Keywords
|
| Lonicera japonica; Phytochemicals; GC-MS analysis; Therapeutical; Pharmacological uses |
Introduction
|
| Medicinal plants, which form the back bone of traditional medicine, in the last few decades, have been the subject for very intense pharmacological studies. Medicinal plants are potential sources of new compounds of therapeutic value and as sources of important compounds in the drug development. In developing countries, it is estimated that about 80% the population really depends on traditional medicine for their primary health care. Traditional medical systems have played an important role in the health care needs in all societies. The most chemically active constituents of plants are alkaloids, tannins, flavonoids and phenolic compounds. Many of these indigenous medicinal plants are also used for medicinal purposes [1,2]. Natural products, which come out from medicinal plants are important for pharmaceutical research and for drug development as a sources of therapeutic agents. At present the demand for herbal or medicinal plant products has increased significantly. In the recent past, there has been growing interest in exploiting the biological activities of different ayurvedic medicinal herbs, owing to their natural origin, cost effectiveness and lesser side effects [3]. Medicinal plants are expensive gift from nature to human. The approval of traditional medicine as an alternative form of health care and improvement of microbial resistance to the existing antibiotics has lead researchers to scrutinize the antimicrobial compounds [4]. Herbal medicines are safer than synthetic medicines because the phytochemicals in the plant extract target the biochemical pathway. Medicinal plants have been used all over the world for the treatment and prevention of various ailments, particularly in developing countries. Various herbs and spices have been reported to exhibit antioxidant activity. |
| Cancer chemoprevention by phytochemicals may be one of the most important approaches for cancer control. Phytochemicals obtained from vegetables, fruits, spices, herbs and medicinal plants, such as alkaloids, terpenoids and other phenolic compounds, have been proven to suppress experimental carcinogenesis in various organs in pre-clinical models. There arises a need to screen medicinal plants for bioactive compounds as a basis for further pharmacological studies. A majority of antioxidant activity is attributed to the flavones, isoflavones, flavonoids, anthocyanin, coumarin, catechin etc. Antioxidant based drug formulations are used for the prevention and treatment of complex diseases like stroke, diabetes, Alzheimer’s disease and cancer [5]. Medicinal plants are considered to be an important source of phytochemicals and therapeutic benefit of many |
| Medicinal plants often attribute to their antioxidant properties [6]. The antioxidants are physiological substances that act against oxidative stress. Flavonoids are a class of polyphenolic compounds usually present in glycosidic form, widely distributed in fruits and vegetables [7], which exert many biological activities [8]. The most frequent sugar found in flavonoid glycosides are glucose, rhamnose, arabinose and glucoronic acid [8,9]. |
