesosaprobi and Le-padella patella is indicator of Alfa-mesosaprobi. However, Rotaria neptunia is indicator of po-limesosaprobi (Kolisko, 1974; Koste, 1978a; Berzins and Pejler, 1989; Pejler and Berzins, 1993a; De Manuel Barrabin, 2000; Tasevska et al., 2004; Shumka and Miho, 2006).
The majority of the species commonly found in Turkish Thrace, inhabit in less and moderately polluted waters. But, some of these species like Brachionus angularis and Brachionus calyciflo-rus which are widely distributed in the region in-habit critically polluted waters. This situation in-dicates that wetland in Turkish Thrace is gradu-ally polluted, when we consider whole Turkish Thrace.
70 of the Rotifera species identified in Turk-ish Thrace are also seen in Balkan countries. (Zarfdjian and Economidis 1989; Zarfdjian et al., 1990; Michaloudi et al., 1997; Zarfdjian et al., 2000; Michaloudi and Kostecka 2004; Tasevska et al., 2004; Djurkovic et al., 2008; Kozuharov et al., 2009, Tasevska et al., 2006). Rotifera species distribute widely in the world because rotifer eggs can be carried easily everywhere by streams, birds, other animals and wind. In addi-tion to these, Northeast South bird migration route passing through Turkish Thrace and Arda from Greece, Tunca and Meriç from Bulgaria pouring into Aegean sea by crossing the region, cause the species which are common in Balkans appear in Turkish Thrace as well. Therefore, the Rotifera fauna of Turkish Thrace is similar to Balkan fauna.
Ustaoglu, (2004) who collected the researches concerning Rotifera in Turkish freshwaters, listed 229 species belonging to Rotifera in Turkey and together with the following studies, this number increased to 297. Number of species in Turkish Thrace increased to 138 together with former studies. If it was considered that Turkish Thrace covers 3% of Turkish mainland, it is concluded that Turkish Thrace is rich in species diversity having border to Balkan countries cause an in-crease in species diversity and spread of the spe-cies in Balkans into Turkish Thrace.
Conclision
As a result, if the former studies performed in the region were also considered, it is visible that the Rotifera fauna of Edirne is represented with 114 species, Tekirdag with 72 species, Kirklareli with 99 species and Turkish Thrace with 138 species. Turkish Thrace and especially Igneada region support high biodiversity. However, ac-cording to the rotifer species identified in the re-gion, it can be said that water bodies in Thrace are eutrophic and are getting polluted.
651
References
- Altindag, A., Sözen, M., (1996). Seyfe (Kirsehir) Gölü Rotifera Faunasinin Taksonomik Yönden Incelenmesi, Turkish Journal of Zo-ology, 20: 221-230
- nAltindag, A., Özkurt, S., (1998). A Study on the Zooplanktonic Fauna of the Dam Lakes Kunduzlar and Çatören (Kirka-Eskisehir), Turkish Journal of Zoology, 22: 323-331
- nBekleyen, A., (2001). A Taxonomical Study on the Rotifera Fauna of Devegeçidi Dam Lake (Diyarbakir-Turkey), Turkish Journal of Zo-ology, 25: 251-255
- nBekleyen, A., (2003). ATaxonomical Study on the Zooplankton of Göksu Dam Lake (Di- yarbakir), Turkish Journal of Zoology, 27: 95-100
- nBekleyen, A., Tas, B., (2008). Çernek Gölü’nün (Samsun) Zooplankton Faunasi, Ekoloji, 17(6): 24-30
