Mini Review - (2022) Volume 14, Issue 4
Update on pleiotropic immunologic and potential antiviral effects of antibiotics and their clinical impact
Romany Helmy Thabet1,2*,
Sana'a Mahmoud Muflih Bani Amer2,
Hamam Hani Nageeb Dwaghreh2,
Ansam Zakaria Issa Baniamer2,
Mohammad Rami Mohammad Tbaishat2 and
Erin Maher Wilson3
1Department of Pharmacology, Faculty of Medicine, Assiut University, Egypt
2Department of Basic Medical Sciences,, Faculty of Medicine, Yarmouk University, Irbid, Jordan
3Assiut Health Directorates, Egypt
*Correspondence:
Romany Helmy Thabet, Department of Pharmacology, Faculty of Medicine, Assiut University,
Egypt,
Email:
Received: 25-Mar-2022, Manuscript No. ipaom-22-12737;
Editor assigned: 28-Mar-2022, Pre QC No. P-12737;
Reviewed: 17-Apr-2022, QC No. Q-12737;
Revised: 22-Apr-2022, Manuscript No. R-12737;
Published:
30-Apr-2022
Abstract
While antibiotics are widely used as antimicrobial agents for treating a variety of bacterial infections, it has been reported that a majority of them also exert antiviral, anti-inflammatory, immunomodulatory, and antioxidant activities that may a significant role in alleviating many clinical disorders such as severe acute respiratory syndrome coronavirus 2 (SAR-SCoV-2). Interestingly, literature mentioned that macrolide antibiotics (e.g., azithromycin) significantly reduce viral replication, downregulate the inflammatory cascade and excessive cytokine production, and decrease mortality. In the same line, fluoroquinolones as moxifloxacin and ciprofloxacin suppress COVID-19 replication, overproduction of nitric oxide in the lungs besides inhibiting inflammatory cell responses. Similarly, various antibiotics, such as doxycycline, clarithromycin, ceftriaxone, amoxicillin, amoxicillin-clavulanic acid, ampicillin, gentamicin, benzylpenicillin, piperacillin/tazobactam, ciprofloxacin, ceftazidime, cefepime, vancomycin, meropenem, and cefuroxime among others, were recommended for use in the management of COVID-19 owing to their pleiotropic activities. The current review illustrates the potential immunologic activities beyond the antibacterial effect of different antibiotics in clinical practice from the pharmacological point of view.
Keywords
Antibiotic; Macrolides; COVID-19; Pleiotropic data
Introduction
Antibiotics are now widely prescribed in various protocols
for treating COVID-19 infections, especially in severe
cases. The data in the literature about the antiviral role of
these antibiotics are greatly contradictory. Despite their
known adverse effects on patients' health, physicians usually
take into consideration the benefits versus risks ratio and
insist on using them in therapeutic protocols during viral
infections. Piperacillin/tazobactam, meropenem, and coamoxiclav
drugs were used mainly in critical care units for
COVID patients [1-3]. there should be good management
while using antibiotics to prevent superinfection and
resistance [4]. Moreover, physicians should always
consider that a virus may be a superinfection [5]. Despite
the use of doxycycline as an antiviral drug, there are
some contraindications between published researches on
whether or not this drug is causing serious side effects such
as esophagitis, ulceration, and resistance to the drug [6].
Azithromycin was found to be effective against COVID-19
due to its antiviral and immunomodulating properties, such
as decreasing the viral load and release, as well as preventing
lung fibrosis so it is helpful in the treatment journey [7-9].
Many virally ill patients are taking cefepime for the claims
that it has antiviral effects although when influenza A study
was conducted, it proved otherwise, so more researches
are needed for this drug [10]. Ceftazidime shows antiviral
activity against COVID-19 so it should be considered as
the first drug of choice according to Chang Dong et al
[11]. Although fluoroquinolones have antiviral effects and
showed effectiveness against CMV, VZV, HSV-1, HSV-2,
HCV, and HIV, their side effects are wild and serious since
they affect the nervous system, tendons, and joints [12,13].
