Keywords
Sperm cells; Micropyle; Oocytes; Morphometric
Introduction
Most of Brazilian native fish species need to migrate up rivers to spawning. These species are known as reofilic fish and are represented by several species, including Piaractus mesopotamicus, Brycon orbygnianus, Salminus brasiliensis and Prochilodus lineatus. When these species are kept in lentic waters (captive), they fail to complete their reproductive cycle, making to use of hormones necessary to induce gonadal final maturation and gametes release (Crepaldi et al., 2006). The hormonal induction process acts in supplementing the amount of gonadotropin hormones which is no longer produced due to the absence of favorable environmental conditions (Zohar and Mylonas, 2001).
The fish species cited are neotropical fish found in major Brazil basins. Alteration in the habitats (hydroelectric dams, deforestation and pollution) and predatory fishery have caused reduction of the populations among the studied species (Brasil- Ministry of Environment, 2007).
Number, shape and size of micropyle and ultrastructure of egg membrane and of the micropyle canal; the number and length of the longest and shortest ridge in the micropyle region beyond the structures of the sperm cell have been used as taxonomic tool in ichthyology (Esmaeili and Johal, 2005; Esmaeili and Gholamifard, 2012).
Most fish species have ovoid shaped eggs and a single micropyle (Ganeco and Nakaghi, 2003) or a single oocyte with several micropyles (Psenicka et al., 2011).The sperm cell is divided into head, midpiece and flagellum (Nagahama, 1983) and in a few other species, such as sturgeon, sperm cells have acrosome and subacrosome (Psenicka et al., 2007; Psenicka et al., 2008). Some spermatozoids can still present at the end of the flagellum a structure similar to a keel (Alavi et al., 2009). The organization structure of the sperm cell also reveals major differences according to fertilization strategy, habitat conditions or most importantly oocytes morphology.
The aim of this work was to identify and measuring the gametes of fish using ultrastructural analysis in Prochilodus lineatus, Salminus brasiliensis, Piaractus mesopotamicus and Brycon orbignyanu.
Material and Methods
Selection of fishes
This study was conducted during the fish breeding season between october and december at the fish culture station of Companhia Energetica de Minas Gerais, Brazil. The fishes were captured in the Rio Grande (Paraná basin), at Itutinga, Minas Gerais, in Brazil, twelve months before the beginning of the experiment and maintained in aquarium.
Animals were fed with a commercial diet consisting of 32% extruded crude protein (8 mm diameter pellets). The animals were selected according to their external reproductive characteristics (bulging of coelomic wall in females and release of sperm by light pressure on the urogenital papilla in males). Six breeding pairs of the following species were used: Piaractus mesopotamicus, Prochilodus lineatus, Brycon orbignyanus and Salminus brasiliensis. The water temperature was maintained at 26°C ± 1°C. Induction of the final maturation of the gonads occurred after administration of two intramuscular doses of a crude extract of carp pituitary (CECP); the first dose 0.25 mL CECP.kg-1 and the second dose, 12 hours later, 0.75 mL CECP.kg-1 .
Eight hours after the second CECP dose, fish were removed from the aquarium and had their urogenital papilla cleaned and dried with a towel. The collection of gametes began after delicate manual massage of the coelomic wall. This procedure was performed in accordance with the Ethical Principles of Animal Experimentation, Protocol 040/2009 of Lavras Federal University-UFLA.
Ultrastructural analysis of gametes
Post-fixation methodology was accomplished in accordance to the methodology employed in the Laboratory of Eletronic Microscopy and Ultrastructural Analysis of Lavras Federal University-UFLA. Samples containing 1mL of semen or 30 oocytes of each species were fixed separately in microtubules containing a solution of 2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.05 M sodium cacodylate buffer, pH 7.2, calcium chloride 0.001 M. The samples were maintained for 24 hours at 4°C. After this, samples were immersed in 1% osmium tetroxide for 4 hours at room temperature and subsequent dehydration through in ascending series of acetone (25%, 50%, 75%, 90% and 100%).
Before evaluation in the electron scanning microscope, the oocytes and spermatozoa were dehydrated with a CPD030 critical point instrument. The samples were coated with gold in a SCD 050 vacuum evaporator according to protocols designed in Ultrastructural Analysis and Electronic Microscopy Laboratory of Federal University of Lavras, Brazil.