| Lonicera japonica (Honeysuckle) belongs to family caprifoliaceae is one of the oldest medicinal herbs in known history. Lonicera japonica possesses many biological functions including hepatoprotective, cryoprotective, antimicrobial, antioxidative, antiviral and antiinflammatory [10]. The major parts of this plant have medicinal properties, flower buds have anticancer and anti-inflammatory properties [7], and leaf has antioxidant and tyrosinase inhibition properties [11]. A few species are used in indigenous medicine as antipyretic, stomachic, diuretic and antidysentric in India [12,13]. This plant is also favoured because of their extreme hardiness to the cold. Lonicera plants have strong tolerance to severe low-temperature conditions. They can survive at temperature of -46°C without damage [14,15]. The freezing tolerance of perennial plants increases in winter to prevent injury under cold conditions. It is known as cold acclimation and seems to be connected with the content and accumulation of specific type of carbohydrates and proteins. The raffinose family of oligosaccharides have been shown to be potential cryoprotectants because of their capacity to modify the freezing behaviour of aqueous solutions [15,16]. Also the presence galactose-containing oligosaccharides strongly correlate with increase in freezing, as well as desiccation tolerance [15]. However in traditional Chinese medicine, Lonicera japonica has been used medicinally for thousands of years. It is an ingredient of herbal tea and has been known for its cooling and detoxification effects. Lonicera caerulea berries are rich sources of phenolic compounds such as phenolic acid as well as anthocyanins, proanthocyanins and other flavonoids, which display potential health promoting effects -chemopreventive, antimicrobial, anti-adherence and antioxidant benefits. It is a commonly used anti-inflammatory herbal medicine. Such a high level of antioxidant activity is due to the high level of polyphenolic compounds especially anthocyanins. The blue berries of Lonicera seem to be prospective sources of health supporting phytochemicals. Biological activities of Lonicera include: protection against the incidence and mortality rates of cancer [17], protection against heart disease and as well as they have antitumorigenic [18], antimicrobial [19], anti-inflammatory-allergic [20] and antimutagenic properties. The aim of this paper was to review the current literature on flower derived biologically active compounds with focus on Lonicera japonica (Figure 1). |
Materials and Methods
|
|
Plant material
|
| Matured flowers of Lonicera japonica were collected in morning hours from the plants maintained in the Botanical garden. The plant was identified on the basis of morphological features with the help of flora and the database present in the library, Karnatak University Dharwad. |
|
Preparation of extract
|
| Fresh flower samples (100 g) were cleaned and naturally dried at room temperature for 10 days. Dried plant samples were further air dried for 24 hr. And then ground into a fine powder using mortar and pestle and passed through a sieve. Powdered sample (10 g) was extracted with 100% ethanol at room temperature in a soxhlet apparatus with 300 ml of solvent for 24 hr. The extract was filtered through a Millipore filter with a 0.45 mm nylon membrane. The extract was concentrated under reduced pressure by a vacuum rotary evaporator to yield an ethanol extract. Solvents (Analytical grade) for extraction were obtained from commercial sources. The samples were stored at -4°C until use. The ethanolic extract prepared from Lonicera japonica flowers (oil) were tested for the presence of various known phytochemicals [21]. |
|
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
|
|
Preparation of plant extracts:
|
| The organic extracts were dried at 60°C protected from light. The residue was weighed and dissolved in dimethyl sulfoxide (DMSO) to obtain a final concentration of 20 mg in 5 μl of DMSO. One μl of the ethanolic flower extract of Lonicera japonica was employed for GC-MS analysis [22]. |
|