However, other researchers state that some types of them
are the best antibiotics to be used for COVID due to their
pharmacokinetic profile [12]. A study aimed to measure
the changes and patterns of national antimicrobial use for
one year preceding and one year during the COVID-19
pandemic in Jordan reported that there was an increase in
the use of several antibiotics during 2020 compared with
2019. Third-generation cephalosporins, carbapenems,
macrolides, and lincosamides are the most frequently used
drug and cephalosporins the least. In 2020 there was a
marked reduction in amoxicillin and on the other hand,
the use of azithromycin highly increased. Moreover, there
was an increase in using hydroxychloroquine in 2020
compared with 2019 [14]. Based on the aforementioned data, it is obvious that most available antibiotics have
significant activities notably potential antiviral effects. The
current review highlights the different possible antiviral
mechanisms that lead to their potential clinical use in
COVID-19 outbreaks and focuses on the current status
protocols used in Jordan compared with those worldwide.
B-Lactams: Beyond Antibacterial
Effects
Antibiotics may be prescribed for patients with
COVID-19 due to confirmed superinfection with
bacteria. Among patients in the intensive care unit (ICU),
Piperacillin/tazobactam was the most commonly used
antibiotic [1]. Many antibiotics are recommended for
COVID-19 management, such as piperacillin/tazobactam.
However, there should be wisdom while using antibiotics to
prevent their evolution in having antimicrobial properties
and thereby becoming inefficient [4]. A case report talked
about an old man diagnosed with end-stage renal disease
(ESRD) on hemodialysis, influenza A and COVID-19
as a co-infection. He was experiencing worsening flulike
symptoms, including fever of up to 38.6°C, nonproductive
cough, generalized abdominal pain, nausea,
vomiting, and liquid green diarrhea. He started taking
oseltamivir for influenza and vancomycin/cefepime for the
probable bacterial cause of his pneumonia and diarrhea.
This patient had a superinfection of COVID-19, so the
probability of the patient having COVID should not fail
to be noticed by the physicians, even when other viruses
are causing the symptoms like influenza in this case [5].
Liu C, et al. [2] made a comparison between the suspected
bacterial infection patients and patients without bacterial
infection but with COVID-19, the risk factors of mortality
and the incidence of acute organ injury were analyzed.
The suspected bacterial infection group had more severely
ill patients, more deaths, and more acute organ injuries.
The death rate increased in suspected bacterial infection
patients taking intravenous moxifloxacin and meropenem,
while oral antibiotics reduced mortality in this group. In
addition to that, the mortality of the patients without
bacterial infection also increased due to penicillin and
meropenem. Suspected bacterial infection patients had
negative clinical outcomes compared to patients without
bacterial infection. Antibiotics were associated with
an increased risk for acute organ injury in hospitalized
patients with COVID-19. Antibiotics were useless for
most patients and correlated with an increase in deaths.
The real use of antibiotics failed to give the expected results.
A comprehensive antimicrobial plan and guidelines should
be applied in the time of COVID-19 [2]. There is a study
that included a comparison between COVID-19 positive
and negative patients and patients on non-critical care
and critical care units. COVID-19 has been a challenge
for antibacterial stewardship due to bacterial co-infection,
which caused recommendations for suspected bacterial
respiratory tract infection complicating COVID-19. In
non-critical care wards, amoxicillin, doxycycline, and
co-amoxiclav accounted for over half of all antibiotics. Moreover, meropenem, piperacillin-tazobactam, and coamoxiclav
accounted for approximately half prescribed
in critical care. Systemic antifungals were prescribed in
9.8% of critical care patients. In non-critical care units,
COVID-19 hospitalized patients had a low proportion of
broad-spectrum antibiotics as well as a low prevalence of
antibiotic prescribing. On the other hand, in the critical care
unit, broad-spectrum antibiotics and antifungal prescribing
was observed indicating the importance of infection
prevention and control and stewardship initiatives in this
setting [3]. Amoxicillin/Clavulanic acid was prescribed for
pneumonia-like lower airway symptoms and persisted for
14 days. A high-flow nasal cannula (HFNC) was initiated
as respiratory supportive therapy and was instrumental in
the clinical recovery of the patient after seven days, proving
its efficacy as a successful treatment for respiratory support
in a patient with active pneumonia from COVID-19
[15]. Even though cefepime is a proven- food and drug
administration (FDA) antibacterial drug, sometimes it
exhibits some antiviral activity. A study was made to explore
new drugs related to the treatment of influenza A virus
endonuclease protein, one of these drugs was cefepime
which exhibited the best interactions with the influenza
A virus endonuclease protein but eventually, the testing
showed no antiviral activity because it showed no reduction
in the viral plaque numbers, but further studies are needed
to prove this in vitro study [10]. A case report of a 51 years
old man with several diseases along with COVID-19 was
administering cefepime because it can decrease secondary
bacterial infections but not the COVID-19 itself so
more studies are needed to discover the relation between
antibiotics and COVID-19 [16]. In another case report of
a 78 years old man who was diagnosed with urinary tract
infection and COVID-19 along with other diseases, the
patient has been told to take cefepime, but it appeared
that there was no effect on the virus and it only reduced
the infection of the urinary tract [17]. Research by Chang
Dong, et al showed that ceftazidime is the best potential
inhibitor for the COVID-19 since it mainly needs to
bind to the ACE2 found on the surface of the host cell to
enter and fuse with the cell. It inhibits the interaction of
ACE2 with the viral spikes of COVID-19 (spike receptorbinding
domain), so ceftazidime can be thought of as an
antibacterial and antiviral effect and should be considered
as a first-line antibiotic for COVID-19 treatment. Also, it
showed negligible cytotoxicity even at high concentrations,
indicating its safety for clinical usage [11].