Morphometric analysis of the gametes
In the morphometry of the oocytes, was valued the major diameter (Md), minor diameter (md), total volume and the sperm head width ratio and the major diameter of major micropyle ostium (MSH/MO). In the morphometry of the micropyle (Figure 1) in the oocytes surface, was measured the diameters of the major ostium (MO) and minor ostium (mO) besides area each one. In the morphometry of sperm was valued head length, midpiece, flagellum, total length and proportion of the flagellum. We used the SRV32 Leosoftware, Windows version for these measurements.
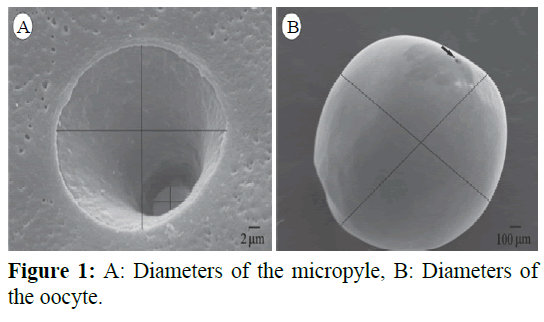
Figure 1: A: Diameters of the micropyle, B: Diameters of the oocyte.
Results
In all species, there was the presence of only one micropyle and it was funnel-shaped. The micropylar region had a porous surface and open pores in the pellucide zone (Figure 2). The ultrastructural analysis also revealed the presence of several ridges and folds around the micropyle in S. brasiliensis, B. orbignyanus and P. mesopotamicus (less intense). The pores near of the micropylar opening of Prochilodus lineatus were more distant from each other compared to the pores of Brycon orbignyanus and Salminus brasiliensis (Figure 2). It is also possible to note that P. mesopotamicus micropyle had the shape of a cylinder with an elliptical base, while P. lineatus oocytes had a more circular base. B. orbignyanus oocytes were the highest and in P. mesopotamicus the lowest ones. The oocytes studied showed different diameters between species. The morphometry of oocytes ranged from 1228.2 ± 36.0 μm for major diameter (B. orbignyanus) and 866.5 ± 70.5 μm for minor diameter (P. mesopotamicus). The major values of volume and MSH / MO were found to B. orbignyanus (6.7 ± 1.0 mm3 and 43.1 ± 3.7%, respectively). In the micropyle, the major value for major ostium (MO) was observed in P. mesopotamicus (29.1 ± 2.1 μm for major diameter, 23.0 ± 1.1 μm and 2109.2 ± 185 mm2 for area). However, the minor value was observed in B. orbignyanus (3.4 ± 0.5 μm for major diameter, 2.5 ± 0.3 μm and 27.2 ± 4.8 mm2 for area) (Tables 1 and 2).
| Species |
Major micropyleostium(MO) |
Minor micropyleostium (mO) |
| Md*(µm) |
md**(µm) |
Area (µm2) |
Md*(µm) |
md**(µm) |
Area (µm2) |
| P. lineatus 18.6 ± 2.4 15.8 ± 2.3 918.1 ± 174.1 5.0 ± 0.5 3.9 ± 0.2 62.4 ± 8.6 |
18.6 ± 2.4 |
15.8 ± 2.3 |
918.1 ± 174.1 |
5.0 ± 0.5 |
3.9 ± 0.2 |
62.4 ± 8.6 |
| B. orbignyanus |
21.9 ± 0.4 |
17.6 ± 0.7 |
1216.1 ± 70 |
3.4 ± 0.5 |
2.5 ± 0.3 |
27.2 ± 4.8 |
| S. brasiliensis |
24.1 ± 2.6 |
20.8 ± 3.1 |
1530.3 ± 290 |
7.3 ± 0.9 |
4.7 ± 0.9 |
108.4 ± 31 |
| P. mesopotamicus |
29.1 ± 2.1 |
23.0 ± 1.1 |
2109.2 ± 185 |
5.7 ± 0. 3 |
4.5 ± 0.1 |
81.0 ± 5.4 |
| *Major diameter (Md); **Minor diameter (md). |
Table 1: Morphometry of the micropyle of Brycon orbignyanus, Piaractus mesopotamicus, Prochilodus lineatus and Salminus brasiliensis. Mean ± standard deviation.
