Keywords
Root surface; Initial caries lesion; Ultrasound; QLF; DIAGNOdent; CLSM; Demineralization; Remineralization
Introduction
Early detection of a root surface lesion may enhance remineralization by an effective treatment [1]. Cervical area of a tooth is very difficult to restore due to being with less hard tissue, aprismatic or disorganized prism structure. Moreover being with more porotic nature compared to enamel, different positions of anatomical region, presence of less and narrow dentinal canals of cementum are the other reasons for not obtaining a sufficient adhesive restoration. Additionally it is a difficult region for any restoration, since centric and eccentric forces concentrate over the tooth. Due to those reasons, detection of early root surface caries and its preventive treatment gain big importance in dentistry [2]. Increments in caries were found to be strongly correlated with immediate or 24 hour immediate measurement of the etched sides [3]. Extensive use of fluoride application decreases the frequency and prevalence of dental caries in developed countries. This important change on disease treatment directed more research on new detection methods, providing maximum tissue prevention for the patient and less operation requirement for the dentist [4].
Preventive treatments may well be obtained on determined lesions. Ultrasound, QLF, DIAGNOdent and CLSM methods were found effective at early stages of a caries lesion on enamel at in vitro conditions, while very few studies were done on root surface caries [5-8]. Application of fluoride treatment at 3, 6 months, 1 year irrespective of the type of fluoride enhanced remineralization of root surface caries [9].
A clinical study of detection of initial root caries lesions and follow up effects of remineralization agents were searched clinically before this study, then QLF and Ultrasound could detect and differentiate any remineralization occurred by fluoride toothpaste and Bifluoride12 varnish [8].
High concentration of fluoride toothpaste increased remineralization while promoting a decrease in demineralization [10]. In a very well-designed in vitro study, fluoride solution enhanced remineralization on root surface caries even at 400 micron depth [5]. Presence of cementum increases resistance to caries with richest fluoride at the 25 micrometer outer layer [11]. Advanced dentinal lesions may be remineralized in spite of demineralized lesion body and surface layers which are the most preferable sites for remineralization. Remineralized surface layer does not inhibit transport of mineral ions.
Remineralization of the lesion refers to a number of crystal growth [6]. On the other side another study, 6 month period, long existing white spot lesions were stable enough, they did not allow fluoride work over [12].
Ultrasonic waves are mechanical pressure waves similar to audible sound waves and must have a medium in which to propagate [13]. Ultrasound has been applied to the evaluation of some structures and the conditions of soft and hard tissues by previous investigators [1,14-16]. Some researchers studied the ultrasound detection of enamel demineralization and small enamel caries [15,17-21] Bab et al. [19] and Ziv et al. [2] evaluated an ultrasonic instrument using surface waves, and the system was found to be sensitive for detection of proximal enamel and dentine caries lesions.
It was not until 1998 that the implications of the observations of Alfano and Yao [22] were fully realised and Hibst and Gall [23] reported that laser light in the region of 655 nm induced fluorescence in the red and near-infrared region of the spectrum and that there were quantifiable differences between sound and carious enamel and dentine.
The hypothesis of this study was that ultrasound may notice any early defect on root tissue of human enamel. Therefore it was aimed that early root caries could be detected and be quantified at the earliest stage, moreover its remineralization. Additional aim was to evaluate the effectiveness of the following devices on diagnosis of early root caries lesion; Ultrasound, QLF, DIAGNOdent and CLSM in vitro.
Materials and Methods
Human maxillary central incisors extracted due to periodontal reason cleaned with 1% detergent and distilled water left in 10% buffered formalin solution (pH 7.0) and was kept in distilled water until the experimental process.
Teeth with caries, cracks, defects or colored at the cervicals were excluded from the study following observation with naked eye and 30 x magnification under the light microscope (Leica MZ 7, 5, Cambridge, England). Cementum with underlying dentin were removed from bucco-cervical surfaces as squared prism blocks in 3 mm thickness by a diamond fissure bur (#012) (RomiDiamondTM Burs, Romidan LTD, Herzeliya, Isreal) with vigorous water at high speed turbid. The specimens were prepared as 3 mm thickness, 4 mm length and 2 mm width by measuring with electronic digital cumpass (Electronic Digital Caliper, T3456, Shan, Guilin, China). 64 specimens with pulpal surface down were placed on cylinder clear polyacrylic blocks with 5 cm length 6 mm in diameter and stuck with a glue (Pattex, Henkel, Düsseldorf, Almanya).
All specimens were numbered from 01 to 64 randomly. Cementum tissue was removed by 600 grain emery paper in wet environment to imitate wear in oral environment. Then the specimens were kept in distilled water in a container with a cover.