Pleiotropic Effects of Macrolide Antibiotics
Macrolides have anti-inflammatory and
immunomodulating actions in addition to their
antibacterial effects, so these properties may ensure the
efficacy of macrolides in respiratory viral infections
especially there is much data showed that the macrolides
reduced the receptors of viruses, excessive cytokines
production, and virus replication as well as downregulated
the inflammatory cascade (Fig.1). Additionally, they may reduce the exacerbation of the virus [18]. There
are different types of macrolides such as azithromycin
which has been proposed as a potential treatment due to
immunomodulating and antiviral properties as there are
vitro studies have demonstrated the capacity of azithromycin
in reducing the production of pro-inflammatory cytokines
such as interleukin-8 (IL-8), interleukin-6 (IL-6), and
tumor necrosis factor α (TNF α), reducing the oxidative
stress and modulating the functions of T helper cells
[7]. CD147 is a receptor of COVID-19 on host cells
and it is a novel route for COVID-19 invasion, but the
azithromycin may be beneficial in reducing the viral load
of hospitalized patients by interfering with ligand/CD147
receptor interactions, may decrease the expression of some
metalloproteinases which are downstream to CD147
and may decrease the viral replication and release [8].
Azithromycin maintains epithelial cell integrity or prevents
lung fibrosis so it can be beneficial in COVID-19 treatment
[9]. Azithromycin prevents COVID-19 infection by
raising the levels of interferons and interferon-stimulated
proteins which helps in reducing the replication and
releasing of the virus [19]. Perhaps, azithromycin antiviral
effects result from interfering with receptor-mediated
binding, viral lysosomal escape, intracellular cell-signaling
pathways, and enhancing type I, III interferon expression
[20]. It is proposed as a medication for COVID-19 in
association with hydroxychloroquine or chloroquine
[21]. A multi-center retrospective observational study
in the United States of consecutive patients hospitalized
with a COVID-19 has reported that the treatment with
hydroxychloroquine alone significantly decreased the
mortality, and hydroxychloroquine in combination with
azithromycin exhibited a highly significant decrease in
mortality [22].
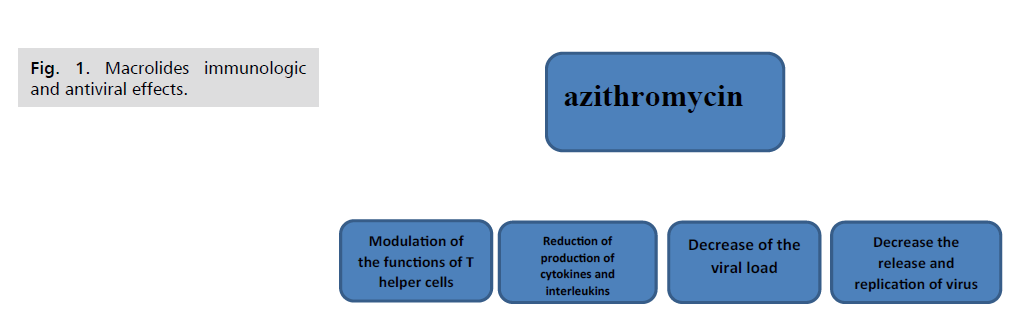
Figure 1: Macrolides immunologic and antiviral effects.