Instruments and chromatographic conditions:
|
| GC-MS analysis of ethanol extract was performed using a GCMS QP2010 Gas Chromotograph Mass Spectrometer (Shimadzu Corp, Japan) resulted in detection and identification of volatile constituents of Lonicera japonica flowers. Sample (1 μl) were analysed on an HP-GCD apparatus equipped with an RtX-5MS (30 m × 0.25 mm) fused-silica capillary column using helium (1 ml/min) as carrier gas. The injector and detector temperatures were 250°C and 280°C respectively, and the oven conditions were 70°C for 2 min, then rising from 70 to 200°C at a rate of 4°C/min and subsequently held at 200°C for 10 min. The mass range was recorded from 45 to 450 m/z, with ionization energy of 70 ev. |
|
Identification of components:
|
| Interpretation of mass spectrum of GC-MS was done using database of National Institute Standard and Technology (NIST). The mass spectrum of unknown component was compared with the spectrum of the known component stored in the NIST library. Major components were identified by with authentic standards and by with recorded from computerized libraries. The constituents of the flower oil were identified by the combination of mass spectral and retention indexes and they were compared with both those of reference authentic standard compounds and from library spectra data and literature [23,24]. The name, molecular weight and structure of the components of the test materials were ascertained [25]. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. |
Results
|
|
Chemical composition of the flower essential oil
|
| To explore the importance of any medicinal plant the initial step is to screen for its phytochemicals as it gives a broad idea regarding the nature of compounds present in it. In the present study, leaf and flower extracts of Lonicera japonica was preliminary screened for the phytochemicals. Among different extracts ethanolic extract was found to be rich in all the phytoconstituents followed by methanolic extract. This phytochemical screening shows presence of alkaloids, flavonoids, steroids, terpenoids and phenols. This phytochemical screening aids as an initial step for future determination of its activity like antioxidant, anticancer, anti-inflammatory, antimutagenic etc. (Figure 2; Tables 1 and 2). Dr. Dukes phytochemical and ethanobotanical database. |
|
GC-MS analysis
|
| GC-MS analysis of the oil led to the identification of different compounds. The hydrodistillation of the air dried floral parts of Lonicera japonica gave dark yellow oil. |
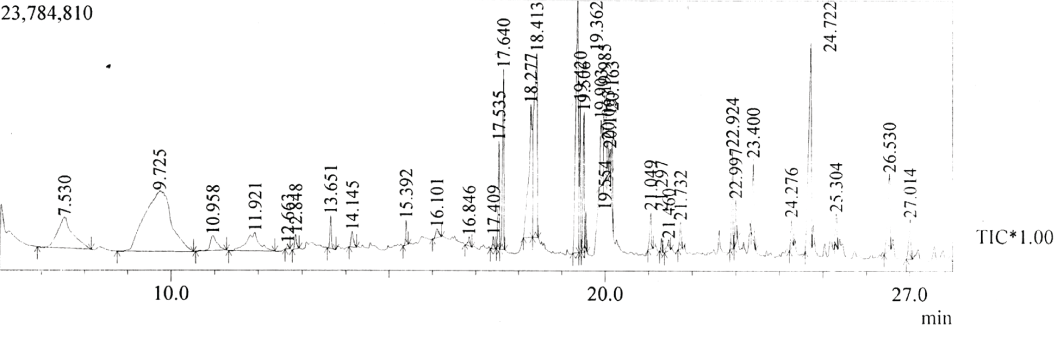
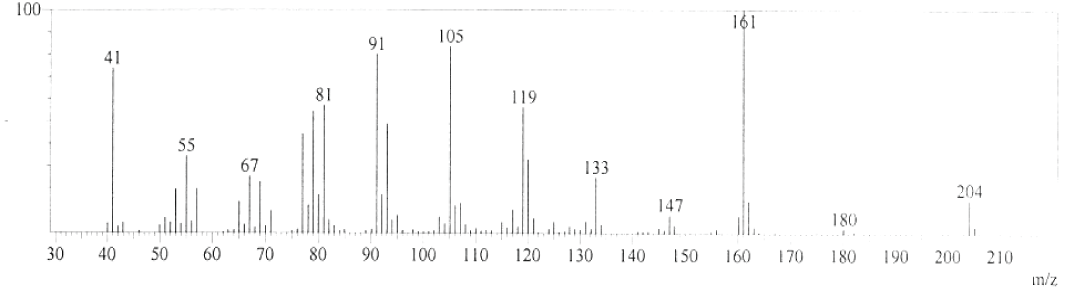
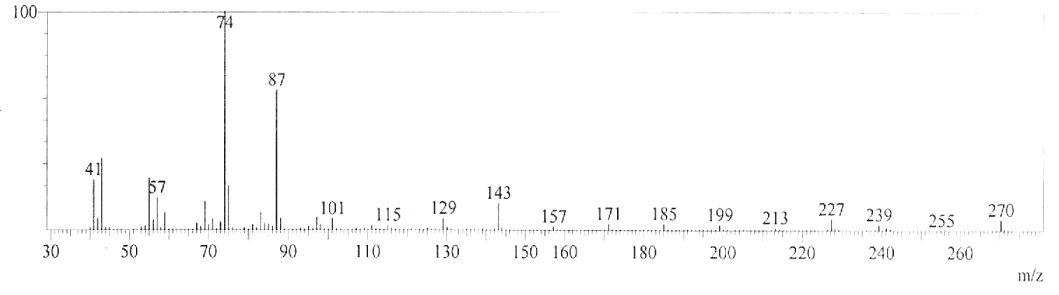
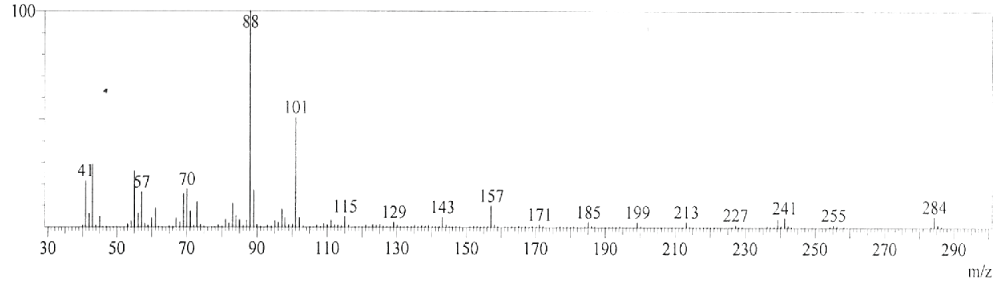
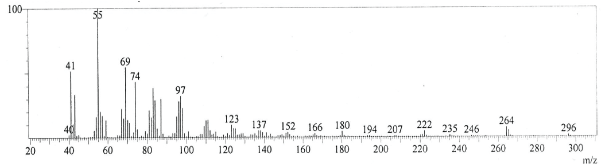
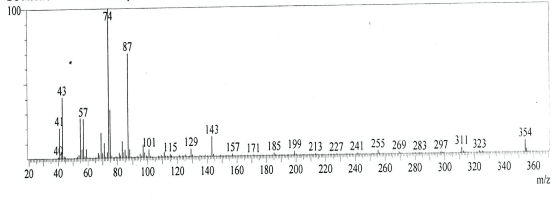
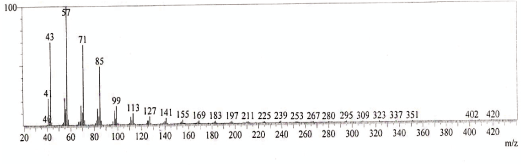
| GC-MS chromatogram of ethanolic flower extract clearly showed 37 (Figure 2) peaks indicating the presence of 37 phytochemical compounds. The identification of the phytochemical compounds was based on the peak area, retention time, and molecular formula. The spectrum sketch out of GC-MS confirmed the presence of 37 components. The individual fragmentation pattern of necessary compounds was illustrated in Figures 3-11. |
| When the mass spectra of these peaks were compared with those of the compiled data for known compounds, 28 major phytochemical constituents were identified in ethanolic flower extract and are presented in Table 1 with their retention time (RT), molecular formula, molecular weight and peak area (%). The phytochemical compounds recognized through GC-MS analysis showed many biological activities. Compounds such as phenols, terpenoids, alkaloids, steroids and flavonoids were recorded. The various phytochemical constituents of Lonicera japonica which contribute to the medicinal activity are presented in Table 2. |
