Research Article - (2024) Volume 16, Issue 3
Unraveling the Molecular Targets: Drug Discovery Strategies for Narcolepsy Treatment
Sheilina Choudhary*
Department of Bioengineering and Food Technology, School of Bioengineering and Food Technology, Shoolini University, Solan, India
*Correspondence:
Sheilina Choudhary, Department of Bioengineering and Food Technology, School of Bioengineering and Food Technology, Shoolini University, Solan,
India,
Tel: 07024992106,
Email:
Received: 10-May-2024, Manuscript No. IJDDR-24-14484;
Editor assigned: 13-May-2024, Pre QC No. IJDDR-24-14484 (PQ);
Reviewed: 27-May-2024, QC No. IJDDR-24-14484;
Revised: 04-Jun-2024, Manuscript No. IJDDR-24-14484 (R);
Published:
11-Jun-2024
Abstract
Objective: The objective of this research paper on narcolepsy is to distinguish and assess novel compounds focusing on particular atomic pathways related to the clutter. Through thorough screening and optimization, the point is to reveal potential drug candidates that illustrate adequacy in moderating narcoleptic indications, counting intemperate daytime languor and cataplexy. The inquiry will dive into the instruments of activity of lead compounds, giving experiences into their intelligence with neural circuits and neurotransmitter frameworks significant to narcolepsy. Moreover, they think about surveying the security profiles of promising sedate candidates, laying the basis for future translational investigations and the improvement of imaginative restorative mediations for narcolepsy.
Methods: In this investigation, target proteins were downloaded from the PDB and docked in Biovia. The ligands' and standard medications' binding affinity with each target protein was compared and assessed. Also, only 3 substances were chosen for SWISS-ADME final results.
Results: The docking result revealed that the ligands selected have the best binding affinity with all the target proteins.
Conclusion: The ligands could potentially be used to treat narcolepsy in future approaches for studying the urge ligands in vitro and in vivo analysis to create novel narcolepsy inhibitors.
Keywords
Narcolepsy; Hypocretin; Orexin; Sleep-wake
cycle; Cataplexy; Neurotransmitters; Autoimmunity;
Hypocretin receptor agonists; Hypnagogic hallucinations
Abbreviations
EDS: Excessive Daytime Sleepiness; REM:
Rapid Eye Movement; MSLT: Multiple Sleep Latency Test;
CFHL: Cerebrospinal Fluid Hypocretin Levels; HLA: Human
Leukocyte Antigen; CSF: Cerebrospinal Fluid; NT: Narcolepsy
Type
Introduction
Narcolepsy, a captivating and complex neurological clutter,
disturbs the sensitive adjustment of rest and alertness,
posturing significant challenges to influenced people. This
clutter is stratified into two essential sorts, NT1 and NT2, each
recognized by particular clinical highlights. NT1 is characterized
by the nearness of cataplexy, and sudden scenes of muscle
shortcoming activated by feelings, and is frequently related to
an insufficiency in CSF hypocretin-1 levels. In differentiation,
NT2 offers likenesses with NT1 but needs cataplexy and is
fundamentally characterized by EDS without the same
hypocretin insufficiency.
A key symptomatic instrument for narcolepsy is the MSLT,
which assesses the time it takes for a person to move from
attentiveness to rest beneath controlled conditions.
Outstandingly, the MSLT too uncovers an abbreviated REM rest
inactivity, a trademark characteristic of narcolepsy.
Complementing this, CSF investigation helps in diagnosing NT1 by
affirming hypocretin insufficiencies, giving pivotal experiences into
the fundamental neurobiological instruments of the clutter [1].
EDS stands as the foundation of narcolepsy's clinical
appearance, with influenced people experiencing sudden and
powerful inclinations to rest during the day. This inescapable
languor can significantly affect every day working, driving
challenges in keeping up efficiency and engagement in day-by-day exercises. Cataplexy, frequently accompanied by hypnagogic
hallucinations-vivid and dreamlike encounters happening at
the move from alertness to sleep-further complicates the
demonstrative scene. These scenes of muscle shortcoming or
loss of motion, activated by feelings, are special to narcolepsy
and contribute to the particular challenges confronted by people
with this clutter [2].