A French study has reported that 100% of patients
treated with a combination of hydroxychloroquine and
azithromycin were virologically cured compared to 57.1%
of patients treated with hydroxychloroquine only, and
12.5% in the control group at day 6 post-inclusion [23].
On the other hand, there must be caution in using the
combination of azithromycin and hydroxychloroquine
due to the potential cardiac harm, especially in more
fragile patients such as those with cardiovascular disease
or prolonged QTc [7]. More recent evidence has raised
serious safety concerns on the use the azithromycin
with hydroxychloroquine or chloroquine in COVID-19
treatment because all these drugs have a role in arrhythmia [21]. Recent evidence suggests that this toxicity may be
related to hydroxychloroquine [9]. Egyptian randomized
trial of patients with mild COVID-19 aims to testify
whether adding azithromycin or clarithromycin to a
standard of care regimen was superior to standard of
supportive care alone in those patients who received only
symptomatic treatment for control of cough and fever.
The results of this trial have shown a signifcant early
improvement of symptoms (fever, dyspnea, and cough)
as well as significant early conversion of COVID-19
polymerase chain reaction (PCR) to negative in patients
who treated with either azithromycin or clarithromycin
compared who standardly cured. Using azithromycin and
clarithromycin in mild COVID-19 could be effective in
early control of fever and PCR negative conversion, but
the safety and efficacy of macrolides and their conjunction
with other therapy modalities need more research [24].
The empirical practice of azithromycin in COVID-19
treatment did not show good quality in clinical data.
Use of Tetracycline in Covid
Infections
Doxycycline works as antiviral, anti-inflammatory,
immunomodulatory drug [25]. It also has a great efficacy
by inhibiting matrix metalloproteinases (MMP) which
was leading to damage to the base plate and increased
vascular permeability and as a result used to prevent lung
damage, alongside reducing pro-inflammatory cytokines in
addition to interleukin-6 [26]. Although there is a study
that proves that there are no side effects of doxycycline
[27]. New research has emerged showing its effects and
negative aspects in the long run such as a decrease in the
body’s ability to absorb it when it is taken with antacid and
iron tablets. It also can cause esophagitis, ulceration and
the reuse of doxycycline in COVID-19 is a major driver of
antimicrobial resistance [6]. Another study was conducted
in New York on patients who were given doxycycline in
the early stage of treatment to assess fever, shortness of
breath, cough, and oxygen saturation/oxidative stress,
the study showed the importance of using doxycycline in
patients to recover from the fever, cough, and shortness of
breath also it is associated with improved clinical outcome,
reduced hospitalization and decrease mortality [25]. In
clinical research that was carried out in Pakistan, it is found
that doxycycline is effective in reducing the symptoms
of COVID-19 and increasing body desire for recovery
and healing process in patients [28]. In addition to the effectiveness and importance of doxycycline as a single
drug, it has also proven its significance as a combination
therapy with other agents, such as the study that was
conducted on the efficacy of ivermectin with doxycycline
for patients with mild to moderate COVID-19, the
treatment was randomized and placebo-controlled for
infected patients, the results showed that patients with
COVID-19 infections who received the aforementioned
combination, recovered earlier and had negative results for
COVID-19 than those who received placebo treatment
[29]. In another randomized trial that has been clinically
tested in Indonesia, the patients who received lopinavir/
ritonavir and doxycycline increased in the c-reactive protein
(CRP) and IL-6 and significantly reduced IL-10 and TNF-a
levels [30]. COVID-19 can cause a variety of neurological
manifestations which may vary in severity, one of the
symptoms that cause is meningitis and doctors managed
the disease pharmacologically by using intravenous
amoxicillin and ceftriaxone to treat bacterial meningitis in
a 70-years old female patient [31]. An experimental study
reported that imipenem is the most widely used antibiotic
in the intensive care unit (ICU) for an average duration of
3 weeks [32].
