Keywords
Congestive Heart Failure; Handheld Ultrasound; Point of Care Ultrasound; Bedside Ultrasound
Introduction
Portable ultrasound technology is now over a decade old, and continues to evolve in rapid fashion. The latest devices are roughly the same size as a smart phone, and can fit inside the pocket of a typical white coat [1]. They are able to provide both high resolution 2-D images (despite a small screen size) and color Doppler. They also provide the user with the ability to adjust sector depth, perform linear measurements, and save images for downstream review [2]. This technology has been studied extensively in the pre-hospital setting, in the emergency room, and in the critical care setting [3-5]. In addition, many medical schools offer an ultrasound curriculum for senior medical students [6]. Yet despite the emerging body of evidence supporting the use of handheld ultrasound by experienced users in the aforementioned settings, there is a relative paucity of literature describing the utility of this technology to assess for congestion in the acute heart failure population.
Heart failure cardiologists are challenged with the expectation of an accurate volume status assessment on every patient they encounter. Often this assessment is relatively straightforward. The presence of an elevated jugular venous pulsation (JVP), positive hepatojugular reflux, an S3, and lower extremity edema, all suggest residual volume overload/congestion [7]. However, in many patients, the physical exam can be quite challenging, and volume status is still in question even after a thorough clinical assessment. This is particularly true in the morbidly obese patient, where the physical exam is often quite limited. An elevated JVP is a highly specific physical exam finding for intravascular congestion [8], but often the JVP is difficult (or impossible) to see in morbidly obese patients. In my home institution, this scenario is unfortunately all too common, as the portion of heart failure patients with BMI>35 is ~25%, and over half these patients of these patients have BMIs >40.
In addition to body habitus, tricuspid regurgitation can make the JVP assessment challenging, and even lead to an erroneous conclusion. Mistakenly basing a clinical assessment on the v wave as opposed to the wave, can lead to over-diuresis and acute kidney injury. Finally, anatomical variations with respect to the location and caliber of the internal jugular vein can also impact the reliability of this exam finding [9].
When the physical exam is of limited utility, the history often gains importance in determining the likelihood of residual congestion. Complaints of persistent orthopnea, early satiety, and residual dyspnea on exertion all point in the same direction, and suggest that a hospitalized heart failure patient may not be ready for discharge. However, many chronically ill patients are not terribly mobile, nor do they have terrific insight on the extent of their symptoms (or lack thereof). In addition, delirium is quite common in the elderly hospitalized patient, which can make history gathering quite challenging. Thus, relying on history can be inappropriate in many heart failure patients.
A strategy that is often invoked as an attempt to identify the end point of a hospitalization for heart failure is the targeting of a fixed amount of fluid loss to get to a known "dry weight" (i.e. The patient's dry weight is 200 lbs, so once they reach 200 lbs we will discharge) [10]. This strategy seems reasonable but its success is predicated on two fundamentals: a) a known, accurate dry weight and b) the ability to obtain reliable daily weights while hospitalized. Unfortunately, in some settings it is quite difficult to obtain both pieces of information in consistent fashion. Serial assessment of weight is a Class I recommendation in the 2013 ACC/AHA Guideline for the Management of Heart Failure and is a Joint Commission standard; yet despite this, many centers struggle with capturing and consistently documenting this information. Staffing challenges (e.g. inadequate training, frequent turnover) and lack of a standardized process for daily weight measurement can both contribute to the lack of reliable and consistent patient weights on inpatient wards.
Due to the aforementioned challenges in assessing congestion in the real-world setting, coupled with the ubiquitous focus on quality of care in the heart failure population, providers should feel empowered to utilize novel diagnostic approaches with the hope of improving the accuracy and efficiency of clinical care. It is my belief that in the hands of an experienced user, the assessment of the inferior vena cava (IVC) using the handheld ultrasound provides a non-invasive, reliable, and reproducible estimate of right atrial pressure as a proxy for residual congestion, and should be routinely used when assessing heart failure patients. According to the 2015 American Society of Echocardiography guideline document for chamber quantification, an IVC diameter of <2.1 cm that collapses >50% with forced inspiration (i.e. sniff) suggests normal RA pressure of 3 mmHg (range, 0 to 5 mmHg), whereas IVC diameter >2.1 cm that collapses <50% with a sniff suggests high RA pressure of 15 mmHg (range, 10 to 20 mmHg). In scenarios in which IVC diameter and collapse do not fit this paradigm, an intermediate value of 8 mmHg (range, 5 to 10 mmHg) may be used [11].