Past these center side effects, narcolepsy habitually coexists
with different comorbidities, extending from psychiatric clutters
to cardiovascular issues, underscoring the multifaceted effect of
the condition on general well-being. Misery, uneasiness, and
weight are among the commonly watched comorbid conditions,
emphasizing the requirement for an all-encompassing approach to narcolepsy administration that addresses both sleep-related
indications and related well-being concerns [3].
The disturbed night time rest experienced by people with
narcolepsy includes another layer of complexity. Divided rest,
characterized by visit enlightenments and challenges keeping up
a nonstop rest design, compounds the challenges confronted by
those living with narcolepsy. This disturbance not only
contributes to daytime drowsiness but also increases the hazard
of other well-being complications, strengthening the
requirement for comprehensive administration methodologies.
Understanding the complexities of divided rest designs in
narcolepsy is vital for creating intercessions that target both
daytime and nighttime indications [4].
In this comprehensive investigation of narcolepsy, we point to
dive into the complicated features of the clutter, including its
subtypes, symptomatic techniques, related indications such as
cataplexy and hypnagogic visualizations, comorbidities, and the
effect of divided rest. By picking up a more profound
understanding of narcolepsy, we trust to contribute to the
continuous endeavors to upgrade symptomatic exactness,
helpful intercessions and generally care for people hooking with
the challenges posed by this captivating neurological condition.
Propels in these ranges hold the potential to altogether move forward the quality of life for those influenced by narcolepsy and
clear the way for more focused and personalized approaches to
treatment [5].
Materials and Methods
Phytocompounds
Phytochemicals are antioxidants, that maintain mitochondrial
function and homeostasis, prevent intrinsic apoptosis and
neuroinflammation, and activate cellular signal pathways to
induce anti-apoptotic and pro-survival genes [6].
Such as the orexin-2 receptor is the main protein associated
with narcolepsy. It plays a significant part in directing alertness
and rest designs. In narcolepsy, a neurological clutter
characterized by intemperate daytime languor, variations from
the norm within the orexin framework, especially the orexin 2
receptor, have been recognized. Diminished action or
brokenness of the orexin 2 receptor is regularly connected to the
pathophysiology of narcolepsy, leading to disturbed sleep-wake
cycles (Table 1).
| S. no. |
Phytocompounds |
| 1 |
Modafinil (Provigil) |
| 2 |
Armodafinil (Nuvigil) |
| 3 |
Sodium-oxybate (Xyrem) |
| 4 |
Vafidemstat (ORY-2001) |
| 5 |
SUVN-G3031 |
| 6 |
Prednisone |
| 7 |
Methylprednisolone |
| 8 |
DORA-22 |
| 9 |
Firazorexton |
| 10 |
Danavorexton (TAK-925) |
Table 1: Main phytocompounds associated with narcolepsy.
Protein extraction and purification
The Three-Dimensional (3D) structure of the protein alpha-synuclein was resolved using the X-ray diffraction method with a
resolution factor of 2.16 Å. The structures were obtained from
the PDB Research Collaboratory for Structural Bioinformatics
(RCSB PDB) in pdb format. The missing residues were replaced
using BIOVIA, which purifies the protein by eliminating the water
molecules hetatm and adding polar hydrogen to the retained
Chain A, while the rest of the chains are eliminated. This purified
protein is then stored in pdb file format, which was utilized to
obtain the 2-dimensional structure and Ramachandran plot using
PDBsum and the hydrophobicity plot from the BIOVIA discovery
studio program.
Ligand retrieval and purification
A total of 10 phytocompounds of different plant specimens
were selected from IMPAAT which has potential anti-narcolepsy
activity based on its antioxidant, anti-mutagenic, anti-hepatotoxic,
anti-inflammatory, anti-aging, and chemopreventive properties.
The canonical SMILES, PubChem CID, and the Two-Dimensional
(2D) models of 2, Modafinil, and Armodafinil were retrieved in SDF
format via the PubChem database, and all the structures were converted to PDB format with the help of Open Babel software.