Emerging benefits of fluoroquinolones in viral infections
Fluoroquinolones may have antiviral actions against
vaccinia virus, papovavirus, CMV, VZV, HSV-1, HSV-
2, HCV and HIV, ciprofloxacin and moxifloxacin may
exhibit some replication inhibitory action by binding to its main protease significantly than chloroquine and
nelfinavir. Noteworthy, levofloxacin and moxifloxacin
are just like ceftazidime that can be thought of as firstline
therapeutic agents for the management of severe
community-acquired pneumonia [12]. In contrast with
FDA in 2018, Irene Karampela and maria dalamaga
said that fluoroquinolones, especially levofloxacin and
moxifloxacin, are considered to be the best drug out of all
antibiotics because of their good pharmacokinetic profile,
increased concentration in the lungs, and excellent safety
profile compared with other antibiotics such as b-lactams
and macrolides [12]. Ciprofloxacin and moxifloxacin exert
a strong capacity for binding to COVID-19 Main protease
and bind to the protein active site more strongly than the
native ligand. This indicates the basis for a possible new
strategy of COVID-19 treatment and ciprofloxacin and
moxifloxacin repositioning to treat COVID-19 infection,
but more studies are needed to clarify their efficacy [33].
In a study where the potency and cellular toxicity of four
fluoroquinolones (enoxacin, ciprofloxacin, levofloxacin,
and moxifloxacin) were assessed in Vero cells and A549 cells
engineered to overexpress ACE2, the COVID-19 entry
receptor, the results showed that all four fluoroquinolones
suppressed COVID-19 replication at a high micromolar
concentration in both cell types. On another hand, they
concluded that Fluoroquinolones are not ideal antiviral
candidates for COVID-19 treatment because the potency
of them while they suppressed the virus replication was
low and there was minimal cellular toxicity following
treatment with them [34]. A case report with moxifloxacin treatment for COVID-19 positive-tested patient, the
patient showed after 8 days of IV injection of 400 mg
of moxifloxacin an obvious reduction of symptoms and
recovered from COVID-19 [35]. another case report with
COVID-19 showed also after treatment with the same
dose of moxifloxacin with the latter case report, the patient
showed a marked clinical and radiological improvement
after several days of moxifloxacin treatment [36]. The side
effects of fluoroquinolones make them the last choice out
of all other antibiotics to treat infections or viruses which
include potential or permanent disabling in tendons,
joints, nerves, and central nervous system as mentioned by
the FDA in 2018 [13].
Discussion and Conclusion
Beyond its antibacterial activities, different antibiotics
elicit pleiotropic effects that have the potential to be of
great benefit in the clinical treatment of viral infection e.g.
COVID-19-induced pneumonia or lung injury preceding
event of secondary infections by bacteria. Impairing
viral entry, interfering with viral replication, attenuating
cytokine storm by reduction of interleukin (IL)-6, IL-
1, tumor necrosis factor (TNF)-α and other combating
oxidative stress; explain the potential efficacy of antibiotics
in the management of severe cases of viral infections (Fig.2).
More experimental studies and randomized clinical trials
are needed for investigating their role in reducing mortality
and improving clinical outcomes
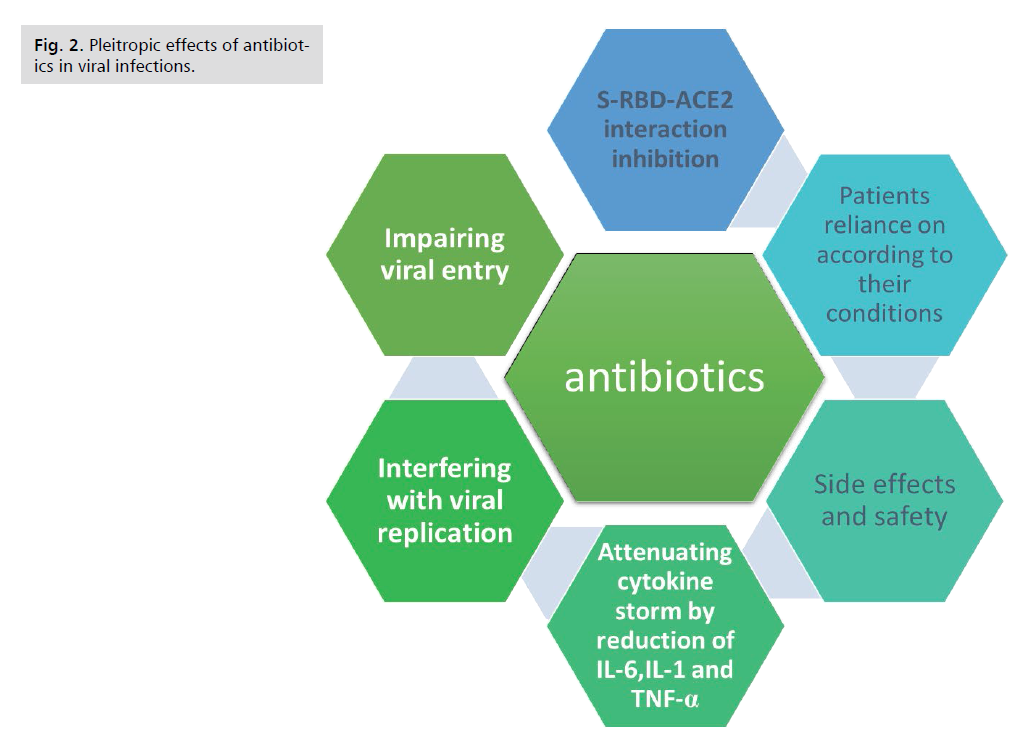
Figure 2: Pleitropic effects of antibiotics in viral infections.