The In Vitro Microbial-Caries Model developed by Fontana et al. [24] was used in this study. Sixty-four human enamel specimens were demineralized for 96 hours (Indiana University, Oral Health Research Institute, Indianapolis, USA), then half of each specimen was covered with an acid-resistant nail varnish (FlorMar® Nail Enamel, France) to keep an area for the control on the healthy surface. The varnished surface was marked with a water resistant pen on clear polyacrylic blocks. The specimens had been sterilized with ethyleneoxide gas (American Hospital Sterilization Department, Ni¸anta¸, Istanbul, Turkey) for 24 hours before processing the in vitro microbial model. Two specimens from each group were randomly chosen before treatment and were examined for the baseline data on Confocal Microscopy.
The 21 specimens in each group were secured in cariesforming vessels by glueing the ends of their plexiglass rods to a round plexiglass base that fit in the bottom of the vessels.
In vitro microbial caries model
This process was performed at Oral Health Research Institute of Indiana University Faculty of Dentistry (Indiana, Indianapolis, USA). All of the media and model components, except for the specimens, were autoclaved at 121°C for 20 min prior to the initiation of each experiment. Each group of 21 specimens was placed in a caries-forming vessel (125-ml Pyrex slow speed stirring vessel; Fisher).
All caries-forming vessels were placed on an electric stirrer inside an incubator at 37°C under aerobic conditions. Each caries vessel had three inlets, one for TSBS or TSB (Tryptic soy broth with sucrose), one for MW (Mineral wash which is a phosphate buffer solution), and one for injection of the antibody and one outlet for drainage tubing. The drainage tubing ended flush in a drainage container, which was also placed inside the incubator. Drainage of fluid from each caries vessel was maintained at 0.7 ml/min by a peristaltic pump (Figure 1).
3
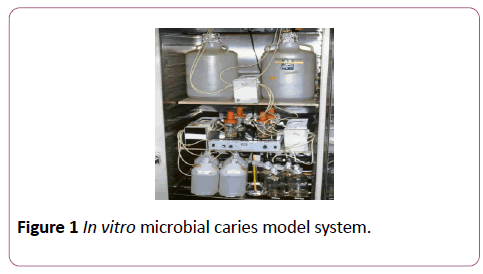
Figure 1: In vitro microbial caries model system.
The demineralized areas were analyzed by the following systems; QLF, Ultrasound, DIAGNOdent and CLSM.
QLF is an optical, visual light-based fluorescence detection and quantification system for assessing early demineralization of human enamel. QLF views were obtained in a dark room at the department of Restorative Dentistry at Marmara University. Analysis processes were carried out by marking the framed demineralized surface and healthy tooth surface. The program compared the demineralized and the healthy tissue area at four different thresholds for ΔF (%), area (mm2) and ΔQ values [25-28]. Multiplying ΔF (%) and the area (mm2), ΔQ (% mm2) values for each lesion were obtained. Standardized rectangle was cut out from remineralized view and placed over demineralized view, thus software QLF 1.96w programme could analyzed them.
Ultrasound measurements were taken at the Turkish Airlines, NDT Division in stanbul, Ye¸ilköy, Atatürk Airtport, Turkey (Figures 2 and 3). Nondestructive testing (NDT) methods aim for detection of a discontinuity and its volumetric changes without any destruction of the material tested.

Figure 2: Ultrasound system used in the study.

Figure 3: Measurement of a specimen using the ultrasound probe.
DIAGNOdent measurements were done at Hacettepe University Department of Restorative Dentistry (Ankara, Turkey). In the present study, the sixty four root surface specimens were measured at baseline, demineralization and remineralization stages by DIAGNOdent system.
CLSM measurement was processed at Indiana University Oral Health Research Center. The surface of each specimen was allowed to air dry before being analyzed with the confocal laser scanning microscope (Leica DM 6000 M, Germany). After being brought into focus (using a 10X Nikon objective, N.A. 0.25), the specimens were illuminated with an argon ion laser using a 488 nm excitation wavelength. Confocal slits were set at 15 μm with a 515 nm long pass carrier filter, and the argon laser intensity was set at 100%. For collection of the images, samples were frame-averaged using 128 frames per imaged (Figures 4 and 5). The surface analysis and Depth analysis of the specimen at Figure 4 was done to illustrate the demineralized areas (Figures 6 and 7).
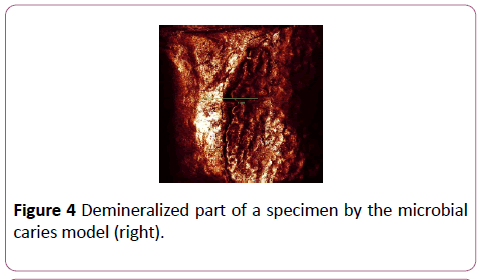
Figure 4: Demineralized part of a specimen by the microbial caries model (right).