| Oocyte |
| |
Md*(µm) |
md**(µm) |
Volume(mm3) |
MSH/MO(%) |
| P. lineatus 18.6 ± 2.4 15.8 ± 2.3 918.1±174.1 5.0 ± 0.5 3.9 ± 0.2 62.4 ±8.6 |
1020.4±94.0 |
888.7±69.3 |
3.4±0.7 |
31.0±4.9 |
| B. orbignyanus |
1228.2±36.0 |
1137.3±79.7 |
6.7±1.0 |
43.1±3.7 |
| S. brasiliensis |
1093.7±91.9 |
985.6±97.8 |
4.5±1.2 |
19.4±2.0 |
| P. mesopotamicus |
1014.2±85.7 |
866.5±70.5 |
3.2±0.7 |
23.6±1.8 |
| *Major diameter (Md); **Minor diameter (md). |
Table 2: Morphometry of the oocytes of Bryconorbignyanus, Piaractusmesopotamicus, Prochiloduslineatusand Salminusbrasiliensisand sperm head width ratio and the major diameter of major micropyleostium (MSH/MO). Mean ± standard deviation.
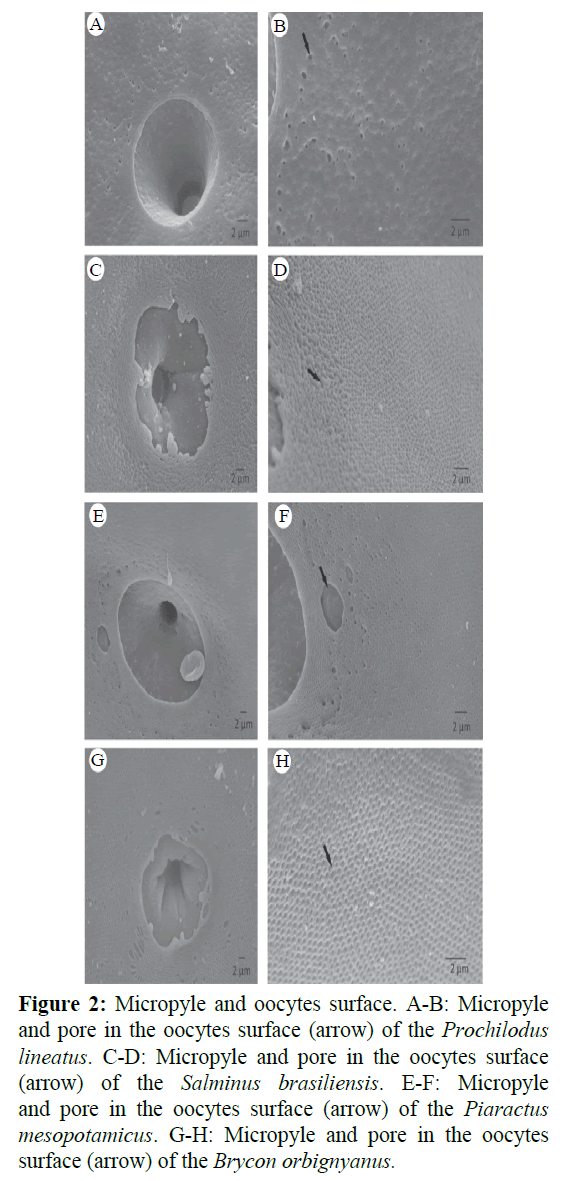
Figure 2: Micropyle and oocytes surface. A-B: Micropyle and pore in the oocytes surface (arrow) of the Prochilodus lineatus. C-D: Micropyle and pore in the oocytes surface (arrow) of the Salminus brasiliensis. E-F: Micropyle and pore in the oocytes surface (arrow) of the Piaractus mesopotamicus. G-H: Micropyle and pore in the oocytes surface (arrow) of the Brycon orbignyanus.