Conflicts of Interest
All authors declare that they have no conflict of interest.
Data Availability
All data generated or analyzed during study are included
in this review.
Authors Contribution
RHT, the corresponding author, has the major
contribution in designing, coordinating the duties of each
co-author and edited the final version the manuscript.
SMMBA, searching in literature about macrolides update
pleiotropic activities; HHND, help in designing figures
and searching about beta lactam antibiotics; AZIB, help
in searching about tetracyclines and fluoroquinolones;
MRMT, help in writing manuscript; EMW, help in revising
manuscript linguistically. All authors read and approved
the final manuscript.
Funding
This study was not funded by any specific grant from
funding agencies in the public, commercial, or not-forprofit
sectors.
Acknowledgments
None.
REFERENCES
- Beović B, Doušak M, Ferreira-Coimbra J, et al. Antibiotic use in patients with COVID-19: A ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75:3386-3390.
Google Scholar, Crossref
- Liu C, Wen Y, Wan W, et al. Clinical characteristics and antibiotics treatment in suspected bacterial infection patients with COVID-19. Int Immunopharmacol. 2021;90:107157.
Google Scholar, Crossref
- Seaton RA, Gibbons CL, Cooper L, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect Chemother. 2020;81:952-960.
Google Scholar, Crossref
- Adebisi YA, Jimoh ND, Ogunkola IO, et al. The use of antibiotics in COVID-19 management: A rapid review of national treatment guidelines in 10 African countries. Trop Medi Health. 2021;49:1-5.
Google Scholar, Crossref
- Jing R, Vunnam RR, Schnaubelt E, et al. Co-infection of COVID-19 and influenza A in a hemodialysis patient: A case report. BMC Infect Dis. 2021; 21:1-6.
Google Scholar, Crossref
- Narendrakumar L, Joseph I, Thomas S. Potential effectiveness and adverse implications of repurposing doxycycline in COVID-19 treatment. Expert Review of Anti-infective Therapy. 2021;19:1001-1008.
Google Scholar, Crossref
- Pani A, Lauriola M, Romandini A, et al. Macrolides and viral infections: Focus on azithromycin in COVID-19 pathology. Int J Antimicrob Agents. 2020;56:106053.
Google Scholar, Crossref
- Ulrich H, Pillat MM. CD147 as a target for COVID-19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434-440.
Google Scholar, Crossref
- Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, et al. Azithromycin in the treatment of COVID-19: A review. Expert Rev Anti-InfectTher. 2021;1:147-163.
Google Scholar, Crossref
- Sai Disha K, Rashmi Puranik, Sudheesh N, et al. Structure-based identification of small molecules against influenza A virus endonuclease: An in silico and in vitro approach. Pathog Dis. 2020;78:ftaa032.
Google Scholar, Crossref
- Lin C, Li Y, Zhang Y, et al. Ceftazidime is a potential drug to inhibit SARS-CoV-2 infection in vitro by blocking spike protein–ACE2 interaction. Signal Transduction and Targeted Therapy. 2021;6:1-4.
Google Scholar, Crossref
- Karampela I, Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch Med Res. 2020;51:741-742.
Google Scholar, Crossref
- US Food and Drug Administration. Drug Safety Communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients. 2018.
Google Scholar, Crossref
- Al-Azzam S, Mhaidat NM, Banat HA, et al. An assessment of the impact of coronavirus disease (COVID-19) pandemic on national antimicrobial consumption in Jordan. Antibiotics. 2021;10:690.
