Zeynep Kuzu1*, Incisule Ozer1, Canan Yucesan1 and Ayse Dursun2
1Department of Neurology, Ankara University School of Medicine, bni Sina Hospital, Samanpazar, Ankara, Turkey
2Department of Pathology, Gazi University School of Medicine, Be¸evler, Ankara, Turkey
*Corresponding Author:
Zeynep Kuzu
Department of Neurology, Ankara University
School of Medicine, Ibni Sina Hospital
Samanpazar±, Ankara, Turkey, 49500
Tel: 9005393823394
E-mail: drzeynepkuzu@hotmail.com
Received Date: Aug 26, 2017 Accepted Date: Nov 26, 2017 Published Date: Nov 28, 2017
Citation: Kuzu Z, Ozer I, Dursun A, Yucesan C (2017) Tumefactive Neuro-Behçet’s Disease: A Case Report and Review of the Literature. J Neurol Neurosci Vol. 8 No: 6: 235. doi: 10.21767/2171-6625.1000235
Keywords
Neuro-Behçet's disease; Tumour-like lesions
Introduction
Behçet's Disease (BD) is a multi-systemic autoimmune disease, characterized with recurrent oral-genital ulcerations, uveitis and skin lesions such as erythema nodosum, folliculitis, ulceration and skin pathergy reaction [1]. Neurological involvement (Neuro-Behçet's Disease, NBD) is one of the leading causes of mortality and morbidity in Behçet's Disease [2]. Central Nervous System (CNS) lesions in BD are polymorph and occur in 5.3% of Turkish cases [3]. In addition, men were 2.8 times more likely to experience NBD than women [2]. Clinical patterns of neurological involvement can be categorized as two main groups; those with parenchymal CNS involvement (encephalitis, encephalomyelitis) and those with non-parenchymal CNS involvement. The latter group consisted of cases who had CNS dysfunction due to involvement of major vessels and those who did not show any CNS parenchymal involvement (essentially cerebral venous thrombosis and, rarely, intracranial aneurysms) [3]. The brain magnetic resonance imaging (MRI) lesions are usually localised in brainstem and basal ganglia in parenchymal NBS. There have been 10 biopsy-proven tumefactive NBD case reports in English based literature so far [4-13]. We evaluated histopathological properties of all the cases including our case. We think this may be helpful to comprehend different neuropathological involvement pattern of NBD and may trigger future researches
Case Presentation
Forty-two-year-old Turkish male admitted to a Neurosurgery clinic with a complaint of weakness in the left extremities, sleepiness and secondary generalized seizures in February 2013. Because of contrast enhancing lesions with necrotic core and extensive oedema around the lesions in brain MRI (Figure 1a) he was undergone an operation and the result of tissue biopsy was compatible with necrotic inflammation, perivascular polymorphonuclear leukocytes (PMNL), lymphocytes and other inflammatory cells infiltration (Figure 1b). Then, he was admitted to our clinic in March 2013.