In all species, the sperm morphology showed considerable similarities, consisting of an ovoid sperm head and had not acrosome, but had a cylindrical midpiece and a single flagellum (Figure 3). Morphometric results for sperm cells are summarized in Table 3.
| Species |
Head length(µm) |
Head width
(µm) |
Midpiece
(µm) |
Flagellum
(µm) |
Total length(µm) |
Proportion of the flagellum(%) |
| P.lineatus |
2.06 ±0.24 |
1.58 ±0.14 |
3.41 ±0.43 |
26.48 ±1.67 |
32.05 ±1.60 |
82.08 ±5.97 |
| B.orbignyanus |
1.82 ±0.21 |
1.50 ±0.21 |
3.07 ±0.49 |
27.75 ±1.92 |
32.66 ±1.81 |
84.32 ±5.93 |
| S.brasiliensis |
1.88 ±0.32 |
1.58 ±0.28 |
1.54 ±0.31 |
27.03 ±2.19 |
29.11 ±2.53 |
93.34 ±9.57 |
| P.mesopotamicus |
2.19 ±0.204 |
1.33 ±0.10 |
1.61 ±0.17 |
26.46 ±2.11 |
29.86 ±1.23 |
88.91 ±6.86 |
Table 3: Morphometry of the various portions of the sperm of B. orbignyanus, P. mesopotamicus, P. lineatusand S. brasiliensis. Mean ± standard deviation.

Figure 3: Sperm cells: A: Prochilodus lineatus, B: Salminus brasiliensis, C: Piaractus mesopotamicus, D: Brycon orbignyanu.
Discussion
Comparing the diameters of oocytes, it was observed that the gametes of B. orbignyanus and S. brasiliensis were more spherical, while the P. mesopotamicus oocytes presented an ellipsoid shape (Figure 2). The same inference can be made for both the ostium and the micropyle.
Working with different species of the genres Prochilodus and Salminus, Honorato-Sampaio et al. (2015) found higher values for the oocyte diameter of these species, that were 1583.5 μm 1613.2 μm respectively. This indicated that there can be oocyte size variation between species of the same genre. On the other hand, Perini et al. (2013), studied other freshwater species, Curimatella lepidua and Elegans steindachnerina, observed lower oocyte size than those found in this study (510.4 μm and 506.3 μm respectively).
The micropyle is a small opening in the zona radiata, composed of the vestibule and micropylar channel and participates in the fertilization process (Ganeco and Nakaghi, 2003).
All species showed only one micropyle. The presence of only one such structure is common in teleost fish, and is a characteristic used to differentiate the oocytes from other species with multiple micropyles like Sturgeon (Psenicka et al., 2011; Siddique et al., 2016).
For all species, the function of the porous surface and open pores in the pellucid zone of the micropylar region is used to optimize gas exchange for the development of the embryo (Rizzo et al., 2002). Ganeco and Nakaghi (2003) also observed several ridges and folds around the micropylar vestibule in B. orbignyanus. According to Sampaio (2006) these folds may represent a strategy to facilitate fertilization of these species. The same author observed that these folds do not occur in the micropilar zone in oocytes of P. lineatus and Prochilodus argenteus, which suggests that the absence of these folds may be a feature of the genus Prochilodus. In addition, the works cited corroborate our findings regarding in P. lineatus.
According to Riehl (1993), the distance among pores of the radiata zone, pore size and micropyle morphology can be used in the identification of the species. This study showed that the pores near of the micropylar opening of P. lineatus were more distant from each other compared to the pores of B. orbignyanus and S. brasiliensis (Figure 2). The pores of the oocytes of these species were larger in diameter with a higher density. These data are consistent with the observations of Ganeco and Nakaghi (2003). They observed that the surface of the oocytes of B. orbignyanus and B. gouldingi displays pores which are close to each other around the micropylar opening. As mentioned before, B. orbignyanus is the only endangered species that was studied. The fact that the micropyle of B. orbignyanus had the smallest mo (27 μm2) and sperm head width ratio and the major diameter of major micropyle ostium (MSH/MO) (43%) reinforces the importance of artificial propagation in the preservation of the species.