Molecular docking
After the retrieval of protein and ligand, molecular docking
was executed using PyRx. PyRx is mainly a virtual molecular
screening application used to dock small-molecule libraries to a
macromolecule to find lead compounds with desired biological
functions. The 15 phytocompounds and two standard drugs
were added as ligands, and the two purified proteins were
uploaded as macromolecules. The added ligands were energy-minimized and were all converted into. pdbqt format. All the
ligands were docked with target proteins discretely and were
evaluated based on binding affinity. The strength of protein-ligand binding is known as binding affinity. The negative values
for binding affinity (or binding free energy) indicate that
the ligand is predicted to bind to a target macromolecule.
The more negative the numerical values for the binding affinity,
the better the predicted binding between a ligand and a
macromolecule. Therefore, the ligand with the least binding
affinity and zero RMSD value was selected for each protein
and visualized in BIOVIA Discovery Studio software. RMSD
value is utilized to evaluate the docked conformation compared
to other docked conformations or the reference conformation.
Based on binding affinity the inhibitory activity of ligands and
standard drugs will be compared.
Here, 10 phytocompounds and two standard drugs were
uploaded as ligands and two target proteins i.e., 4S0V, and 6D26.
The ligands loaded were with minimum energy and were
converted to .pdbqt format and the grid was generated for the
targeted protein as follows. The grid for the center is shown in
the Table 2 and the values obtained for grid dimensions are as
follows: X=15 Å Y=15 Å and Z=15 Å. This was similar for all the
three points.
| PDB id of protein |
X |
Y |
Z |
| 4S0V |
28 |
14 |
20 |
| 6D26 |
28 |
16 |
20 |
Table 2: PDB ID of selected proteins and their specifications.
Visualization
The top ligands with the lowest binding affinity for each
protein were chosen, and the best model of each ligand was
saved in PyRx in pdb file format. The Three-Dimensional (3D)
structure and non-bond interaction were observed using BIOVIA
discovery studio software, and the 3D model was retrieved in
PNG file format.
Physiochemical studies (ADMET analysis)
The pharmacokinetics were evaluated in ADMET using Lipinski’s
Rule of Five (RO5). Pharmacodynamic properties were predicted
by parameters such as physiochemical properties, absorption,
distribution, metabolism, medical chemistry, toxicity, and
excretion. The highest four docked ligands having the least
binding affinity for each protein were evaluated ADMET analysis
was performed using ADMETlab 2.0 (Table 3).
| Drug |
Formula |
No. of H-bond acceptors |
No. of H- bond donors |
Blood-brain-barrier permeability |
Lipinski |
| Modafinil |
C15H15N2O3S |
3 |
1 |
Yes |
Yes |
| Armodafinil |
C15H14ClN2O3S |
3 |
1 |
Yes |
Yes |
| Na-Oxybate |
C4H6NaO3 |
3 |
3 |
Yes |
Yes |
| Vafidemstat |
C17H15F4N3O3 |
4 |
2 |
No |
No |
| SUVN-G3031 |
C11H16BrNO3 |
4 |
1 |
No |
No |
| Prednisone |
C21H26O5 |
5 |
3 |
Yes |
No |
| Methylprednisolone |
C22H28O5 |
5 |
3 |
Yes |
No |
| DORA-22 |
C16H14N2O4 |
4 |
2 |
No |
No |
| Firazorexton |
C19H15ClF2N3O3 |
6 |
2 |
No |
No |
| Danavorexton |
C18H16BrNO3 |
4 |
1 |
No |
No |
Table 3: Drugs used in narcolepsy treatment and their specifications.
Results
A total of 16 phytocompounds were chosen, out of which only
10 were selected based on docking results. The two-dimensional
chemical structure of the following is shown in Figure 1. To
test these compounds' inhibitory efficacy against the target
proteins, two standard medicines, Modafinil and
Armodafinil, were obtained, as shown in Figure 1. Also,
Modafinil and Armodafinil are the two phytocompounds found
to have the best binding affinity with all the proteins selected.