Discussion
|
|
GC-MS analysis
|
| The analysis of the phytochemicals in the alcoholic extracts revealed phenolics, alkaloids, flavonoids and terpenes to be active components which are probably the major players in the antioxidant responses. Further studies are needed to be conducted to understand the structural features of the compounds predicted from phytochemical analysis. Characterization of phenolic compounds present in the extracts was achieved by analysis of the retention times and by database matching. This procedure revealed the presence of many bioactive compounds few are Caryophylline, Diterpene, Chlorogenic acid, Apigenin, Linolenic acid, Cyanidine-3-glucoside, Phytol, Gallocatechin, Squaline etc. |
| Spectral study i.e., gaschromatography-mass spectrometry (GCMS) analysis of the flower sample of Lonicera japonica was carried out to identify the nature of the components present. The fragmentation pattern provides useful information for a tentative determination of the location of the compounds. Table 1 summarizes the mass spectrometric data of all the peaks shown in the chromatograms. The GC-MS output also showed the presence of few major components at retention times. The respective fragmentation patterns of the components are shown in Figures 3-11. The prediction of the biological activities by applying the NIST and Duke’s databases was confirmed with previous observations. The gas chromatogram shows the relative concentrations various compounds getting eluted as a function of retention time. The heights of the peak indicate the relative concentrations of the components present in L. japonica flower. The mass spectrometer analyzes the compounds eluted at different times to identify the nature and structure of the compounds. The large compound fragments into small compounds giving rise to appearance of peaks at different m/z ratios. These mass spectra are fingerprint of that compound which can be identified from data library. |
| The presence of phytochemicals have been shown to possess antifungal, antibacterial, antioxidant, antiinflammatory, antitumour, anticancer, immunostimulant and chemopreventive. Most of these compounds have demonstrated positive effects against chronic degenerative diseases. Apigenin is a nontoxic dietary flavonoid that has been shown to have antitumour and anti-inflammatory activities. Apigenin can block the formation of uric acid leading to beneficial effects in gout [26]. Hence, the results of the GC-MS profile can be used as pharmacognostical tool for the identification of phytochemicals of L. japonica. The present study helps to predict the formula and structure of biomolecules which can be used as drugs. This also enhances the traditional usage of L. japonica which possesses several known and unknown bioactive compounds. Further investigation may lead to the development of drug formulation. It is necessary to point out that the chemical compounds of any plant greatly depend on geographical region, age of plant, local climatic seasonal and experimental conditions. Genetic differences are also responsible for changes of chemical compounds [27], thereby altering the biological activities studied [28]. |
|
Traditional use
|
| Recent research has supported some of the folkloric claims for the therapeutic uses of blue honeysuckle berries in atherosclerosis, hypertension gastrointestinal disorders and bacterial infection. The main beneficial effect is due to the presence of vitaolon, vitamin C and high levels of polyphenolics [29,30].L. caerulea phenolics, as secondary metabolites have been shown to provide defence against oxidative stress from endogenous ROS (Reactive Oxygen Species) and free radicals. |