I describe 3 real-world examples where the handheld ultrasound proved clinically valuable in assessing volume status in the heart failure population.
Case 1
Patient 1 is a 77 year-old male with a non-ischemic cardiomyopathy and a history of systolic heart failure (LVEF 35 to 40%), diabetes mellitus, obstructive sleep apnea, paroxysmal atrial fibrillation and morbid obesity. He had been accumulating volume as an outpatient for several months, and at the time of his admission for typical heart failure symptoms, he was approximately 350 lbs (height of 69 inches, BMI 51.7). His “dry weight” was unknown. He was started on a continuous infusion of furosemide at the time of admission. I met the patient on hospital day two and I was unable to see his jugular venous pulsation despite concerted effort; all other aspects of his exam were equivocal. Neither fluid intake/output nor a daily weight had been documented on the morning of our first encounter. Utilizing the handheld ultrasound (General Electric Vscan device) I was able to visualize his IVC, which measured 3.0 cm in diameter, with reduced collapse (<50%). It is worth highlighting that despite the morbid obesity, the IVC was readily accessible using handheld ultrasound. Since the traditional clinical exam was essentially useless in assessing intravascular volume status, I utilized the handheld ultrasound each day, to track progress. On hospital day 6 his IVC was 2.1 cm with normal collapse (Figure 1). At this time we made the transition to PO diuretics. The patient and family were quite pleased with the thorough nature of our care, and at the time of this writing, he has not been rehospitalized.
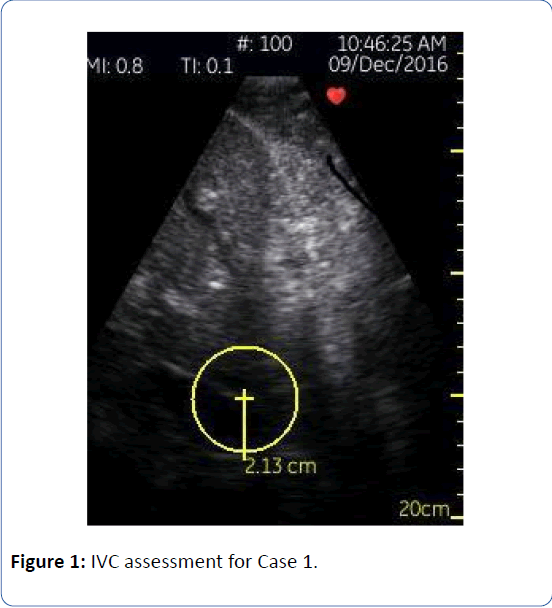
Figure 1: IVC assessment for Case 1.
Case 2
Patient 2 is a 36 year-old obese male (BMI 42) with a Stage D non-ischemic cardiomyopathy, being evaluated for an LVAD; he was seen in clinic 4 days after being discharged home on milrinone at 0.375 mcg/kg/min, after failing a trial of weaning. He arrived to clinic feeling progressively lethargic and fatigued, and his creatinine had increased from 1.4 mg/dL at discharge to 1.8 mg/dL on the day of follow up. His blood pressure was 110/70, with a heart rate of 110 bpm (HR 90s during hospitalization). His JVP was difficult to interpret, but thought to be visible at 6 to 7 cm of H2O, his lungs were clear, he had no hepatojugular reflux, and he had no edema. A similar exam was documented on day of hospital discharge. The leading suspicion as to the cause of his worsening symptoms, tachycardia, and acute kidney injury was overdiuresis, but the possibility of progressive low output heart failure due to inadequate milrinone support was also entertained. In addition, his weights had actually trended up since discharge, which confounded the picture. In order to secure the diagnosis, we utilized the handheld ultrasound. The IVC was barely visible. It was measured at 1.2 cm but this is likely an overestimate (Figure 2), and it collapsed to completely flat with quiet breathing. In Stage D patients, the cost of making an erroneous clinical conclusion can be quite high; these ultrasound findings made us more comfortable with the decision to administer crystalloid in clinic and reduce his diuretic regiment. We deferred an increase in his inotropic support and ultimately deferred emergent rehospitalization. He was seen in clinic 4 days later feeling well, with a down trend in both creatinine and heart rate to baseline. He is now status post Heartmate 3 LVAD implantation.