- nBerzins, B., Pejler, B., (1989). Rotifer Occur-rence and trophic degree, Hydrobiologia, 182: 171-180
- nBranco, C.W.C., Rocha, M.I.A., Pinto, G.F.S., Gômara, G.A., De Filippo, R., (2002). Lim-nological Features of Funil Reservoir (R.J., Brazil) and Indicator Properties of Rotifers and Cladocerans of the Zooplankton Com-munity, Lakes & Reservoirs: Research And Management, 7: 87-92
- nDe Manuel Barrabin, J., (2000). The Rotifers of Spanish Reservoirs: Ecological, Systemati-cal and Zoogeographical Remarks, Lim-netica, 19: 91-167
- nDemir, N., U.Kirkagaç, M., Topçu, A., Zencir, Ö., Pulatsu, S., Karasu Benli, Ç., (2007). Sarisu-Mamuca Göleti Su Kalitesi ve Besin Düzeyi, Tarim Bilimleri Dergisi, 13(4): 385-390
- nDjurkovic, A., Mijovic, S., Cadjo, S., (2008). Balwois 2008-Ohrid. Republic of Macedo-nia-27, 31 May 2008
- nD.S.I., (1986). Gala Gölü Limnolojik Arastirma Raporu. T.C. Enerji ve Tabii Kaynaklar Ba-kanligi, ANKARA
- nEmir, N., (1989). Samsun Bafra Gölü Rotatoria Türlerinin Mevsimsel Degisimi Üzerine Ekolojik Bir Çalisma, Doga Türk Zooloji Dergisi, 13(3): 219-227
- nEmir, N., Demirsoy, A., (1996). Karamuk Gölü Zooplanktonik Organizmalarinin Mevsimsel Degisimleri, Turkish Journal of Zoology, 20: 137-144
- nErdogan, S., Güher, H., (2005). The Rotifera Fauna of Gala Lake, Pakistan Journal of Biological Sciences, 8(11): 1579-1583
- nFontaneto, D., De Smet, W.H., Melone, G., (2008). Identification Key to the Genera of Marine Rotifers Worldwide, Meiofauna Ma-rina, 16: 75-99
- nGüher, H., (2003). Mert, Erikli, Hamam ve Pedina Gölleri’nin (Igneada/ Kirklareli) Zooplanktonik Organizmalari Kommunite Yapisi, Ege Üniversitesi Su Ürünleri Dergisi, 20(1-2): 51-62
- nGüher, H., Kirgiz, T., Çamur, B., Güner, U., (2004). A Study on Zooplankton Organisms Community Structures of Lake Terkos (Is-tanbul-Turkey), Pakistan Journal of Bio-logical Sciences, 7(4) : 566-570
- nGüher, H., Erdogan, S., (2008). Aliç Göleti Peri-fitik Zooplankton (Cladocera, Copepoda, Rotifera) Türleri Üzerine Bir Arastirma, Journal of FisheriesSciences.com, 2(3): 516-523ndoi: 10.3153/jfscom.mug.200749
- nJersabek, C.D., Segers, H., Morris, P.J., (2003). An illustrated online catalog of the Rotifera in the Academy of Naturel Sciences of Philadelphia (version 1.0). [WWW database] URL https://rotifer.acnatsci.org/rotifer.php.
- nKaya, M., Altindag, A., (2007a). Zooplankton Fauna and Seasonal Changes of Gelingüllü Dam Lake (Yozgat, Turkey), Turkish Jour-nal of Zoology, 31: 347-351
- nKehayias, G., Chalkia, E., Chalkia, S., Nistikakis, G., Zacharias, I., Zotos, A., (2008). Zoo-plankton dynamics in the upstream part of Stratos reservoir (Greece), Biologia, 63(5): 699-710
- nKolisko, A., (1974). Plankton Rotifers, Biology and Taxonomy, Die Binnengenwasser, Cilt XXVI/I. Supplement, pp. 144
- nKoste, W., (1978). Die Radertiere Mitteleuropas I.Tafelband, Berlin, Studgart, 670
- nKoste, W., (1978). Die Radertiere Mitteleuropas II. Tafelband, Berlin, Stutgart, 670
- nKoste, W., Shiel, R.J., (1989). Rotifera from Australian Inland waters. III. Euchlanidae, Mytilinidae and Trichotriidae (Rotifera: Monogononta), Transactions of the Royal Society of South, 113: 85-114
- nKoste, W., Shiel, R.J., (1990). Rotifera from Australian Inland Waters. VI. Proalidae, Lindiidae (Rotifera: Monogononta), Trans-actions of the Royal Society of South, 114(3): 129-143