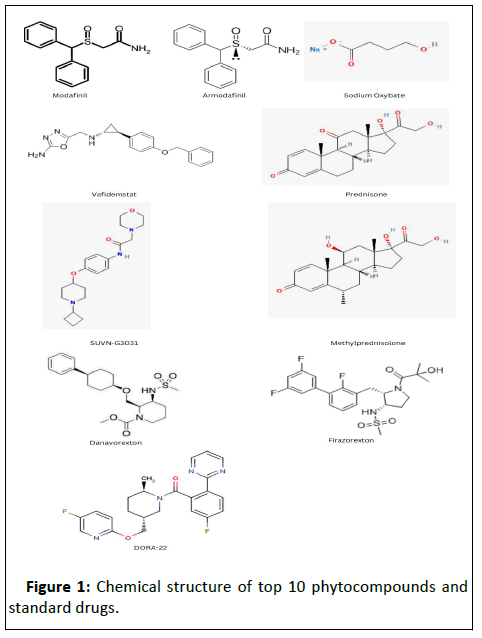
Figure 1: Chemical structure of top 10 phytocompounds and
standard drugs.
Protein retrieval and purification
PDB retrieved the two target proteins' Three-Dimensional (3D)
crystal structure, after getting its proper sources, as discussed in Table 1 and 2. After that, the proteins 4S0V and 6D26 were
purified in the BIOVIA Discovery software which is subjected to
its structure analysis which is seen in Figure 2 and 3 respectively.
In structural analysis, the Ramachandran plot, secondary
structure, and hydropathy plot are analyzed. Then, as shown in Table 3, several drugs used in narcolepsy treatment with their
specifications are discussed.
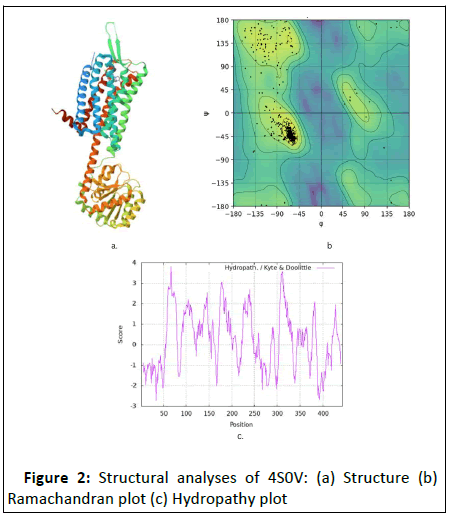
Figure 2: Structural analyses of 4S0V: (a) Structure (b)
Ramachandran plot (c) Hydropathy plot
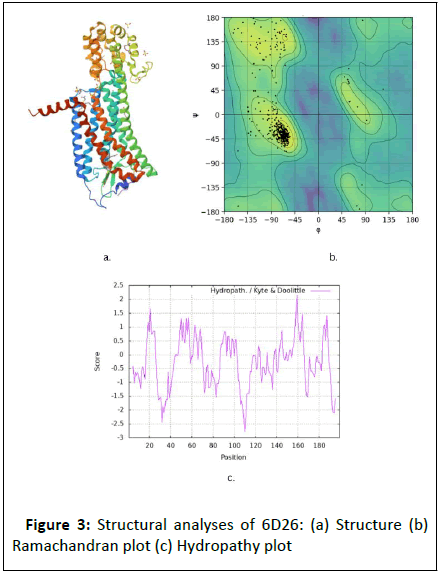
Figure 3: Structural analyses of 6D26: (a) Structure (b)
Ramachandran plot (c) Hydropathy plot
Molecular docking
Ten ligands were docked in the PyRx software against the
proteins 4S0V and 6D26 for this docking study. The
conformation with the lowest binding affinity and zero Root
Mean Square Deviation (RMSD) was selected as the compound's
optimal docking orientation upon the docking. Once the docking
was completed, the RMSD and binding affinity were recorded.
Out of all the fifteen phytocompounds, the common
phytocompounds for all the three target proteins that had
lower binding affinity than 7 and above were selected along
with these 10 phytocompounds the standard drugs were also
docked with each protein and their binding affinity was
recorded.
ADMET analysis
SwissADME is a web-based program that predicts and
calculates the properties of small organic compounds'
Absorption, Distribution, Metabolism, Excretion, and Toxicity
(ADMET). It focuses mostly on drug-like molecules and is
commonly utilized in drug discovery and development.