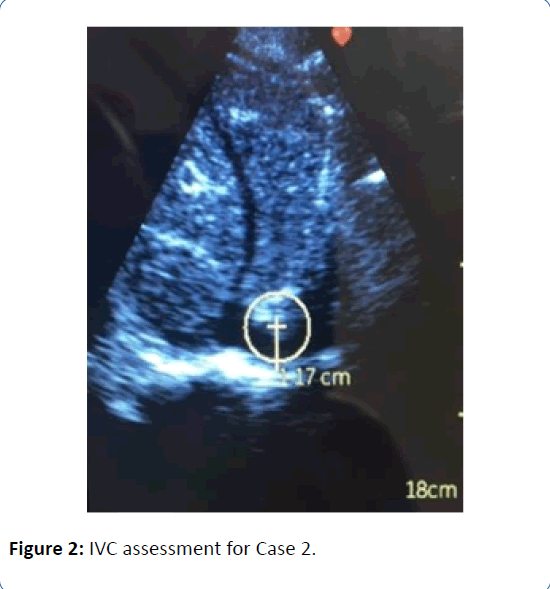
Figure 2: IVC assessment for Case 2.
Case 3
Patient 3 is a frail 83 year-old woman with an ischemic cardiomyopathy (LVEF 30 to 35%), and significant valvular disease (severe mitral regurgitation and moderate to severe tricuspid regurgitation); I met her on hospital day 7 of a readmission for heart failure. She was being considered for a MitraClip due to frequent heart failure hospitalizations. Her JVP was persistently elevated, and she was persistently dyspneic, but she was felt to be “euvolemic” based on evaluation by prior providers, due to the lack of abdominal distension, lack of lower extremity edema, and clear lungs. Her elevated JVP had been attributed to her significant tricuspid regurgitation. As a result, she had already been transitioned to oral diuretics prior to our first encounter. IVC assessment during my initial evaluation using the handheld ultrasound revealed a severely dilated inferior vena cava at 3.7 cm, with essentially no collapse on inspiration (Figure 3a). IV diuresis was reinitiated (furosemide infusion with intermittent chlorthiazide for sequential nephron blockade), and daily ultrasound images were obtained to assess progress (Figures 3b and 3c). Four days later her IVC was 2.4 cm, and collapsed to totally flat on quiet breathing (Figure 3d). She was transitioned to oral diuretics and underwent right and left heart catheterization two days later to complete her MitraClip evaluation. Her hemodynamics were as follows: mean RA 5 mmHg, PA 22/10 (mean of 16 mmHg), mean PCWP 9 mmHg [5]. She did have intermittent, mild orthostatic symptoms towards the latter portion of her hospitalization (around the time of the right heart catheterization), so she was given a 250 cc fluid bolus with symptomatic relief. Her dyspnea had resolved completely. Of note, the severe IVC dilatation in this patient was likely longstanding; in these cases, I find that the extent of IVC collapse is a more reliable marker for volume status, as compared to the diameter. Collapse of the IVC to totally flat on quiet breathing (regardless of IVC diameter at end expiration) appears to suggest that diuresis is adequate, and further volume removal may result in hypovolemia and potential hypotension/ acute kidney injury.
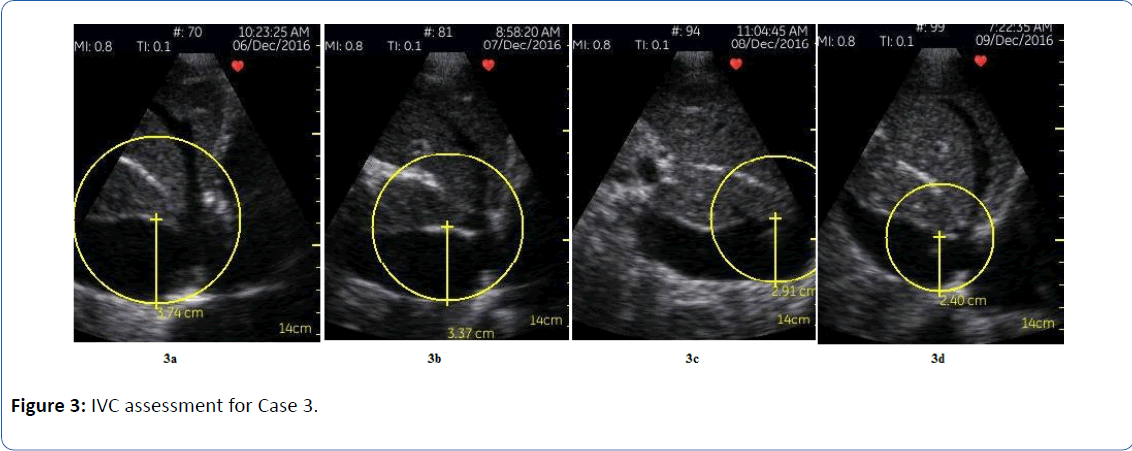
Figure 3: IVC assessment for Case 3.