In species whose sperm cells are devoid of an acrosome, as is the case of all the species in this study, the micropylar apparatus allows the sperm direct access to the oocyte membrane (Redding and Patino, 1993). Sperm measurements were similar to those observed by Verissimo-Silveira et al. (2006), who analyzed the structures of the sperm of B. orbignyanus and S. brasiliensis and revealed that the midpiece is thin, long and asymmetrical. However, the average length of the midpiece in the spermatozoa of S. brasiliensis measured by the same authors was larger than in the present study (1.539 ± 161, 0.309 μm), suggesting that there are genetic differences between groups of specimens in the different basins. Since the mitochondrial content of the midpiece is proportional to its size, there could be differences in the quality of motile sperm cells between these populations.
This study revealed that the head of the sperm in all investigated species had a spherical core of 1.5 μm in diameter with no acrosome, corroborating results obtained by Veríssimo-Silveira et al. (2006). The difference in the morphology of the spermatozoa head of different species may be related to fertilization. The fact that species such as sturgeon present acrosome and subacrosome (Psenicka et al., 2008) and oocytes with many micropyles (Psenicka et al., 2011; Siddique et al., 2014), may induce polyspermy.
These structures were not observed in gametes of the studied species because the sperm has no acrosome at the simple head and only one micropyle per oocyte, since the smaller diameter of the ostium only permit the entry of a single sperm cell. In African catfish (Mansour et al., 2002), similarities were only observed in terms of head size (1.55 ± 0.10 μm in length and 1.36 9 ± 0.4 μm in width) and the flagellum (37.8 ± 1.3 μm in length), but not the size of the midpiece, showing that, despite its cylindrical shape, it was smaller in size (0.5 ± 0.06 μm 177 in length).
In Brycon nattereri (Viveiros et al., 2012) similar results could also be observed with to the head shape (2.00 ± 0.15 μm in length and 1.22 ± 0.09 μm in width) and the flagellum (30.50 ± 2.32 μm in length), however at the end of the intermediate piece was observed the presence of three membranous concentric rings delimited by four membranes.
Some sturgeon species may exhibit similar sized sperm, but that differ in their structure. In Acipenser sinensis, although measures of the head, midpiece and flagellum of spermatozoa were similar to those of the species studied here (1.84 ± 0.45 μm in 181 width and 3.27 ± 0.20 μm in length vs. 2.17 ± 0.36 μm and 33.26 ± 2.74 μm, 182 respectively), morphology differs regarding the acrosome inserted in the head and elongated in the presence of a short middle piece (Wei et al., 2007). In Acipenser beriberi, the structure is similar to that of the other species of the genus Acipenser (Psenicka et al., 2008; Psenicka et al., 2010); the midpiece is relatively smaller (1.09 ± 0.42 μm and the flagellum is long (44.75 ± 4.93 μm) (Psenicka et al., 2007).
Some species sturgeon may exhibit similar sized sperm, but differ in their structure. In Acipenser sinensis, although measures of the head, midpiece and flagellum of spermatozoa were similar to those of the species studied here (1.84 ± 0.45 μm in 181 width and 3.27 ± 0.20 μm in length vs. 2.17 ± 0.36 μm and 33.26 ± 2.74 μm respectively), morphology differs regarding the acrosome inserted in the head and elongated in the presence of a short middle piece (Wei et al., 2007). In another species, Acipenser beriberi, the structure is similar to that other species of the genus Acipenser (Psenicka et al., 2008); the midpiece is relatively smaller (1.09 ± 0.42 μm and the flagellum is long (44.75 ± 4.93 μm) (Psenicka et al., 2007) It was observed that the sperm cells of P. lineatus and B. orbignyanus had similar overall lengths, although the former species had a longer midpiece. Morphologically, the head of the P. mesopotamicus sperm present a more cylindrical shape. The standard deviations for the lengths of the scourge were high, which indicates large differences between the cells and the sperm tails, and which cannot suggest that this variable is important from an evolutionary point of view.
Taking into account that the surface morphology of the oocyte and the micropyle are criteria for identifying different species, and that the length of the flagellum can influence sperm motility, this preliminary study may contribute to phylogenetic analysis and genetic conservation through germplasm banks. Moreover, these methods constitute an important tool in the study of genetic improvement through the observation of the gametes structures. It may be noted that the midpiece of S. brasiliensis and P. mesopotamicus sperm presented was larger than of the other studied species. In contrast, P. lineatus and B. orbignyanus sperm present lower intermediate part. The size of the midpiece may be related to the time of tail activity, because the mitochondria are concentrated in that region. Therefore it may be inferred that smaller midpieces have fewer mitochondria and this may influence the sperm motility (Baccetti et al., 1984; Ishijima et al., 1998; Lahnsteiner and Patzner, 2008).