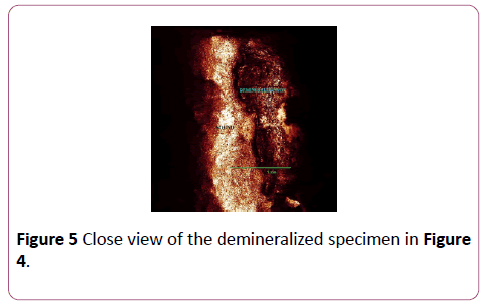
Figure 5: Close view of the demineralized specimen in Figure 4.
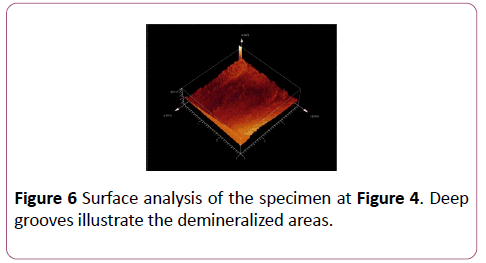
Figure 6: Surface analysis of the specimen at Figure 4. Deep grooves illustrate the demineralized areas.
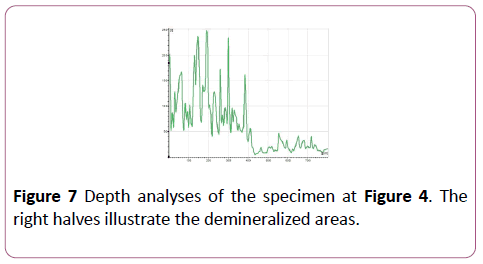
Figure 7: Depth analyses of the specimen at Figure 4. The right halves illustrate the demineralized areas.
Half of the surfaces were covered with acid resistant nail varnish (Figure 8). The demineralized specimens were randomly distributed into three groups, 21 in each with two containers.
Figure 8: The prepared specimens.
During remineralization process, group one was left in artificial saliva without any treatment. Group two was treated with 1100 ppm fluoride (Colgate Total, Turkey) twice a day. Group three was also treated with 1100 ppm fluoride (Colgate Total, Turkey) twice a day besides application of a fluoride varnish once a week (Bifluoride12, Voco, Germany). The specimens were stabilized into the cover of each container by a glue. It was then possible to embed the specimens bond to clear sticks into the treatment solution. The containers were kept on the magnetic stirrer plate (Multipoint HP15P, Variomag, USA). Saliva was changed every morning to prevent any bacterial contamination in the room temperature. The saliva was constantly mixed over the stirring plate during the treatment process.
The cyclic remineralization regimen [29] consisted of a 3 hours/day acid challenge in 50% saturated hydroxyapatite (HAP)/0.1 M lactic acid carbopol solution (pH 5.0), with 21 min dentifrices treatment periods per day, at room temperature. The specimens were kept in artificial saliva rest of the time (Table 1). During treatment period, the specimens were immersed in dentifrice slurries to simulate daily brushing. The slurries were prepared by adding 5.0 mg of dentifrice to 10 ml of water in a beaker with a magnetic stirrer. Bifluoride12 varnish was applied on the specimens starting at the first day once in every seven days, five times in total at group three.
| Hours |
Applied solutions |
| 08.00–09.00 |
Acidc |
| 09.00–09.01 |
Toothpastea |
| 09.01–13.00 |
Saliva |
| 13.00–14.00 |
Acid |
| 14.00–19.00 |
Saliva |
| 19.00–20.00 |
Acid |
| 20.00–20.01 |
Toothpaste |
| 20.01–08.00 |
Saliva |
aToothpaste slurries=5 mg of toothpaste to 10 ml water;
bArtificial saliva; c0.1 M lactic acid, pH 5.0
Table 1: The 30-day treatment regimen applied on the specimens.
The analyses following remineralization
The specimens were washed out with distilled water during two minutes and dried by airblasting, then, the varnish was removed carefully using aceton before processing the analyses of the remineralized specimens by the systems; QLF, ultrasound, Diagnodent and CLSM. The QLF results were evaluated by Repeated-measures ANOVA (α=0.05) statistical analysis method. Tukey statistical analysis was used as well.
Results
The lesion shows less fluorescence than the healthy tissue. Therefore the difference between fluorescence of healthy tissue and lesion were measured of healthy tissue surrounds the lesion with less fluorescence. The difference between the fl. of healthy tissue surrounding lesion is measured, to obtain loss on lesion (%), maximum fluorescence loss in the lesion (%) and size of the lesion area (mm2) were obtained following the analyses of the views. ΔQ value was calculated as (ΔF) × (mm2). ΔQ values were considerably different in statistics for the mean values of data obtained at recalls (p<0.001).