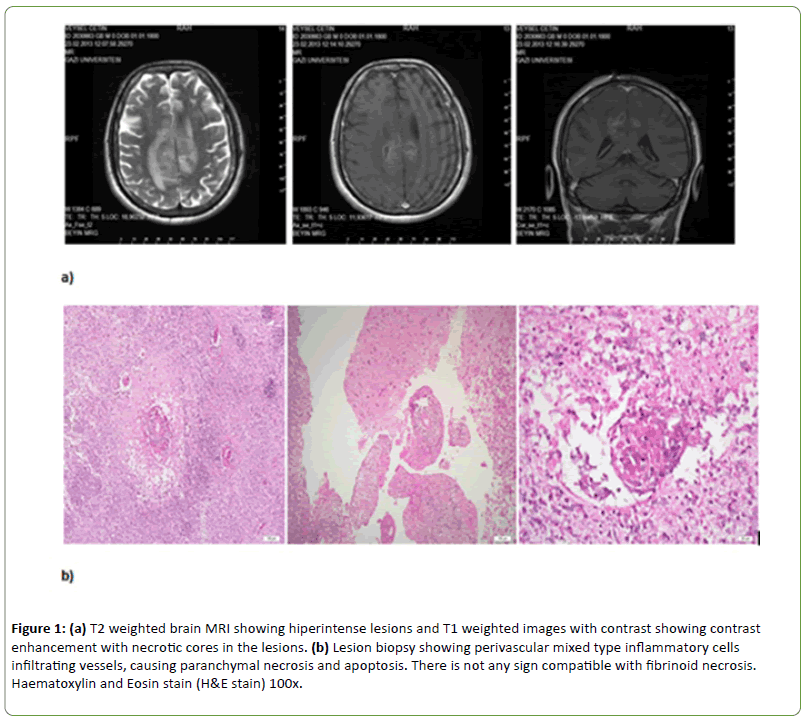
Figure 1: (a) T2 weighted brain MRI showing hiperintense lesions and T1 weighted images with contrast showing contrast enhancement with necrotic cores in the lesions. (b) Lesion biopsy showing perivascular mixed type inflammatory cells infiltrating vessels, causing paranchymal necrosis and apoptosis. There is not any sign compatible with fibrinoid necrosis. Haematoxylin and Eosin stain (H&E stain) 100x.
Except for erythema nodosum his physical examination was normal. Confusion, amaurosis in the both eyes, left spastic hemiparesis (1/5 in the upper and 3/5 in the lower extremity), Babinski's and Hoffman's signs in the left were found in the neurologic examination. Past medical history revealed that he had Behçet's disease since 1998 and had blindness due to eye involvement of BD since 2009.
Routine complete blood counting and biochemical tests were all normal. C-reactive protein level (45 mg/dl) and erythrocyte sedimentation rate (30 mm/h) were relatively high. Bacterial and fungal blood cultures were negative. Infectious causes including toxoplasma, galactomannan and criptococcus neoformans were also excluded. Brain MRI showed that there was huge increase of previous lesions sizes and numbers (Figure 2).
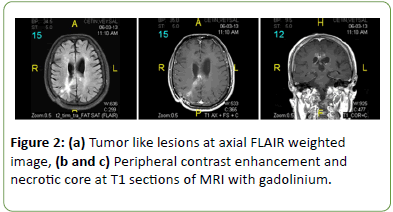
Figure 2: (a) Tumor like lesions at axial FLAIR weighted image, (b and c) Peripheral contrast enhancement and necrotic core at T1 sections of MRI with gadolinium.
The patient was diagnosed with NBD and was treated with intravenous (IV) methylprednisolone 1000 mg/day for 5 days. His left spastic hemiparesis improved significantly; muscle power became 3/5 and 4/5 in the upper and lower left extremities, respectively, on the fifth day of the treatment. He was discharged from hospital with oral steroid and pulse cyclophosphamide as maintenance treatment. Control brain MRI three months later showed significant reduction in all the lesions (Figure 3).
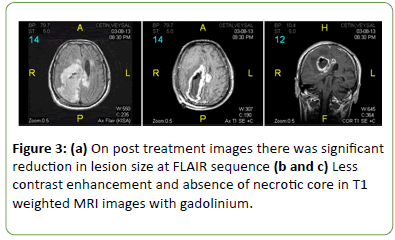
Figure 3: (a) On post treatment images there was significant reduction in lesion size at FLAIR sequence (b and c) Less contrast enhancement and absence of necrotic core in T1 weighted MRI images with gadolinium.
Discussion
CNS lesions in BD are polymorph and occur in 5.3% of cases in Turkey [3]. CNS involvement is one of the most severe manifestations of BD [14]. Sometimes it is difficult to differentiate from multiple sclerosis, stroke, intracranial hypertension, meningoencephalitis and myelitis. Concomitant systemic signs and symptoms are helpful in differential diagnosis [15].