SwissADME provides a variety of ADMET-related features and
predictions, including:
• Lipophilicity: It computes the octanol/water partition
coefficient, which shows the compound's hydrophobicity and
ability to pass biological membranes.
• Pharmacokinetics: It predicts parameters such as chemical
absorption, distribution, metabolism, and excretion.
• Drug-likeness: SwissADME assesses the compound's
adherence to Lipinski's rule of five, a commonly used guideline
for evaluating drug-like qualities based on molecular weight,
lipophilicity, hydrogen bonding, and polar surface area.
• Toxicity predictions: It predicts numerous qualities such as
mutagenicity, tumorigenicity, irritability, and reproductive
impacts to determine possible toxicity.
• Bioavailability: SwissADME forecasts the compound's oral
bioavailability, an important aspect of drug development. Table
4 provides information on Human Intestinal Absorption (HIA),
the logarithm of molar solubility in water (log S), and the
solubility of various ligands.
| Ligand |
HIA |
Log S (ESOL) |
Solubility |
| Modafinil |
High |
-2.76 |
4.80e-01 mg/ml |
| Armodafinil |
High |
-2.76 |
4.80e-01 mg/ml |
| Na-Oxybate |
High |
-0.03 |
1.90e+02 mg/ml |
| Vafidemstat |
High |
-3.37 |
1.11e-04 mg/ml |
| SUVN-G3031 |
High |
-3.29 |
1.92e-01 mg/ml |
| Prednisone |
High |
-2.85 |
4.91e-01 mg/ml |
| Methylprednisolone |
High |
-3.26 |
2.07e-01mg/ml |
| DORA-22 |
High |
-4.79 |
1.29e-01 mg/ml |
| Firazorexton |
High |
-4.41 |
1.31e-04 mg/ml |
| Danavorexton |
High |
-3.81 |
1.89e-02 mg/ml |
Table 4: Absorption of phytocompounds.
Modafinil, armodafinil, and sodium oxybate all have high HIA,
indicating that they are readily absorbed in the human small
intestine.
The log S values of these compounds are −2.76, −2.76, and
0.03, respectively, reflecting their molar solubility in water.
Modafinil and armodafinil have a log S of -2.76, which corresponds
to a solubility of 4.80e-01 mg/ml, while sodium oxybate has a log S
of -0.03 and a higher solubility of 1.90e+02 mg/ml.
These properties suggest that these compounds are likely to
be well absorbed and have varying degrees of solubility. This is
an important factor in pharmacokinetic behavior and potential
therapeutic efficacy.
Table 5 demonstrates that modafinil, armodafinil, and sodium
oxybate have passed the Lipinski and pain alert tests. Lipinski's
rule of 5 could be a valuable rule for foreseeing the retention,
dispersion, and end of drugs, whereas torment alarms are
important for recognizing potential medication candidates that
will cause torment or distress to the organization. The reality
that these compounds have passed these tests suggests that
they show alluring pharmacokinetic and pharmacodynamic
properties, which may contribute to their adequacy in treating
narcolepsy and related indications.
| Ligands |
Lipinski |
Pain alerts |
| Modafinil |
Accepted |
0 |
| Armodafinil |
Accepted |
0 |
| Na-Oxybate |
Accepted |
0 |
| Vafidemstat |
Accepted |
0 |
| SUVN-G3031 |
Accepted |
0 |
| Prednisone |
Accepted |
0 |
| Methylprednisolone |
Accepted |
0 |
| DORA-22 |
Accepted |
0 |
| Firazorexton |
Accepted |
0 |
| Danavorexton |
Accepted |
0 |
Table 5: Medicinal chemistry of phytochemicals.
Table 6 presents the atomic weight, the number of hydrogen
acceptors, and the number of hydrogen givers for different
ligands, counting modafinil, armodafinil, sodium oxybate,
vafidemstat, SUVN-G30313031, prednisone, methylprednisolone,
DORA-22, firazorexton, and danavorexton. The atomic weight
values run from 273.35 g/mol to 424.44 g/mol, showing the
differing atomic sizes of these ligands. The number of hydrogen
acceptors and hydrogen givers gives experience in the chemical properties and potential official destinations of these ligands. For
illustration, modafinil and armodafinil have two hydrogen
acceptors and one hydrogen benefactor, whereas sodium oxybate
has three hydrogen acceptors and one hydrogen benefactor.