Table 2 presents average fluorescence loss and total volumetric mineral loss (ΔQ) at the control, demineralization and remineralization groups.
| Corresponding groups |
Average Fluorescence % |
DQ(mm2 x%) |
| Control group (n=64) |
-26.82 ± 12.48 |
-9.83 ± 4.11 |
| Demineralization (n=64) |
-38.36 ± 10.81 |
-15.51 ± 3.85 |
| Remineralization with 0 ppm (artificial saliva) (n=21) |
-36.19 ± 10.56 |
-13.71 ± 4.33 |
| Remineralization with 1100 (n=21) |
-31.65 ± 7.66 |
-11.61 ± 4.25 |
| Remineralization 1100 ppm + Fluoride varnish (n=21) |
-28.33 ± 9.53 |
-10.81 ± 3.45 |
Table 2: presents average fluorescence loss and total volumetric mineral loss (Q) at the control, demineralization and remineralization groups.
The QLF results were evaluated by Repeated-measures ANOVA (α=0.05) statistical analysis method. QLF differentiated demineralization and baseline, also demineralization and second, third remineralization groups according to ΔF values (Table 3). Statistically significant differences were in average fluorescence loss and total volumetric mineral loss (ΔQ) between demineralization and second/third remineralization groups (p< 0.01). Second group showed 57% decrease on demineralization of lesion by toothpaste application. The third group gave 72% decrease on demineralization by toothpaste and varnish application. There was no significant difference between demineralization and 0 ppm fluoride groups (p< 0.01). Two remineralization groups, 1100 ppm floride and 1100 ppm fluoride and varnish were not different from each other statistically (Table 3).
| Corresponding groups |
Diffrences of average fluorescence radiance D F |
D Q |
| T |
p |
T |
p |
| Control group |
Demineralization |
3.86 |
0.0003* |
2.52 |
0.014 |
| Demineralization |
0 ppm |
1.31 |
0.37 |
1.58 |
0.12 |
| Demineralization |
1100 ppm |
3.35 |
0.0003* |
2.48 |
0.012 |
| Demineralization |
1100 ppm +Bifluoride |
2.45 |
0.0004* |
2.34 |
0,018 |
| 0 ppm |
1100 ppm |
2.14 |
0,0006* |
2.26 |
0.017 |
| 0 ppm |
1100 ppm +Bifluoride |
2.63 |
0.0005* |
2,06 |
0.019 |
| 1100 ppm |
1100 ppm +Bifluoride |
0.54 |
0.58 |
0.87 |
0.38 |
Statistically Significant (P<0.001)
Table 3: The data of QLF parameters showed that according to statistical correspondence between control group (=64), demineralization group (64) and remineralization groups (63).
According to Tukey analyses (F=9.789, p< 0.001), there was statistical differences between baseline group and demineralization (p< 0.001); ad the remineralization groups separately; group 1 (p< 0.01); group ll (p< 0.01); and group lll (p< 0.01). There was no correlation between demineralization and remineralization groups (p>0.05), (Table 4).
| Average ultrasound value |
Baseline group |
Demineralization group |
0 ppm (group 1) |
1100 ppm (group II) |
1100 ppm +Bifluoride (group III) |
| 0.95 ± 0.12 |
1.18 ± 0.25 |
1.16 ± 0.32 |
1.15 ± 0.24 |
1.15 ± 0.28 |
Table 4: The average ultrasound value for the baseline, demineralization and remineralization groups.
DIAGNOdent measured the difference between baseline and demineralization by Tukey analysis (F=437.30 p< 0.001), (Control group vs demin group: p< 0.001). Also control group and groups l-lll were differentiated separately according to Tukey analyses (p< 0.001). Additionally, there was difference between demineralization and group lll (p< 0.001). However, any significant correlation was found between the remineralization groups (p>0.05), (Table 5).
| Average DIAGNOdent value |
Baseline group |
Demineraliza-tion group |
0 ppm (group 1) |
1100 ppm (group II) |
1100 ppm +Bifluoride (group III) |
| 8 ± 4 |
30 ± 3 |
29 ± 3 |
28 ± 2 |
27 ± 3 |
Table 5: The average DIAGNOdent value for the baseline, demineralization and remineralization groups.
The average depth of the lesions as measured by Confocal Microscopy are presented in Table 6. CLSM differentiated statistically significant demineralization from baseline (F=93.324, p< 0.001), (Control group vs demin group: p< 0.001); group l-lll (p< 0.001); but it could not differentiate any difference from demineralization and any remineralization ((p>0.05). Additionally, no correlation was found between remineralization groups (Table 6).
| Average depth of lesions |
Baseline Group |
Demineraliza-tion Group |
0 ppm |
1100 ppm |
1100 ppm +Bifluoride |
| 6.8 ± 3 |
71.81 ± 27.22 |
69.28 ± 30.41 |
68.63 ± 12.42 |
66.97 ± 28.52 |
Table 6: The average depth of the lesions for baseline, demineralization and remineralization groups (µm) by CLSM.