Alavi and Cosson (2006) also reports that the number of mitochondria may be a possible cause of the difference in the duration of sperm motility that occur among species. Another issue that must be taken into account is that the length of the flagellum may also affect sperm motility. However, studies that relate the time of sperm motility with the length of the flagellum and the number of mitochondria present in the intermediate part are scarce.
Conclusion
The gametes of the studied species showed similarities, although the oocytes of P. lineatus showed differences in the micropylar region and S. brasiliensis and P. mesopotamicus sperm were midpieces smaller than on the other species. Taking into account that the morphology of the micropyle and sperm are criteria for species identification, conservation and breeding, this study may contribute in this regard.
Acknowledgements
The authors thank the Companhia Energética de Minas Gerais- CEMIG and the Laboratory of Microscopy and Ultrastructural Analysis of Lavras Federal University-UFLA for their technical expertise.
Fundings
This work was supported by the FAPEMIG (Foundation of research of Minas Gerais), CNPq (National Counsel of Technological and Scientific Development), CAPES (Higher Education Personnel Improvement Coordination) and FUNDECC (Fundação de Desenvolvimento Científico e Cultural-UFLA).
18121
References
- Alavi, S.M.H., Cosson. J. (2006) Sperm motility in fishes: Effect of ions and osmolality: Review. Cell Biol International 30, 1-14.n
- Alavi, S.M.H., Rondina, M., Viveiros, A.T.M, Cosson, J., Gela, D. (2009) Effects of osmolality on sperm morphoçlogy, motility and flagellar wave parameters in Northern pike Esoxlucius L. Theriogenology72,32-43.
- Baccetti, B., Burrini, A.G., Callaini, G., Mazzini, M., Zerunian, S. (1984) Fish germinal cells. I. Comparative spermatology of seven cyprinid species. Gamete Res 10,373-396.
- Crepaldi, D.V., Faria, M.C., Teixeira A.L.P., Costa, A.A.P., Melo, D.C. (2006) Utilização de hormônios na reprodução induzida de surubim (Pseudoplatystomaspp). Rev Bras Reprod Animal Belo Horizonte 30,168-173.
- Esmaeili, H.R., Johal, M.S. (2005) Ultrastructural features of the egg envelope of silver carp, Hypophthalmichthys molitrix (Osteichthyes, Cyprinidae). Environ Biol of Fish72, 373-377.
- Esmaeili, H.R., Gholamifard, A. (2012) Ultrastructure of the chorion and the micropyle of an endemic cyprinid fish, Cyprinion tenuiradius Heckel, 1849 (Teleostei: Cyprinidae) from southern Iran. Iranian J Fisheries Sci11, 657-665.
- Ganeco, L.N., Nakaghi, L.S.O. (2003) Morfologia da micrópila e da superfície dos ovócitos de piracanjuba, Bryconorbignyanus (Osteichthyes, Characidae), sobmicroscopia eletrônica de varredura. ActaScientiarum: Biological Sciences Maringá 25,227-231.
- Honorato-Sampaio, K., Prado, P.S., Sato, Y., Bazzoli, N., Rizzo, E. (2015) Comparative morphology of the oocyte surface and early development in four characiformes from the São Francisco River, Brazil. J Morphol276, 1258-1272.
- Ishijima, S., Hara, M., Okiyama, M. (1998) Comparative studies on spermatozoa and motility of Japanese fishes. Bull Ocean Res InstUni Tokyo 33,139-152.
- Lahnsteiner, F., Patzner, R.A. (2008) Sperm morphology and ultrastructure in fish. In: Fish Spermatology. S.M.H. Alavi, J.J. Cosson, K. Coward and G. Rafiee (Eds). Alpha Science Ltd.,Oxford, UK pp: 1-62.
- Mansour, N.,Lahnsteiner, F., Patzner, R.A. (2002) The spermatozoon of the African catfish: fine structure, motility, viability and its behaviour in seminal vesicle secretion. J Fish Biol 60,545-560.