Because of space-occupying lesion our case was operated at first. Since histopathologic examination revealed perivascular inflammatory infiltration, after excluding infectious causes, he was diagnosed with tumefactive NBD. As previously suggested, he was given high dose steroid and he improved significantly [4].
NBD may rarely cause tumour-like lesion, tumefactive NBD. We searched tumefactive NBD case reports in English based literature and found 10 biopsy proven cases that had mass effect in brain MRI by means of midline shift and/or ventricular compression so far [4-13]. Data regarding age, gender, complaints at admission, neurological symptoms, CNS imaging, histopathologic findings and treatment response were summarized including our case in Table 1 (11 cases).
| Case No |
Age/gender |
Complaints at admission |
Neurological examination |
Brain MRI |
Biopsy |
Treatment Acute attack/prophylactic |
Response to steroid |
| 1(4) |
59/F |
Headache, progressive left hemiparesis, lethargy and temporal fits as characterized by sensations of bad smell for the last 24 hours |
Left hemiparesis and bilateral papilloedema |
Right fronto-temporal mass causing shift of the midline structures suggestive of high-grade glioma |
Reactive gliosis associated with panvasculitis, thrombosis, extensive infarction and vascular proliferation |
Surgery |
Not given |
| 2(5) |
47/M |
Right hemiparesis, dysarthria, headache and vomiting |
Mild right hemiparesis of MRC grade IV and dysarthria |
Ring enhancing lesions with subtle perilesional oedema both at the left side of the pons and at the left parietal cortex |
Perivascular lymphocytic cuffing, focal necrotic lesion, microglial nodules, foci of necrosis, foamy histiocytic collections, and reactive gliosis |
Steroid |
Recovery |
| 3(6) |
43/M |
Headache, photophobia and asthenia |
Drowsiness, mild left arm and facial paresis and right eyelid ptosis, without pupillary abnormalities. |
2 cm thalamic lesion with mass effect and contrast enhancement |
Gliosis with gemistocytic astrocytes, without signs of tumour or vasculitis |
Steroid/cyclophosphamide |
Recovery |
| 4(7) |
50/M |
Left hemiparesis and dysarthria |
Left hemiparesis and dysarthria |
Large neoplasm-like lesion affecting the brainstem, basal ganglia, and white matter of the cerebral hemisphere |
Invasion of inflammatory cells in the white matter, especially around the blood vessels. |
Steroid |
Recovery |
| 5(8) |
34/M |
Right hemipyramidal syndrome and diffuse headache |
Right hemipyramidal signs |
Large left capsulo-thalamic lesion with oedema and peripheral contrast enhancement |
Necrotic lesions with inflammatory infiltration in the white matter. Multifocal lymphocytic, neutrophilic and macrophagic infiltrate with perivascular distribution. |
Steroid/cyclophosphamide |
Recovery |
| 6 (9) |
33/M |
Nausea, vomiting, and headache |
Mild right hemiparesis, somnolans |
Mass lesion at the left basal ganglia extending to the ventral site of the midbrain, causing compression to the surrounding tissue with cerebral edema. |
Mild reactive gliosis without inflammatory cell infiltration |
Steroid |
Recovery |
| 7(10) |
23/M |
Headache,fever and progressive right-sided weakness. |
Right homonymous hemianopia and mild right spastic hemiparesis |
Mass lesion involving theleft temporal lobe with signal change extending into the left cerebral peduncle, thalamus, internal capsule, basal ganglia, and corona radiata posteriorly |
Perivascular inflammatory infiltrate |
Steroid/azathiophirin |
Recovery |
| 8(11) |
41/F |
Mental deterioration and right hemiparesis |
Poor mental activity, dysarthria and right hemiparesis. |
Lesion of the left lenticulo-thalamic region |
Tissue ruled out a tumor but did not show any specific diagnosis |
Steroid |
Recovery |
| 9(12) |
52/F |
Dizziness, nausea, and headache, confusion, poor feeding andvomiting |
Brun’s nystagmus, mild attention deficit, immediate memory impairment, visuo-spatial disorientation, and wide-based unsteady gait |
In the right cerebellar hemisphere, posterior medulla, and pons with an isosignal central mass. |