Discussion
The microbial caries model used in this study is one of the best imitations of caries production at in vitro conditions. Uniqueness of this lesion production is the first study of root caries lesion produced by the microbial model. The lesions were produced very successfully on root surfaces with an average of 72-micron thickness (Table 6). Conventional caries detection methods are not competent on determination of the initial lesions on enamel also on root surfaces and their clinical follow ups. Lussi [30] reported in his study that 34 dentists joined to the study for determination of caries using mirror and probe by naked eye and were right only 42%. DIAGNOdent measurements are as valuable as clinical ones in this research, since the caries lesions were produced here by the microbial model. Hellyer and Lynch [31] suggested that early detection and repairement of the initial defects should be done with fluoridated agents. Billing et al. [32] reported that shallow root caries lesions could be reshaped, saved and successfully treated by topical fluoride applications.
Lesion area, depth and mineral loss are the parameters that have to be evaluated quantitatively in order to be determined objectively in the QLF system [28]. Detection of early caries lesion has been worked mostly on enamel tissue. First in 1928 Benedic, has determined the fluorescence effects of teeth. van der Veen and ten Bosch [33] studied the fluorescence principle first on root surface demineralization in 1996 in vitro. QLF system was developed to measure the fluorescence amount of dental tissues, and aimed to measure the loss of fluorescence [34]. In that study, lazer fluorescence showed a high correlation with TMR the control method (r=0.91). Shi et al. [35] compared DIAGNOdent and QLF on smooth surface enamel caries and both systems showed good performance on detection of mineral loss, but QLF giving closer correlation. Their study showed that both methods could record mineral loss quantitatively. Moreover, active caries lesion could turn into inactive (arrested) lesion. Our previous study demonstrated the effectiveness of the QLF system on detection of cervical initial caries lesions in clinics [8]. Correlating the findings of the present in vitro study we could find similar results in ΔQ values showing mineral loss in area and fluorescence differences could be observed in demineralization and remineralization groups.
Van der Veen [36] reported that QLF could determine root surface lesions at least 80 μm in depth in her thesis. Fluorescence radiance of carious lesion was weaker than the fluorescence of intact enamel tissue on QLF evaluation. There was a strong relation between fluorescence radiance and mineral loss. The author mentioned that change in fluorescence radiance may be followed to control mineral changes by time. Following up the mineral changes in time would be so much beneficial for reducing demineralization and determination of any remineralization. The benefit of follow ups would be believe in of patients how deep the lesion was, how much the demineralization was reduced by constant application of fluoride products such as varnish, Bifluoride12, in the previous study [8]. It was determined in present in vitro study that, remineralization of root surface incipient lesions could be detected and quantified only by the QLF system used. QLF system was based on 87% mineral consistency of healthy enamel tissue. Since root dentin has less mineral, the control group, healthy root dentin was recorded as -26,82% change in fluorescence radiance. This data actually is the mean of the base of healthy root dentin as -9,83 (ΔQ), while demineralized mean data was -38,36, and -15,51 (ΔQ). The difference between healthy and demineralized samples was meaningful towards lost mineral (Table 3). Remineralization without and with fluoride (-13,71; -11,61) was comparable (p=0.0006). However combined use of fluoride as 1100 ppm toothpaste and varnish were not more effective than sodiumfluoride toothpaste alone (p=0.58; (Table 3). Meantime CLSM found sound dentin as 6,8 μm, while demineralization was 71,81 μm. No difference was there among both fluoride treatments and no fluoride group. Since both QLF system and CLSM as control test methods did not see difference between remineralization groups, two opinions may appear; one is that fluoride treatments used in this study may not remineralize enough to see clear difference from each other. The second is that remineralization is not enough to be differentiated from demineralization in CLSM datas. May be remineralization is not produced on root dentin in this study? May be it is not enough to be count on as in enamel. How much mineral gain is enough for remineralization?
Actually differentiation of remineralization by QLF does not mean there is a good amount of remineralization either. Since QLF may detect fluorescence loss on a large surface area, fluoride mineral may not fill out whole lesion enough to be differentiated by CLSM, Ultrasound and DIAGNOdent. Presence of a very few amount of fluoride may not be accepted as mineralization. As Fure and Lingström [9] reported remineralization of root dentin at 3 months as Durmu¸lu reported as one month. Ultrasound could feel the porotic nature of root dentin.