Conclusion
The previous examples document how a portable ultrasound can provide invaluable information regarding volume status, when the physical exam is challenging. It is not feasible or practical to order a daily limited echocardiogram on all patients with a difficult exam. Nor is it practical (or cost-effective) to perform a right heart catheterization on every patient where the volume status is in question. The portable ultrasound represents a technological advancement that allows the experienced user to have a reasonably accurate assessment of right atrial pressure, typically in less than 1 minute. In addition to Cardiologists, Cardiology fellows and appropriately trained heart failure nurses can also utilize this technology with accurate findings that can potentially benefit patients [12,13].
Despite my profound enthusiasm for this technology, I do agree with Dr. Barry Silverman, who contends that the handheld ultrasound is a valuable bedside tool which can supplement the bedside cardiac exam but not replace it [14]. The handheld device should not be thought of as a “short cut” to be used instead of a thorough history and physical examination. However, in complex clinical situations where volume assessment is uncertain, and the presence of residual congestion is unclear, I do believe IVC assessment is an invaluable tool. I believe every institution should give providers the ability to utilize this high-yield technology. Further research is certainly warranted to evaluate the cost-effectiveness of this device and its impact on quality, particularly heart failure readmissions. Clinical trials are currently underway.
22433
References
- Mirabel M, Celermajer D, Beraud A (2015) Pocket-sized focused ultrasound: Strengths and limitations. Arch Cardiovascular Dis 108: 197-205.
- Di Bello V, La Carrubba S, Conte L (2015) Incremental value of pocket-sized echocardiography in additional to physical examination during cardiology evaluation. Echocardiogr 32: 1463-1470.
- Biais M, Carrie C, Delaunay F (2012) Evaluation of a new pocket echoscopic device for focused cardiac ultrasonography in an emergency setting. Crit Care 16: R82.
- De Backer D, Fagnoul D (2012) Pocket ultrasound for focused echocardiography. Crit Care 16: 134.
- Ferre R, Chionciel O, Pang P (2015) Acute heart failure: The role of focused emergency cardiopulmonary ultrasound in identification and early management. Euro J Heart Failure 17: 1223-1227.
- Solomon S, Saldana F (2014) Point-of-care ultrasound in medical education- stop listening and look. New Eng J Med 370: 12.
- Shamsham F, Mitchell J (2000) Essentials of the diagnosis of heart failure. Am Fam Physician 61: 131913-131928.
- Stevenson LW, Perloff JK (1989) The limited reliability of the physical signs for estimating hemodynamics in chronic heart failure. JAMA 261: 884-888.
- O’Brien T, Menon S, Stephens T (2012) Algorithm based assessment of target weight removal in acute decompensated heart failure. J Card Fail 18: 43-46.
- Lang R, Badano L, Mor-Avi V (2015) recommendations for chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 2015; 28:1-39.
- Khan H, Wineinger N, Uddin P (2014) Can hospital rounds with pocket ultrasound by cardiologists reduce standard echocardiography?. Am J Med 127: 669e1-669e7.
- Gundersen GH, Norekval T, Haug H (2016) Adding point-of-care ultrasound to assess volume status in heart failure patients in a nurse led outpatient clinic. A randomised study. Heart 102: 29-34.
- Silverman B (2015) Handheld ultrasound is a valuable bedside tool which can supplement the bedside cardiac exam but not replace it. JACC: Cardiovasc Imaging 5:621-622.