Lymphocyticvasculitis involving small and medium-sized vessels (mainly veins) of white matter |
Steroid/azathioprin |
Not responded and died |
| 10 (13) |
38/M |
Confusion |
Left third nerve palsy, right spastic hemiparesis |
Mass-like lesion deep in the left side of the brain. The epicenter was in the left cerebral peduncle with extension into the basal ganglia and the frontal corona radiata, left optic tract, midbrain, left pons, left inferior cerebellar peduncle and left cerebellar hemisphere |
Patchy inflammatory infiltrates composed primarily of mononuclear lymphocytic cells and microglial rod cells. |
Steroid/azathioprine |
Recovery |
| 11 (own case) |
47/M |
Weakness in the left extremities, sleepiness and seizure |
Confusio, amaurosis in the both eyes, left spastic hemiparesis |
Right parietal subcortical region, ranging to the body of and corpus callosum and leading to mass effect |
Necrotic inflammation, perivascular PMNL, lenfocytes and other inflamatory cells |
Steroid/cyclophosphamide |
Recovery |
CYC: Cyclophosphamide; AZA: Azathioprine
Table 1: Characteristics of biopsy proven tumour-like NBD.
The median age was 43 (range 23-59) years, with a male predominance (72.7%) in patients with tumefactive NBD (Table 1). Admission complaints included headache (n=7), paresis (n=7), ataxia (n=5), seizure (n=2), and mental changes (n=5). Other abnormal findings in neurologic examination were aphasia, disturbed consciousness, cerebellar ataxia, hemianopia, memory deficit (Table 1). Lesion localisation was mainly in brain stem, basal ganglia or thalamus. Only two cases had lobar lesions (frontotemporal and parietal) without brain stem involvement (1st and 11th cases).
Seventy-three percent of all the cases had inflammatory cell infiltration in the tumour like lesion area. However, 27% of the cases did not have any inflammatory infiltration in spite of acute brain lesion; biopsy may not have taken from acute lesion area in those cases. Otherwise, although blood brain barrier was broken in acute lesion (because all tumefactive lesions were contrast enhancing) inflammatory cell infiltration might not be seen in all cases. May this be related pathologic investigation technique, or may inflammatory infiltration not develop in some acute lesion area? This issue needs to be explained in future researches.
The chosen treatment modalities were mainly corticosteroids during acute attacks (n=10; 91%) and immunosuppressive agents (n=6; 54.5%) such as cyclophosphamide and azathiopurine for preventive treatment (5-13). One patient who had panvasculitis in biopsy material was just operated, not given any medical treatment [4]. Nine out of ten patients (90%) improved completely or partially with steroid; however, one patient did not respond the treatment in her third attack and since she had transtentorial herniation, temporal lobectomy was done and she died later on [12].
For differential diagnosis of tumefactive NBD, space occupying lesions such as glioblastoma multiforme, lymphoma, bacterial abscess or tuberculoma must be considered. Rarely, an inflammatory condition such as sarcoidosis, Wegener's disease, multiple sclerosis (Balo's type) or granulomatous pseudotumour may lead to similar lesions [8]. It has been suggested that histopathological examination may be done to rule out infectious or tumoural diseases and to avoid the inadequate use of immunosuppressive agents in previous reports [14,16,17].
In summary, headache and paresis were the most frequent admission complaints, just compatible with classical parenchymal NBD. The most frequent lesion localisation was brain stem, basal ganglia or thalamus, also was compatible with classical parenchymal NBD; lobar lesions were seen less commonly in tumefactive NBD. Most cases had perivascular inflammatory cell infiltration in biopsy material and respond steroid very well. However, it should be kept in mind that inflammation may not be seen in biopsy material and some cases may not give good response to steroid treatment. Panvasculitis may be seen rarely (9%) in acute tumefactive NBD lesion.