Ultrasound results also gave very few remineralization same as control method CLSM. The reason for that there may be very few remineralization could not make any difference on sound waves. In this case may be there is not enough remineralization on root dentin sound vawes propagate through dentin tissue. Interestingly all three methods; CLSM, Ultrasound and DIAGNOdent gave similar results as very close numbers of demineralization and remineralization of root dentin. Since ultrasound may see the differences even at 50 μm thickness [37,38], CLSM is very sensitive on microscopic level, and DIAGNOdent is also a fluorescence sensitive method, then it could be conclude that there is some remineralization by fluoride treatment groups but not enough to be detected by the systems used; Ultrasound, DIAGNOdent and Confocal microscopy, instead of saying these methods cannot detect the remineralization. This resting method, ultrasonography has a probe with longitudinal waves working in a straight way by applying on the surface direct. Therefore, it is more useful for the lesion on smooth surfaces, including root region of human teeth. On the other hand interproximal region to detect any initial caries lesion, would have to be worked either by separating the teeth by a dental wedge and/or by another probe with circumferential waves which is the one not used by us before.
Both systems; CLSM, which is control method and Ultrasound could not differentiate remineralization and remineralization differences between the fluoride treatment groups. The obtained values for both system showed very close numbers there may be some remineralization, but not be detected. DIAGNOdent values gave similar results by determining demineralization but remineralization, although only remineralization group with both remineralization; bifluoride varnish and toothpaste could be different from demineralization. QLF could detect differences between demineralization and remineralization as well.
Under the in vivo conditions, porphirin could be detected by DIAGNOdent and fluorescence differences could be evaluated as numbers by the system. In the present study, the lesions were produced by the microbial model imitating the in vivo conditions, therefore DIAGNOdent results obtained in the present study are as valuable as any in vivo study.
Ekenback et al. [39] reported the reduced amount of St. mutans by Cervitec treatment on root surface caries and evaluated at one week, one-month duration. Bifluoride12 fluoride varnish used in the present study gave some remineralization but could not be more successful than 1100 ppm fluoride toothpaste alone (P=0.0006; Table 3). Moreover, remineralized lesion area did not show any difference between only 1100 ppm fluoride toothpaste and both 1100 ppm fluoride toothpaste and Bifluoride12 varnish groups (Table 3; p>0.58).
In our previous study, QLF could determine the difference between fluoridation effectiveness of Bifluoride12 varnish and/or 1000 ppm fluoride toothpaste [8]. High concentration of fluoride incorporate into tooth structure on surface probably producing CaF2 resembling globules, either immediately or later as deposits enhanced remineralization [40]. But in this in vitro study it seems there is not over protection by the varnish except acting as a seal over initial lesion (Tables 3-6).
Tranaeus et al. [41] showed an important decrease in fluorescence and lesion area in the fluoridated group. Al- Khateeb et al. [42], Ogaard and Bosch [43] used QLF to demonstrate white spot lesions in orthodontic patients following removal of braces and the lesion size was reduced, moreover enamel fluorescence was partially regained at oneyear recall. Our results were in agreement with what Al- Khateeb’s study [42] on detecting and monitoring initial lesions. It was also understood that root surface caries lesions could be remineralized following application of a fluoride varnish during one month period [8]. Demineralization groups with 1100 ppm fluoride and/or Bifluoride12 varnish both showed the root surface lesions were remineralized. However in the group applied both, remineralization was not more compared to either one, most probable reason for that the varnish had covered the lesion and prevented it from remineralization cycle in the oral cavity. What ultrasound measurement mechanism is based on reflection of sound waves back to the originating point where the waves hit on a different media. Therefore at the control group what measured was enamel thickness from the most outer surface of cementum to the cemento-dentinal junction [37,38,44,45]. In Table 4 it was illustrated as 0.95 ± 0.12 mm value at the control group with sound cementum tissue.
In the demineralization group; 1.18 ± 0.25 micrometer value refers to through demineralization of both cementum and dentin tissues. Then the groups 1, 2, 3 gave few numerical changes due to close mineralization in the groups [46]. However, these numbers were similar with Confocal and DIAGNOdent datas being few number differences in remineralization groups (Tables 5 and 6).
Root surface caries can be prevented first by good oral hygiene, periodic recalls at the dental office and a constant toothbrushing habit [46] as demonstrated in the present study by chemical effect of fluoridated toothpaste alone. The lesion produced by microbial model may not be thick but was detected by all systems used in this study. However remineralization was either less by application of only one minute each time a day for dentine tissue, or varnish does not make any difference on remineralization of root dentine under the conditions of this study. It probably only works for sealing the surface. Then why there is a need for high amount of fluoride in it? Application of fluoridated varnish does not show additional remineralization (Table 3), on the contrary, as declared by the company very high amount of fluoride with 56,000-70,000 ppm fluoride, would be left on the tooth surface prone to uncontrolled exposure of high amount at once or in time under thermal or chemical changes.
In the present study the aim; detection of demineralization of initial root surface caries by Ultrasound, QLF, DIAGNOdent, and CLSM were obtained successfully and Ultrasound, QLF and DIAGNOdent could quantify root surface initial lesion as nondestructive methods.