| These phytochemicals may exert their anticarcenogenic effect by modulating the enzyme systems that metabolize carcinogens or procarcinogens to genotoxins. Numerous reports shows quercetin ability to inhibit proliferation of cancer cells derived from various tissues including breast, colon, pancreas cancer and leukemia [31]. Berry anthocyanins appear to benefit vision in several ways in diabetes including improving night vision by enhanced generation of retina, decreasing muscular degeneration and diabetic retinopathy. This plant is used as a medicine. According to the work, there are so many bioactive compounds that can be used to prepare medicine. The berries of edible honey suckle have been widely used in folk medicine in northern Russia, China and Japan since ancient times. |
Conclusions
|
| The presence of bioactive compounds justifies the use of the whole plant for various ailments by traditional practioners. Phytochemicals isolated from Lonicera japonica have been shown to have potent invitro antibacterial activity. Several studies have shown that the higher antioxidant activity associated with medicinal plants is attributed to the total phenolic compounds. The present study aimed at identifying the nature of the components responsible for their antioxidant activity. This study clearly shows that GCMS is a powerful technique enabling fast separation and characterization of bioactive metabolites. The high sensitivity of this technique helps in characterization of active compounds in Lonicera japonica. |
| Further studies are needed to be conducted to understand the structural features of the compounds predicted from phytochemical analysis. Overall, the present study represents a contribution of the chemical characterization of alcoholic extract from flowers of Lonicera japonica with reported antioxidant activity and traditionally used for several medicinal applications. Gas chromatography and mass spectroscopy analysis showed the existence of various compounds with variable chemical structures and the in vivo studies on biological systems can open up new way for natural drugs that can also be employed for clinical trials which may generate successful results in future. Lonicera japonica can be used as antibacterial supplement in the development of new therapeutic agents. Further pharmacological and clinical studies are required to understand the mechanism and the actual efficacy of the phytochemicals in treating infectious diseases. |
| However isolation of individual phytochemical constituents and subjecting it to biological activity will definitely give fruitful results. However, further studies will need to be undertaken to ascertain fully its bioactivity and other benefits and to elute novel active compounds from medicinal plants which may create new way to treat many incurable diseases. It could be concluded that Lonicera japonica contain various bioactive compounds. So it is recommended as a plant of phytopharmaceutical importance. |
Acknowledgements
|
| Author is thankful to university grants commission (UGC) New Delhi, India for providing financial assistance and also grateful to Dr. Kumar, Deptartment of Biochemistry, Davangere University and Mrs. R V. Murgod, Karnatak University, Dharwad for GC-MS analysis of the samples. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
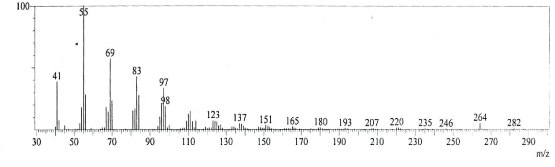 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
 |
 |
 |
| Figure 9 |
Figure 10 |
Figure 11 |
|

