Conclusion
We thought that when tumour-like lesions were seen in patients who had Behçet's disease in past medical history, NBD should be considered for preventing unnecessary brain operation. After excluding infectious aetiology with appropriate investigations, high dose IV methylprednisolone should be begun as soon as possible; the good response to steroid supports to the diagnosis of tumefactive NBD. If there is not good response, then, surgery should be planned as soon as possible. Although most patients have inflammatory infiltration in biopsy material why some cases do not have any inflammatory cell infiltration in acute lesion area needs to be clarified in further research.
Ethics Considerations
On behalf of all authors, the corresponding author states that this research complies with the guidelines for human studies and animal welfare regulations. The subject in our case report has given informed consent and we confirm that the study protocol has been approved by the institute's committee on human research.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
21137
References
- International Study Group for Behcet's Disease (1990) Criteria for diagnosis of Behçet's disease. Lancet 335: 1078-1080.
- Al-Araji A, Kidd DP (2009) Neuro-Behçet's disease: epidemiology, clinical characteristics, and management. The Lancet Neurology 8: 192-204.
- Akman-Demir G, Serdaroglu P, Tasçi B (1999) Clinical patterns of neurological involvement in Behçet's disease: Evaluation of 200 patients. Brain 122: 2171-2182.
- Tuzgen S, Kaya AH, Erdincler D, Oguzoglu SA, Ulu O, et al. (2003) Two cases of Neuro-Behçet's disease mimicking cerebral tumor. Neurol India 51: 376-378.
- Heo JH, Lee ST, Chu K, Kim M (2008) Neuro-Behçet's disease mimicking multiple brain tumors: diffusion-weighted MR study and literature review. J Neurol Sci 264: 177-181.
- Darmoul M, Bouhaouala MH, Smida H, Dougui HM (2006) Pseudo-tumoral neuro-Behcet's disease. Rev Neurol 162: 643-647.
- Imoto H, Nishizaki T, Nogami K (2002) Neuro-Behcet's disease manifesting as a neoplasm-like lesion. Neurol Med Chir 42: 406-409.
- Noel N, Hutie M, Wechsler B (2012) Pseudotumoural presentation of neuro-Behçet's disease: case series and review of literature. Rheumatology (Oxford) 51: 1216-1225.
- Matsuo K, Yamada K, Nakajima K, Nakagawa M (2005) Neuro-Behcet disease mimicking brain tumor. AJNR Am J Neuroradiol 26: 650-653.
- Bennett DL, McCabe DJ, Stevens JM, Mifsud V, Kitchen ND, et al. (2004) Tumefactiveneuro-Behcet disease. Neurology 63: 709.
- Yoshimura J, Toyama M, Sekihara Y (2001) Behcet disease mimicking a thalamic tumor. No Shinkei Geka 29: 527-531.
- Park JH, Jung MK, Bang CO (2002) Neuro-Behcet's disease mimicking a cerebral tumor: A case report. J Korean Med Sci 17: 718-722
- Gertner E (2004) Severe recurrent neurological disease in the MAGIC syndrome. J Rheumatol 31: 1018-1019.
- BorhaniHaghighi A, Sarhadi S, Farahangiz S (2011) MRI findings of neuro-Behçet's disease. Clin Rheumatol 30: 76570.
- Dutra LA, Barsottini OGP (2016) Neuro-Behçet’s Disease: A review of neurological manifestations and its treatment. J Vasc 2: 112.
- Arai Y, Kohno S, Takahashi Y (2006) Autopsy case of neuro-Behçet's disease with multifocal neutrophilic perivascular inflammation. Neuropathology 26: 579-585.
- Hirohata S (2008) Histopathology of central nervous system lesions in Behçet's disease. J Neurol Sci 267: 41-47s.