Demineralization on root dentin could be differentiated by the Ultrasound system was proven as the hypothesis of this study under the present conditions.
Conclusion
It was concluded that demineralization of root surface incipient lesions could be detected and quantified by Ultrasound, QLF, DIAGNOdent and CLSM systems under the conditions of this in vitro.
Acknowledgements
We would like to thank to Monique van der Veen, Elbert Joseling de jong, Margharita Fontana, Carlos Cabezas, George K Stookey and Saadet Gökalp, Meserret Tiritlio¸lu for their valuable help on some methods used in the study and to Berrin Yaniko¸lu for her valuable contribution to the paper.
21524
References
- Fukukita H, Yano T, Fukumoto A, Sawada K, Fujimosa T, et al. (1985) Development and application of an ultrasonic imaging system for dental diagnosis. J Clin Ultasound 13: 597-600.
- Ziv V, Gazit D, Beris D, Feuerstein O, Aharonov L, et al. (1998). Correlative ultrasonic histologic and roentgenographic assessement of approximal caries. Caries Res 32: 294.
- Meller C, Santamaria RM, Connert T, Splieth C (2012) Predicting caries by measuring its activity using quantitative light-induced fluorescence in vivo: A 2-year caries increment analysis. Caries Res 46:361–367.
- Yassen GH, Lippert F, Eckert G, Eder J, Zandoná AF (2012) The effect of strontium and combinations of strontium and fluoride on the remineralization of artificial caries lesions in vitro. Quintessence Int. 43: e95-103.
- Mukai Y, Lagerweij MD, Ten Cate JM (2001) Effect of a solution with high fluoride concentration on remineralization of shallow and deep root surface caries in vitro. Caries Res 35: 317-24.
- Mukai Y, Ten Cate JM (2002) Remineralization of advanced root dentin lesions in vitro. Caries Res 36: 275-80.
- Hang L, Watkins CA, Ettinger RL, Wefel JS (2005) Effect of topical flüoride and flüoride varnish on in vitro root surface lesions. Am J Dent 18: 182-187.
- Durmu¸oglu O, Tagtekin D, Yankoglu F (2012) Clinical evaluation of demineralization and remineralization of intact root surface lesions in the clinic by a quantitative light-induced fluorescence system. Laser Med Sci 27: 397-402.
- Fure S, Lingström P (2009) Evaluation of different fluoride treatments of initial root carious lesions in vivo. Health Prev Dent 7: 147-154.
- Diamanti I, Koletsi-Kounari H, Mamai-Homata E, Vougiouklakis G (2011) In vitro evaluation of fluoride and calcium sodium phosphosilicate toothpastes, on root dentine caries lesions. J Dent 39: 619-28.
- Dietz W, Kraft U, Hoyer I, Klingberg G (2002) Influence of cementum on the demineralization and remineralization processes of root surface caries in vitro. Acta Odontol Scand 60: 241-247.
- Zantner C, Martus P, Kielbassa AM (2006) Clinical monitoring of the effect of fluorides on long-existing white spot lesions. Acta Odontol Scand 64: 115-122.
- Krautkramer J, Krautkramer H (1977) Ultrasonic testing of materials. Berlin, Springer-Verlag, Germany.
- Baum G, Greenwood L, Slawski S, Smirnow R (1963) Observation of internal structures of teeth by ultrasonography. Science 139: 495-496.
- Lees S, Barber FE, Lobene RR (1970) Dental enamel: Detection of surface changes by ultrasound. Science 169: 1314-1316.
- Spranger H (1971) Ultra-sonic diagnosis of marginal periodontal diseases. Int Dent J 21: 442-455.
- Lees S, Barber FE (1971) Looking into the tooth and its surfaces with ultasonics. Ultrasonics 9: 95-100.
- Ng SY, Ferguson MW, Payne PA, Slater P (1998) Ultrasonic studies of unblemished and artificially demineralized enamel in extracted human teeth: a new method for detecting early caries. J Dent 16: 201-209.
- Bab IA, Feuerstein O, Gazit D (1997) Ultrasonic detector of proximal caries. Caries Res 31: 322.
- Gazit D, Ziv V, Bab I, Feuerstein O, Findler M, et al. (1998) In vitro/in vivo assesment of approximal caries using ultasonic surface waves. J Dent Res 77: 766.
- Yan¸lu FÇ, Ozturk F, Hayran O, Analoui M, Stookey GK (2000) Detection of natural white spot lesions by an ultrasonic system. Caries Res 34: 225-232.
- Alfano RR, Yao SS (1981) Human teeth with and without dental caries studied by visible luminescent spectroscopy. J Dent Res 60: 120-122.
- Hibst R, Gall R (1998) Development of a diode laser-based fluorescence caries detector. Caries Res 32: 80.