Vafidemstat has five hydrogen acceptors two hydrogen givers, and
so on for the other ligands.
| Ligands |
Molecular weight |
No. of hydrogen acceptors |
No. of hydrogen donors |
| Modafinil |
273.35 gms |
2 |
1 |
| Armodafinil |
273.35 gms |
2 |
1 |
| Na-Oxybate |
126.09 gms |
3 |
1 |
| Vafidemstat |
336.39 gms |
5 |
2 |
| SUVN-G3031 |
373.49 gms |
5 |
1 |
| Prednisone |
358.43 gms |
5 |
2 |
| Methylprednisolone |
374.47 gms |
5 |
3 |
| DORA-22 |
424.44 gms |
7 |
0 |
| Firazorexton |
470.51 gms |
8 |
2 |
| Danavorexton |
424.55 gms |
6 |
1 |
Table 6: Physiochemical properties of phytochemicals.
Table 7 gives data on the Blood-Brain Barrier (BBB) and skin
permeation of different ligands, counting modafinil, armodafinil,
sodium oxybate, vafidemstat, suvn-g3031, prednisone,
methylprednisolone, DORA-22, firazorexton, and danavorexton.
The BBB could be a particular boundary that isolates the
circulating blood from the extracellular liquid, securing the brain
from possibly hurtful substances. The table demonstrates that
modafinil, armodafinil, sodium oxybate, vafidemstat, prednisone, methylprednisolone, firazorexton, and danavorexton
don't cross the BBB, while suvn-g3031 do. Skin penetration is a
vital calculation within the transdermal conveyance of drugs, and the table shows that all the recorded ligands have comparative
skin saturation rates, extending from -6.31 cm/s to -7.52 cm/s
[7].
| Ligands |
Blood-brain-barrier |
Skin permeation |
| Modafinil |
No |
-6.75 cm/s |
| Armodafinil |
No |
-6.75 cm/s |
| Na-Oxybate |
No |
-7.52 cm/s |
| Vafidemstat |
No |
-6.78 cm/s |
| SUVN-G3031 |
Yes |
-6.97 cm/s |
| Prednisone |
No |
-7.45 cm/s |
| Methylprednisolone |
No |
-7.20 cm/s |
| DORA-22 |
Yes |
-6.31 cm/s |
| Firazorexton |
No |
-7.10 cm/s |
| Danavorexton |
No |
-6.96 cm/s |
Table 7: Distribution of phytochemicals.
As depicted in Table 8, CYP1A2, CYP2C19, CYP2C9, CYP2D6,
and CYP3A4 are all specific CYP450 chemicals. These are protein
iotas found on a very basic level inside the liver, even though
they can additionally be found in other tissues, like the inner
parts and lungs. CYPs play an imperative role within the steady
assimilation framework, breaking down and changing diverse,
inaccessible substances [5]. A few points of interest in
cytochromes are portrayed in Table 8 are:
• CYP1A2: Metabolizes caffeine, nicotine, and other
environmental toxins.
• CYP2C19: Metabolizes proton pump inhibitors,
antidepressants, and antiplatelets.
• CYP2C9: Metabolizes warfarin, NSAIDs, and some antiviral
drugs.
• CYP2D6: Metabolizes codeine, opioids, and antidepressants.
• CYP3A4: Metabolizes a wide range of drugs including statins,
antibiotics, and some anti-cancer drugs.
| Ligands |
CYP1A2 |
CYP2C19 |
CYP2C9 |
CYP2D6 |
CYP3A4 |
| Modafinil |
No |
No |
No |
No |
No |
| Armodafinil |
No |
No |
No |
No |
No |
| Na-Oxybate |
No |
No |
No |
No |
No |
| Vafidemstat |
No |
Yes |
No |
Yes |
Yes |
| SUVN-G3031 |
No |
No |
No |
Yes |
No |
| Prednisone |
No |
No |
No |
No |
No |
| Methylprednisolone |
No |
No |
No |
No |
No |
| DORA-22 |
No |
Yes |
Yes |
Yes |
Yes |
| Firazorexton |
No |
No |
No |
Yes |
Yes |
| Danavorexton |
No |
No |
No |
Yes |
Yes |
Table 8: Toxicity and excretion of phytochemicals.