- Van Gorp GA, Sanders PJ, Van Waardenburg DA, et al. COVID-19 pneumonia successfully managed with high-flow nasal cannula in a 15-year-old boy. BMJ Case Rep CP. 2021;14:e239682.
Google Scholar, Crossref
- Beraldo RF, Marcondes MB, Dos Santos MN, et al. COVID-19 in a patient with liver cirrhosis. Am J Med Case Rep. 2021;22:e929948-1.
Google Scholar, Crossref
- Haraszti S, Sendil S, Jensen N. Delayed presentation of acute generalized exanthematous pustulosis following treatment with cefepime in a patient with COVID-19 without the use of hydroxychloroquine. Am J Med Case Rep. 2020;21:e926901-1.
Google Scholar
- Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediat Inflamm. 2012.
Google Scholar, Crossref
- Batiha GE, Zayed MA, Awad AA, et al. Management of SARS-CoV-2 infection: Key focus in macrolides efficacy for COVID-19. Front Med. 2021;8.
Google Scholar, Crossref
- Gyselinck I, Janssens W, Verhamme P, et al. Rationale for azithromycin in COVID-19: An overview of existing evidence. BMJ Open Respir Res. 2021;8(1):e000806.
Google Scholar, Crossref
- Sultana J, Cutroneo PM, Crisafulli S, et al. Azithromycin in COVID-19 patients: Pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Safety. 2020;43:691-698.
Google Scholar, Crossref
- Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. IntJ Infect Dis. 2020;97:396-403.
Google Scholar, Crossref
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Anti Microb Agents. 2020;56:105949.
Google Scholar, Crossref
- Rashad A, Nafady A, Hassan MH, et al. Therapeutic efficacy of macrolides in management of patients with mild COVID-19. Sci Rep. 2021;11:1-7.
- Alam MM, Mahmud S, Rahman MM, et al. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020;12(8).
Google Scholar, Crossref
- Malek AE, Granwehr BP, Kontoyiannis DP. Doxycycline as a potential partner of COVID-19 therapies. ID Cases. 2020;21:e00864.
Google Scholar, Crossref
- Yates PA, Newman SA, Oshry LJ, et al. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther Adv Respir Dis. 2020;14:1753466620951053.
Google Scholar, Crossref
- Ahsan T, Rani B, Siddiqui R, et al. Clinical variants, characteristics, and outcomes among COVID-19 patients: A case series analysis at a tertiary care hospital in Karachi, Pakistan. Cureus. 2021;13.
Google Scholar, Crossref
- Mahmud R, Rahman MM, Alam I, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: A randomized trial. J Int Med Res. 2021;49(5):03000605211013550.
Google Scholar, Crossref
- Rachman BE, Miatmoko A, Lardo S, et al. A randomized, double-blind, multicenter clinical study comparing the efficacy and safety of a drug combination of lopinavir/ritonavir-azithromycin, lopinavir/ritonavir-doxycycline, and azithromycin-hydroxychloroquine for patients diagnosed with mild to moderate COVID-19 infections. Biochem Res Int. 2021:6685921.
Google Scholar
- Hurn E, Dickinson L, Abraham JA. Bacterial meningitis and COVID-19: A complex patient journey. BMJ Case Rep CP. 2021;14:e239533.
Google Scholar, Crossref
- Mustafa L, Tolaj I, Baftiu N, et al. Use of antibiotics in COVID-19 ICU patients. J Infect Dev Ctries. 2021;15:501-505.
Google Scholar, Crossref
- Marciniec K, Beberok A, Pęcak P, et al. Ciprofloxacin and moxifloxacin could interact with SARS-CoV-2 protease: preliminary in silico analysis. Pharmacol Rep. 2020; 72:1553-1561.
- Scroggs SL, Offerdahl DK, Flather DP, et al. Fluoroquinolone antibiotics exhibit low antiviral activity against SARS-CoV-2 and MERS-CoV. Viruses. 2020;13:8.
Google Scholar, Crossref
- Ding D, Zhu C, Yao W. A cured patient with 2019-nCoV pneumonia. Am J Med. 2020;133:1291-1292.
- Zhu F, Cao Y, Xu S, et al. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529-530.
Google Scholar, Crossref