- Fontana M, Çal¸kan Yankoglu F, Özturk F, Ando M (2000) Comporison of QLF, ultrasound and confocal microscopy in the measurement of demineralization/remineralization of enamel lesions developed on natural smooth surfaces. Proceedings of 4th Indiana Conference, early detection of dental caries II, Iniana University, Inidianapolis. pp. 280-301.
- Ten Bosch JJ, Angmar-Mansson B (1991) A review of quantitative methods for studies of mineral content of intraoral incipient caries lesions. J Dent Res 70: 2-14.
- Bayne CS, Thompson YJ, Taylor FD (2002) Dental Materials. Roberson MT, Heymann HO, Swift JE (eds) Operative Dentistry, (4th edn) The CV Mosby, St. Louis, USA. pp. 33-235.
- Ashley PF, Blinkhorn AS, Davies RM (1998) Occlusal caries diagnosis: An in vitro histological validation of the electronic caries monitor (ECM) and other methods. J Dent 26: 83-88.
- Bozkurt FÖ, Ta¸tekin DA, Küçükkele¸ N, Sur H, Yan¸lu F, et al. (2003) An ultrasonic system for the detection of dental caries. Stookey GK (ed) Early Detection of Dental Caries III Proceedings of the 4th Annual Indana Conference, s., SpringDot Ohio, USA. pp. 107-120
- Dunipace AJ, Zhang W, Beiswanger AJ, Stookey GK (1994) An in vitro model for studying the efficacy of fluoride dentifrices in preventing root caries. Caries Res 28: 315-321.
- Lussi A (1991) Validity of diagnostic and treatment decisions of fissure caries. Caries Res 25: 296-303.
- Hellyer PH, Lynch E (1992) Root caries an Old problem. In: General Dental Treatment Edinburgh: Churchill Living-stone. UK. pp. 15–81.
- Billings RJ, Brown LR, Kaster AG (1984) In vivo studies on incipient and shallow root caries. J Dent Res 63: 257
- Van Der Veen MH, Ten Bosch JJ (1996) A fiber optic setup for quantification of root surface demineralization. Eur J Oral Sci 104: 118-122.
- Angmar-Månsson B, Ten Bosch JJ (2001) Quantitative light-induced fluorescence (QLF): A method for assessment of incipient caries lesions. Dentomaxillofac Radiol 30: 298-307.
- Shi XO, Tranaeus S, Angmar-Mansson B (2001) Comprasion of QLF and DIAGNOdent for quantification of smooth surface caries. Caries Res 35: 21-26.
- Van der Veen M (1995) Quantification of root surface carious lesions. Groningen University, Doctora Thesis, Netharland.
- Korkut B, Yanikoglu F, Tagtekin D (2015) In vitro assessment of dimensional alterations of worn human incisors. Paripex. Ind J Res 4: 218-222.
- Korkut B (2015) Assessment of measurability of dimensional alterations of worn teeth by various methods at different time periods. (doctora thesis) Marmara University, Faculty of Dentistry, stanbul.
- Ekenback SB, Linde LE, Lönnies H (2000) Effect of four dental varnishes on the colonization of cariogenic bacteria on exposed sound root surfaces. Caries Res 34: 70-74.
- Chow LC, Takagi S, Carey CM, Sieck BA (2000) Remineralization effects of a two-solution flüoride mounthrinse: An in situ study. J Dent Res 79: 991-995.
- Tranaeus S, Al-Khateeb S, Björkman S, Tweetman S, Angmar-Mansson B (1999) Comparison of fluoride varnish treatment and professional tooth cleaning in caries-active children using the QLF method- A 6- month pilot study (abstract 69). Caries Res 33:304.
- Al-Khateeb S, Forsberg CM, De Josselin de Jong E, Angmar-Månsson B (1998) A longitudinal laser fluorescence study of white spot lesions in orthodontic patients. Am J Orthod Dentofacial Orthop 113: 595-602.
- Ogaard B, Ten Bosch JJ (1994) Regression of white spot enamel lesions: A new optical method for quantitative longitudinal evaluation in vivo. Am J Orthod Dentofacial Orthop 106: 238-242.
- Tagtekin DA, Ozturk F, Lagerweij M, Hayran O, Stookey GK, et al. (2005) Thickness measurement of worn molar cusp by ultrasound. Caries Res 39: 139-143.
- Ozturk FB, Tagtekin DA, Hayran O, Stookey GK, Yankoglu FÇ (2005) Accuracy of Ultrasound measurement of progressive change in occlusal enamel thickness. Oral Sur Oral Med Oral Path Oral Radio 99: 101-105.
- Youngs G (1994). Risk factors for and the prevention of root caries in older adults. Spec Care Dentist 14: 68–70.