- nKoste, W., Terlutter, H., (2001). Die Rotatorien-fauna einiger Gewasser des Naturschutzge-bietes “Heiliges Meer” im Kreis Steinfurt, Osnabrücker Naturwissenschaftliche Mit-teilungen Band, 27: 113-117
- nKozuharov, D., Trichkova, T., Borisova, P., Stanachkova, M., (2009). The Zooplankton Compositionin Two Reservoir in the North-West Bulgaria in Relation to Dreissena spp. Occurence. Biotechnology & Biotechno-logical Equipment, EQ.23/2009/SE Special Edition/On-line
- nLucinda, I., Moreno, I.H., Melão, M.G.G., Matsumura, T.T., (2004). Rotifers in Fresh-water Habitats in Upper Tietê River Basin, São Paulo State, Brazil, Acta Limnologica Brasiliensia, 16(3): 203-224
- nMichaloudi, E., Zarfdjian, M.H., Economidis, P.S., (1997). The Zooplankton of Lake Mikri Prespa), Hydrobiologia, 351: 77-94
- nMichaloudi, E., Kostecka, M., (2004). Zoo-plankton of Lake Koroneia (Macedonia, Greece). Biologia, Bratislava, 59(2): 165-172
- nPejler, B., Berzins, B., (1993a). On the Ecology of Trichocercidae (Rotifera), Hydrobiologia, 263: 55-59
- nPennak, R.W., (1989). Freshwater Invertebrates of the United States, Protozoa to Mollusca. 3rd Ed., A Wiley Interscience Publication, John-Wiley Son, New York
- nPontin, M.R., (1978). A Key to the freshwater Planktonic and Semi-Planktonic Rotifera of the British Isles. No: 38.178
- nSegers, H., Emir, N., Mertens, J., (1992). Rotifera From North and Northeasth Anatolia (Tur-key), Hydrobiologia, 245: 174-189
- nSegers, H., (1995). Guides to the Identification of the Microinvertebrates of the Continental waters of the World; The Lecanidae (Monogononta). SPB Academic Publishing bv., Volume : 2, ISSN 0928-2440
- nShiel, R.J., Koste, W., (1992). Rotifera from Australian inland waters VIII. Trichocerci-dae (Monogononta), Transactions of the Royal Society of South Australia, 116(1): 1-27
- nShumka, S., Miho, A., (2006). Assessment of the water quality and trends at the Drini cascade system based on plankton data. BALWOIS 2006, International Conference on Water Observation and Information System for Decision Support, Ohrid, Macedonia, 23-26 May 2006, Full Paper. 10 pp
- nhttps://balwois.com/balwois/administration/full_paper/ffp-822.pdf, 06.03.2012
- nTasevska, O., Kostoski, G., Guseska, D., (2004). Compozition and Dynamic of Rotifera Fauna from Eastern Littoral Zone of Lake Ohrid as Parameter of Water quality, Ohrid. FY Republic of Macedonia, 25-29 May 2004, BALWOIS
- nTasevska, O., Kostoski, G., Guseska, D., (2006). Recent Species Composition Of Rotifera Fauna Of The Lake Dojran (R. Macedonia)
- nUstaoglu, M.R., (2004). A Check-list for Zoo-plankton of Turkish Inland Waters, EgeUniversity Journal of Fisheries & Aquatic Sciences, 21(3-4): 191-199
- nZarfdjian, M.H., Economidis, P.S., (1989). Listes Provisoires Des Rotiferes, Cladocéres & Copépodes Des Eaux Continentales Grec-ques, Biologia Gallo- Hellenica, 15: 129-146
- nZarfdjian, M., Vranovský, M., Economidis, P.S. (1990). Les Invertébrés Planctoniques du Lac Volvi (Macédoine, Gréce. Internatio-nale Revue gesamten, Hydrobiologie, 75(3): 403-412
- nZarfdjian, M.H., Evangelia, M., Dimitra, B.C., Spiros, M., (2000). Zooplankton Abundance In The Aliakmon River, Greece, Belgian Journal of Zoology, 130(1): 29-33.