- Brasil-MMA (Ministry of Environment) (2007) Biodiversidade e florestas: espécies ameaçadas.
- Nagahama, Y. (1983) The functional morphology of teleost gonads. In: Hoar, W.S. et al. (Ed) Fish Physiology IXa. Reproduction New York: Academic Press 9,pp:223-275.
- Perini, V.R., Sato, Y., Rizzo, E.,Bazzoli, N. (2013) Comparative analysis of the oocytes and early development of two species of curimatidae teleost fish. Journal of Veterinary Medicine Series C: AnatomiaHistologiaEmbryologia42, 40-47.
- Psenicka, M., Alavi, H.S.M., Rondina, M., Cicova, Z., Gela, D.(2007) Morphology and ultrastructure of Siberian sturgeon (Acipenserbaerii) spermatozoa using scanning and transmission electron microscopy Biol Cell99,103-115.
- Psenicka, M., HadiAlavi, S.M., Rondina, M., Cicova, Z., Gela, D. (2008) Morphology, chemical contents and physiology of chondrostean fish sperm: a comparative study between Siberian sturgeon (Acipenserbaerii) and sterlet (Acipenserruthenus). J Appl Ichthyology 24,371-377.
- Psenicka, M.,Rodina, M.,Linhart, O. (2010) Ultrastructural study on the fertilisation process in sturgeon (Acipenser), function of acrosome and prevention of polyspermy. Animal Reproduction Science 117, 147-154.
- Psenicka, M., Kaspar, V., Alavi, S.M.H.,Rondina, M., Gela, D.et al. (2011) Potential role of the acrosome of sturgeon spermatozoa in the fertilization process. JApplIchthyolVerlag, Berlim27,678-682.
- Redding, J.M., Patino, R. (1993) Reproductive physiology. In: Evans, D.H. (Ed) the Physiology of fishes CRC Press, Inc, USA,592.
- Riehl, R. (1993) Surface morphology and micropyle as a tool for identifying fish eggs by scanning electron microscopy.MicroscAnalyisis 29-31.
- Rizzo, E., Sato, Y.,Barreto, B.P.,Godinho, H.P.(2002) Adhesiveness and surface patterns of eggs in neotropical freshwater teleosts. J Fish BiolLondon61,615-632.
- Sampaio, K.H. (2006) Superfície ovocitária e desenvolvimento inicial de quatro espécies de peixes de interesse comercial da bacia do Rio São Francisco. Dissertation, Universidade Federal de Minas Gerais, Belo Horizonte53.
- Siddique, M.A.M., Cosson, J., Psenicka, M., Linhart, O. (2014) A review of the structure of sturgeon egg membranes and of the associated terminology. Journal of Applied Ichthyology30, 1246-1255.
- Siddique, M.A.M., Psenicka, M., Cosson, J., Dzyuba, B., Rodina, M et al. (2016) Egg stickiness in artificial reproduction of sturgeon: An overview. RevAquacul8, 18-29.
- Veríssimo-Silveira, R., Gusmão-Pompiani, P., Vicentini, C.A., Quagio- Qrassiotto, I. (2006) Spermiogenesis and spermatozoa ultrastructure in Salminus and Brycon, two primitive genera in Characidae (Teleostei: Ostariophysi: Characiformes). ActaZoologica87,305-313.
- Viveiros, A.T.M.,Maria, A.N.; Amaral, T.B., Orfão, L.H.,Isau, Z.A. (2012) Spermatozoon ultrastructure and sperm cryopreservation of the Brazilian dry season spawner fish pirapitinga, Bryconnattereri.Aquacult Res43, 546-555.
- Wei, Q., Li, P., Psenicka, M., Alavi, H.S.M., Shen, L,et al. (2007) Ultrastructure and morphology of spermatozoa in Chinese sturgeon (AcipensersinensisGray 1835) using scanning and transmission electron microscopy. Theriogenology 67, 1269-1278.
- Zohar, Y., Mylonas, C.C. (2001) Endocrine manipulations of spawning in cultured fish fromhormones to genes. Aquaculture,Amesterdam197, 99-136.