Discussion
The above results highlight the potential of modafinil,
armodafinil, and sodium oxybate as viable medications for this
neurological clutter. The investigation centers on the use of these
wake-promoting specialists in overseeing the two essential side
effects of narcolepsy, Excessive Daytime Sleepiness (EDS) and
cataplexy. The discoveries of the investigation can be summarized
as follows:
• Modafinil, armodafinil, and sodium oxybate have been
recognized as potential drugs for treating narcolepsy, with
modafinil and armodafinil fundamentally focusing on EDS and
sodium oxybate tending to both EDS and cataplexy.
• The pharmacokinetic properties of these compounds play an
important role in their efficacy and safety in the treatment of
narcolepsy.
• Modafinil and armodafinil are readily absorbed, reaching peak
plasma concentrations 2 to 4 hours after administration
Sodium oxybate is rapidly absorbed within the clinical dose
range, with an absolute bioavailability of approximately
88D44.
• Human Intestinal Absorption (HIA) of these compounds is
high indicating that they are easily absorbed in the human
small intestine. The log S values of these compounds were
−2.76, −2.76, and -0.03, respectively, reflecting their molar
solubility in water.
• These ligands' Blood-Brain Barrier (BBB) and skin permeability
ds have been studied, providing insight into their
pharmacokinetic properties and potential therapeutic
applications. Modafinil, armodafinil, sodium oxybate,
vafidemstat, prednisone, methylprednisolone, firazorexton,
and danavorexton do not cross the BBB, whereas suvn-g3031
do. The rate of permeation through the skin ranges from
-6.31 cm/s to -7.52 cm/s.
• The above findings also highlight the importance of clinical
practice patterns and the need for comparative studies to
determine which drugs are most effective for specific patient
subgroups.
Conclusion
In conclusion, the research paper on drug discovery for
narcolepsy has given compelling proof supporting the potential
of modafinil, armodafinil, and sodium oxybate as viable
medications for this weakening neurological clutter. The
discoveries from different considerations, including randomized
controlled trials and efficient reviews, consistently demonstrate
the viability of these compounds in managing the essential side
effects of narcolepsy, especially Excessive Daytime Sleepiness
(EDS) and cataplexy. The pharmacokinetic properties of these
compounds, such as their tall Human Intestinal Absorption (HIA)
and favorable Blood-Brain obstruction (BBB) characteristics,
advance support their potential as first-line medicines for
narcolepsy. Moreover, the term paper emphasizes the
significance of evidence based hone parameters and the
requirement for proceeded investigation to optimize the
utilization of these compounds in clinical hone. Generally, the
paper's discoveries emphasize the significant role of modafinil,
armodafinil, and sodium oxybate within the current and future
pharmacological administration of narcolepsy, providing hope
for the progressed quality of life for people living with this
condition.
References
- Bassetti CC, Davis JA (2020) Narcolepsy: A Guide for Patients and Families.
- Khan IA (2018) Functional Foods: Bioactive Components and Applications.
- Hobson JA, Wilson MT, Stickgold R (2017). The Oxford Handbook of Sleep Physiology.
- Thorpy MJ (2019) Dreaming and Sleep Disorders.
- Paul R, de Montellano O (2015) Cytochrome P450: Evolution, Structure, Function and Biochemistry.
- Anders RA, Thompson WC (2014) Brain in Motion: The Neuroscience of Movement, Posture, and Balance.
- Timothy I (2016) Morgenstern: Narcolepsy and Hypersomnia.
Citation: Choudhary S (2024) Unraveling the Molecular Targets: Drug Discovery Strategies for Narcolepsy Treatment. Int J Drug Dev Res Vol: 16 No:3









